 Found 439 hits with Last Name = 'masuda' and Initial = 't'
Found 439 hits with Last Name = 'masuda' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50005414
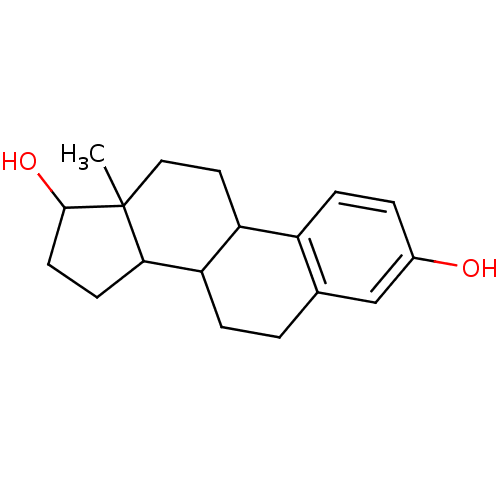
((estradiol)13-Methyl-7,8,9,11,12,13,14,15,16,17-de...)Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260712

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-iso...)Show SMILES CC(C)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O3/c1-12(2)10-11-22-13-8-6-7-9-14(13)23(18(22)26)17(25)21-15(16(20)24)19(3,4)5/h6-9,12,15H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220612
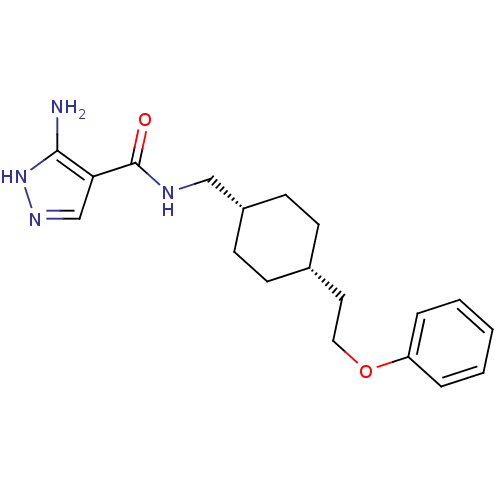
(3-amino-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)m...)Show SMILES Nc1[nH]ncc1C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:10.10,13.14,(18.38,-36.74,;18.71,-38.24,;17.68,-39.39,;18.45,-40.72,;19.96,-40.4,;20.12,-38.87,;21.45,-38.09,;21.44,-36.55,;22.78,-38.86,;24.11,-38.08,;25.45,-38.85,;25.45,-40.39,;26.78,-41.15,;28.11,-40.39,;29.44,-41.16,;30.77,-40.39,;32.11,-41.17,;33.44,-40.4,;34.77,-41.17,;36.11,-40.41,;36.11,-38.86,;34.77,-38.09,;33.44,-38.86,;28.11,-38.85,;26.78,-38.07,)| Show InChI InChI=1S/C19H26N4O2/c20-18-17(13-22-23-18)19(24)21-12-15-8-6-14(7-9-15)10-11-25-16-4-2-1-3-5-16/h1-5,13-15H,6-12H2,(H,21,24)(H3,20,22,23)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50377918

(CHEMBL1162994)Show SMILES CC(C)(C)C(NC(=O)n1c2ccccc2n(CCC2CCOCC2)c1=O)C(N)=O Show InChI InChI=1S/C21H30N4O4/c1-21(2,3)17(18(22)26)23-19(27)25-16-7-5-4-6-15(16)24(20(25)28)11-8-14-9-12-29-13-10-14/h4-7,14,17H,8-13H2,1-3H3,(H2,22,26)(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220606
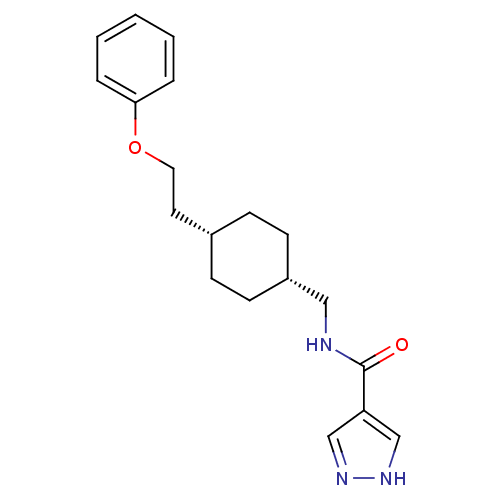
(CHEMBL249093 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1cn[nH]c1 |wU:4.3,7.7,(17.52,-15.71,;17.53,-17.25,;18.86,-18.01,;20.19,-17.24,;21.53,-18.01,;21.53,-19.55,;22.86,-20.31,;24.19,-19.55,;25.52,-20.32,;26.85,-19.55,;28.19,-20.32,;29.52,-19.55,;30.85,-20.33,;32.19,-19.56,;32.19,-18.02,;30.85,-17.25,;29.52,-18.02,;24.19,-18.01,;22.86,-17.23,;16.2,-18.02,;14.79,-17.4,;13.76,-18.55,;14.53,-19.88,;16.04,-19.55,)| Show InChI InChI=1S/C19H25N3O2/c23-19(17-13-21-22-14-17)20-12-16-8-6-15(7-9-16)10-11-24-18-4-2-1-3-5-18/h1-5,13-16H,6-12H2,(H,20,23)(H,21,22)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260627

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-(2-...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(N)=O |r| Show InChI InChI=1S/C20H29N5O4/c1-20(2,3)16(17(21)26)22-18(27)25-15-7-5-4-6-14(15)24(19(25)28)9-8-23-10-12-29-13-11-23/h4-7,16H,8-13H2,1-3H3,(H2,21,26)(H,22,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260672
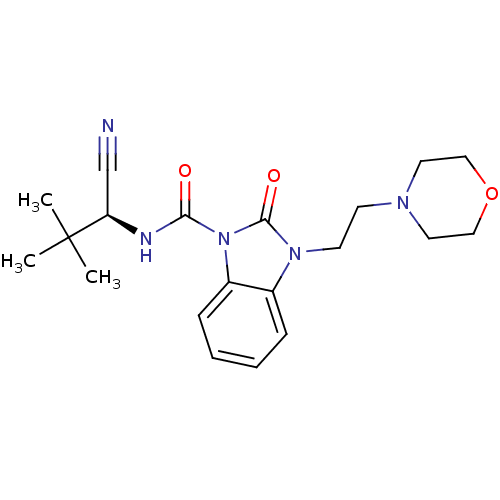
((S)-N-(1-cyano-2,2-dimethylpropyl)-3-(2-morpholino...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C#N |r| Show InChI InChI=1S/C20H27N5O3/c1-20(2,3)17(14-21)22-18(26)25-16-7-5-4-6-15(16)24(19(25)27)9-8-23-10-12-28-13-11-23/h4-7,17H,8-13H2,1-3H3,(H,22,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260673

((S)-N-(2,2-dimethyl-1-(2-methyl-2H-tetrazol-5-yl)p...)Show SMILES Cn1nnc(n1)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C21H30N8O3/c1-21(2,3)17(18-23-25-26(4)24-18)22-19(30)29-16-8-6-5-7-15(16)28(20(29)31)10-9-27-11-13-32-14-12-27/h5-8,17H,9-14H2,1-4H3,(H,22,30)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220592
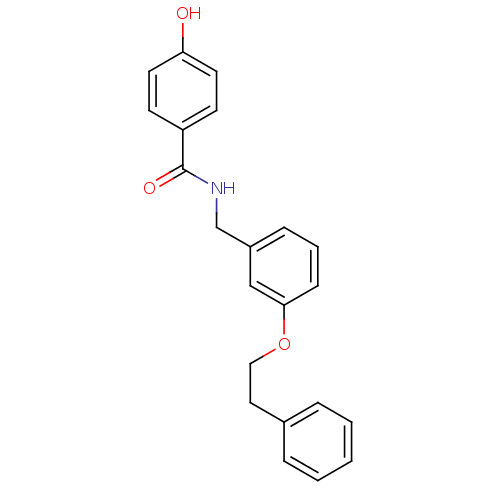
(CHEMBL248875 | N-(3-phenethoxybenzyl)-4-hydroxyben...)Show InChI InChI=1S/C22H21NO3/c24-20-11-9-19(10-12-20)22(25)23-16-18-7-4-8-21(15-18)26-14-13-17-5-2-1-3-6-17/h1-12,15,24H,13-14,16H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220611
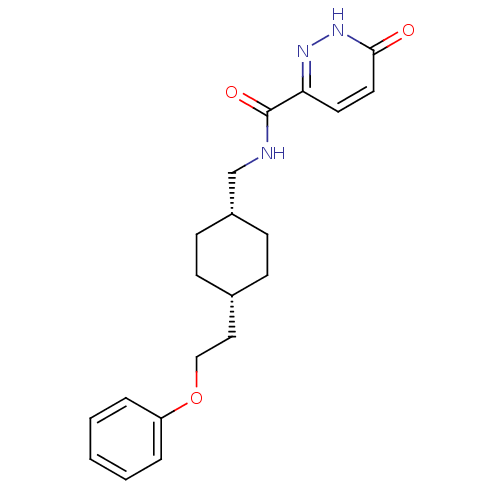
(6-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccc(=O)[nH]n1 |wU:4.3,7.7,(20.2,-34.76,;20.2,-36.3,;21.54,-37.06,;22.87,-36.29,;24.2,-37.05,;24.2,-38.59,;25.53,-39.36,;26.86,-38.59,;28.2,-39.37,;29.53,-38.6,;30.86,-39.37,;32.2,-38.6,;33.53,-39.38,;34.86,-38.61,;34.87,-37.07,;33.53,-36.3,;32.2,-37.07,;26.86,-37.05,;25.53,-36.28,;18.87,-37.07,;17.54,-36.3,;16.2,-37.07,;16.21,-38.61,;14.88,-39.39,;17.55,-39.38,;18.88,-38.6,)| Show InChI InChI=1S/C20H25N3O3/c24-19-11-10-18(22-23-19)20(25)21-14-16-8-6-15(7-9-16)12-13-26-17-4-2-1-3-5-17/h1-5,10-11,15-16H,6-9,12-14H2,(H,21,25)(H,23,24)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220608
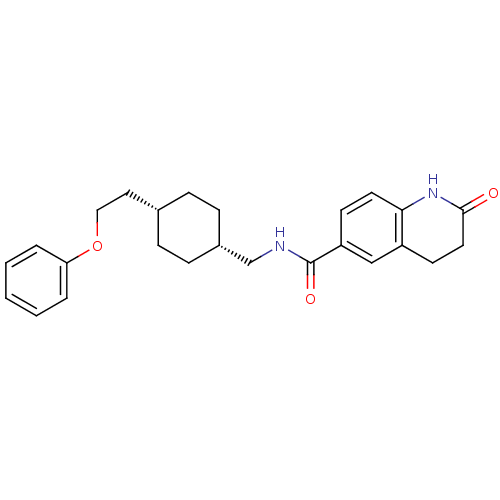
(2-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccc2NC(=O)CCc2c1 |wU:7.7,4.3,(21.1,-26.08,;21.1,-27.62,;22.44,-28.39,;23.77,-27.62,;25.11,-28.38,;25.11,-29.92,;26.44,-30.68,;27.76,-29.92,;29.1,-30.69,;30.43,-29.92,;31.76,-30.7,;33.1,-29.93,;34.43,-30.7,;35.77,-29.94,;35.77,-28.4,;34.43,-27.62,;33.1,-28.4,;27.76,-28.38,;26.44,-27.6,;19.77,-28.39,;19.78,-29.93,;18.46,-30.7,;17.11,-29.94,;15.78,-30.71,;14.45,-29.94,;13.11,-30.71,;14.45,-28.4,;15.78,-27.63,;17.11,-28.4,;18.44,-27.62,)| Show InChI InChI=1S/C25H30N2O3/c28-24-13-11-20-16-21(10-12-23(20)27-24)25(29)26-17-19-8-6-18(7-9-19)14-15-30-22-4-2-1-3-5-22/h1-5,10,12,16,18-19H,6-9,11,13-15,17H2,(H,26,29)(H,27,28)/t18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260709
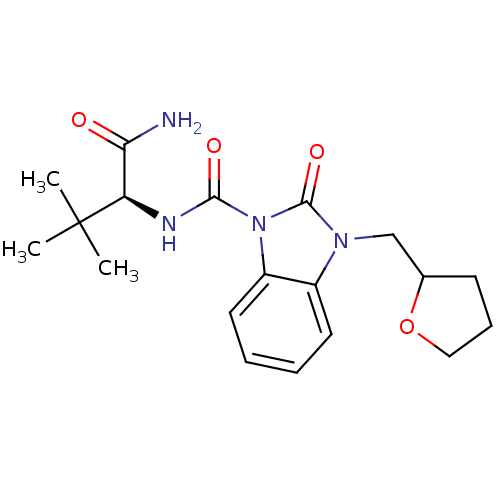
(CHEMBL496497 | N-((S)-1-amino-3,3-dimethyl-1-oxobu...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CC2CCCO2)c1=O)C(N)=O |r| Show InChI InChI=1S/C19H26N4O4/c1-19(2,3)15(16(20)24)21-17(25)23-14-9-5-4-8-13(14)22(18(23)26)11-12-7-6-10-27-12/h4-5,8-9,12,15H,6-7,10-11H2,1-3H3,(H2,20,24)(H,21,25)/t12?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50025452

((testosterone)17-Hydroxy-10,13-dimethyl-1,2,6,7,8,...)Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220602
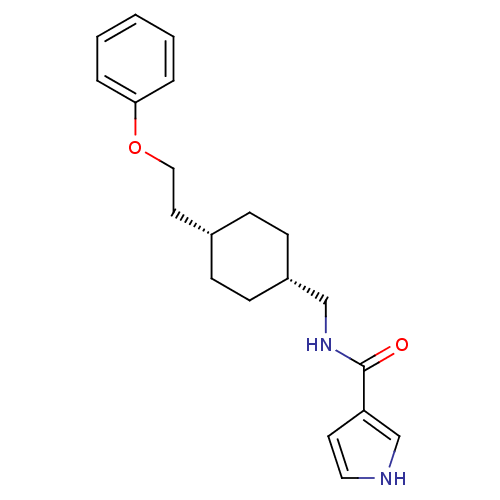
(CHEMBL249307 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1cc[nH]c1 |wU:4.3,7.7,(16.39,-6.18,;16.39,-7.72,;17.73,-8.49,;19.06,-7.71,;20.39,-8.48,;20.39,-10.02,;21.72,-10.78,;23.05,-10.02,;24.38,-10.79,;25.72,-10.02,;27.05,-10.79,;28.39,-10.03,;29.72,-10.8,;31.05,-10.03,;31.06,-8.49,;29.71,-7.72,;28.39,-8.49,;23.05,-8.48,;21.72,-7.7,;15.06,-8.49,;13.65,-7.87,;12.62,-9.02,;13.4,-10.35,;14.9,-10.03,)| Show InChI InChI=1S/C20H26N2O2/c23-20(18-10-12-21-15-18)22-14-17-8-6-16(7-9-17)11-13-24-19-4-2-1-3-5-19/h1-5,10,12,15-17,21H,6-9,11,13-14H2,(H,22,23)/t16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220603

(4-hydroxy-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)...)Show SMILES Oc1ccc(cc1)C(=O)NC[C@H]1CC[C@@H](COc2ccccc2)CC1 |wU:11.11,14.15,(-9.26,-9.14,;-7.93,-8.36,;-7.94,-6.82,;-6.61,-6.05,;-5.27,-6.82,;-5.26,-8.35,;-6.59,-9.13,;-3.94,-6.05,;-3.95,-4.51,;-2.61,-6.81,;-1.27,-6.04,;.06,-6.81,;1.39,-6.03,;2.72,-6.81,;2.72,-8.35,;4.05,-9.12,;5.39,-8.35,;6.72,-9.12,;6.71,-10.66,;8.04,-11.43,;9.38,-10.66,;9.38,-9.11,;8.05,-8.35,;1.39,-9.11,;.06,-8.35,)| Show InChI InChI=1S/C21H25NO3/c23-19-12-10-18(11-13-19)21(24)22-14-16-6-8-17(9-7-16)15-25-20-4-2-1-3-5-20/h1-5,10-13,16-17,23H,6-9,14-15H2,(H,22,24)/t16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261052
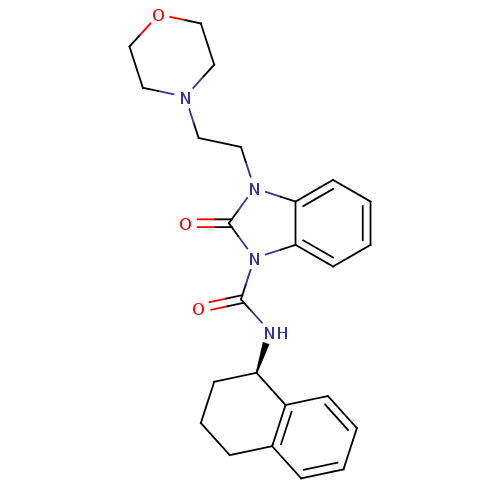
((R)-3-(2-morpholinoethyl)-2-oxo-N-(1,2,3,4-tetrahy...)Show SMILES O=C(N[C@@H]1CCCc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O |r| Show InChI InChI=1S/C24H28N4O3/c29-23(25-20-9-5-7-18-6-1-2-8-19(18)20)28-22-11-4-3-10-21(22)27(24(28)30)13-12-26-14-16-31-17-15-26/h1-4,6,8,10-11,20H,5,7,9,12-17H2,(H,25,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260753
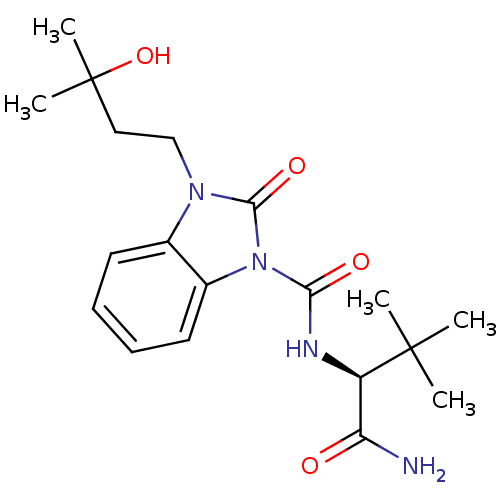
((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-(3-...)Show SMILES CC(C)(O)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O4/c1-18(2,3)14(15(20)24)21-16(25)23-13-9-7-6-8-12(13)22(17(23)26)11-10-19(4,5)27/h6-9,14,27H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261051

(3-(2-morpholinoethyl)-N-(naphthalen-1-yl)-2-oxo-2,...)Show SMILES O=C(Nc1cccc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O Show InChI InChI=1S/C24H24N4O3/c29-23(25-20-9-5-7-18-6-1-2-8-19(18)20)28-22-11-4-3-10-21(22)27(24(28)30)13-12-26-14-16-31-17-15-26/h1-11H,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220595
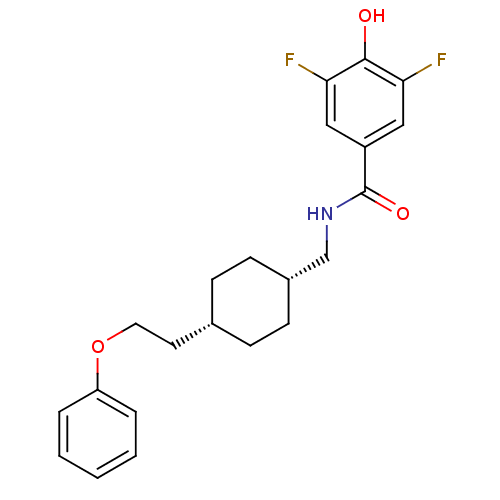
(3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(2-phenoxyeth...)Show SMILES Oc1c(F)cc(cc1F)C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:13.13,16.17,(13.63,-21.21,;14.96,-20.43,;14.96,-18.89,;13.62,-18.13,;16.29,-18.12,;17.63,-18.89,;17.63,-20.42,;16.31,-21.2,;16.32,-22.74,;18.96,-18.12,;18.95,-16.58,;20.29,-18.88,;21.62,-18.11,;22.96,-18.88,;22.96,-20.42,;24.29,-21.18,;25.62,-20.42,;26.95,-21.19,;28.29,-20.42,;29.62,-21.19,;30.95,-20.42,;32.29,-21.2,;33.62,-20.43,;33.62,-18.89,;32.28,-18.12,;30.95,-18.89,;25.62,-18.88,;24.29,-18.1,)| Show InChI InChI=1S/C22H25F2NO3/c23-19-12-17(13-20(24)21(19)26)22(27)25-14-16-8-6-15(7-9-16)10-11-28-18-4-2-1-3-5-18/h1-5,12-13,15-16,26H,6-11,14H2,(H,25,27)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220591
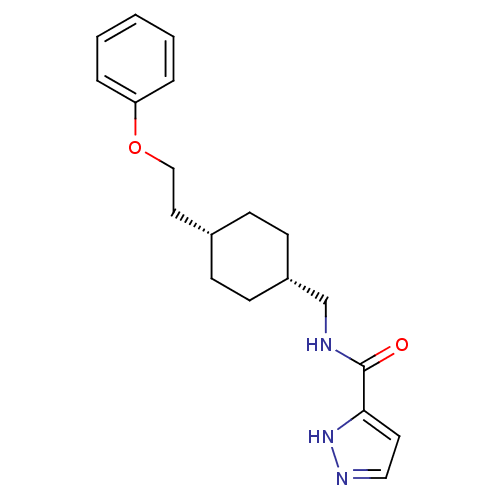
(CHEMBL249909 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccn[nH]1 |wU:4.3,7.7,(17.4,-25.44,;17.41,-26.98,;18.74,-27.74,;20.08,-26.97,;21.41,-27.74,;21.41,-29.28,;22.74,-30.04,;24.07,-29.28,;25.4,-30.05,;26.74,-29.28,;28.07,-30.05,;29.41,-29.28,;30.74,-30.06,;32.07,-29.29,;32.08,-27.75,;30.73,-26.98,;29.4,-27.75,;24.07,-27.74,;22.74,-26.96,;16.08,-27.75,;14.67,-27.13,;13.64,-28.28,;14.42,-29.61,;15.92,-29.28,)| Show InChI InChI=1S/C19H25N3O2/c23-19(18-10-12-21-22-18)20-14-16-8-6-15(7-9-16)11-13-24-17-4-2-1-3-5-17/h1-5,10,12,15-16H,6-9,11,13-14H2,(H,20,23)(H,21,22)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261099

((R)-1-(2-morpholinoethyl)-2-oxo-N-(1,2,3,4-tetrahy...)Show SMILES O=C(N[C@@H]1CCCc2ccccc12)N1Cc2ccccc2N(CCN2CCOCC2)C1=O |r| Show InChI InChI=1S/C25H30N4O3/c30-24(26-22-10-5-8-19-6-1-3-9-21(19)22)29-18-20-7-2-4-11-23(20)28(25(29)31)13-12-27-14-16-32-17-15-27/h1-4,6-7,9,11,22H,5,8,10,12-18H2,(H,26,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220598
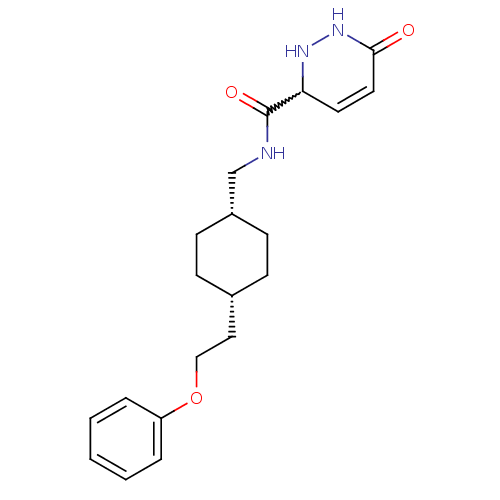
(6-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)C1NNC(=O)C=C1 |w:19.20,wU:4.3,7.7,c:26,(18.91,-43.44,;18.92,-44.98,;20.25,-45.74,;21.59,-44.97,;22.92,-45.73,;22.92,-47.27,;24.25,-48.04,;25.58,-47.27,;26.91,-48.05,;28.25,-47.28,;29.58,-48.05,;30.91,-47.28,;32.25,-48.06,;33.58,-47.29,;33.58,-45.75,;32.24,-44.98,;30.91,-45.75,;25.58,-45.73,;24.25,-44.96,;17.59,-45.75,;17.6,-47.29,;16.26,-48.06,;14.92,-47.29,;13.59,-48.05,;14.92,-45.75,;16.25,-44.98,)| Show InChI InChI=1S/C20H27N3O3/c24-19-11-10-18(22-23-19)20(25)21-14-16-8-6-15(7-9-16)12-13-26-17-4-2-1-3-5-17/h1-5,10-11,15-16,18,22H,6-9,12-14H2,(H,21,25)(H,23,24)/t15-,16+,18? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220599

(3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(phenoxymethy...)Show SMILES Oc1c(F)cc(cc1F)C(=O)NC[C@H]1CC[C@@H](COc2ccccc2)CC1 |wU:13.13,16.17,(-9.57,-19.5,;-8.24,-18.72,;-8.24,-17.18,;-9.58,-16.42,;-6.91,-16.41,;-5.57,-17.18,;-5.57,-18.71,;-6.89,-19.49,;-6.88,-21.03,;-4.24,-16.41,;-4.25,-14.87,;-2.91,-17.17,;-1.58,-16.4,;-.24,-17.17,;1.09,-16.39,;2.42,-17.17,;2.42,-18.71,;3.75,-19.48,;5.08,-18.71,;6.42,-19.48,;6.41,-21.02,;7.74,-21.79,;9.08,-21.02,;9.07,-19.47,;7.74,-18.71,;1.09,-19.47,;-.24,-18.71,)| Show InChI InChI=1S/C21H23F2NO3/c22-18-10-16(11-19(23)20(18)25)21(26)24-12-14-6-8-15(9-7-14)13-27-17-4-2-1-3-5-17/h1-5,10-11,14-15,25H,6-9,12-13H2,(H,24,26)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM86680

(CAS_4042 | Mpa | NSC_4042)Show SMILES CC1CC2C3CCC(OC(C)=O)(C(C)=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220610

(CHEMBL250108 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1cn[nH]c1 |wU:4.3,7.7,(-4.35,-14.31,;-4.35,-15.85,;-3.01,-16.61,;-1.68,-15.84,;-.34,-16.61,;.99,-15.83,;2.32,-16.61,;2.32,-18.15,;3.65,-18.92,;4.98,-18.15,;6.32,-18.92,;6.31,-20.46,;7.64,-21.23,;8.98,-20.46,;8.97,-18.91,;7.64,-18.15,;.99,-18.91,;-.34,-18.15,;-5.68,-16.62,;-7.09,-16,;-8.12,-17.14,;-7.35,-18.47,;-5.84,-18.15,)| Show InChI InChI=1S/C18H23N3O2/c22-18(16-11-20-21-12-16)19-10-14-6-8-15(9-7-14)13-23-17-4-2-1-3-5-17/h1-5,11-12,14-15H,6-10,13H2,(H,19,22)(H,20,21)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220601
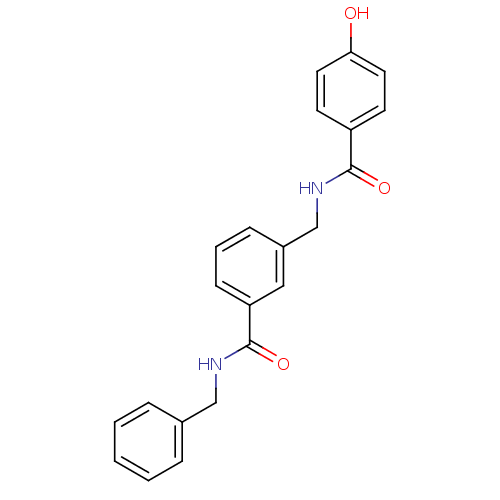
(CHEMBL400917 | N-benzyl-3-{[(4-hydroxybenzoyl)amin...)Show SMILES Oc1ccc(cc1)C(=O)NCc1cccc(c1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C22H20N2O3/c25-20-11-9-18(10-12-20)21(26)24-15-17-7-4-8-19(13-17)22(27)23-14-16-5-2-1-3-6-16/h1-13,25H,14-15H2,(H,23,27)(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220597
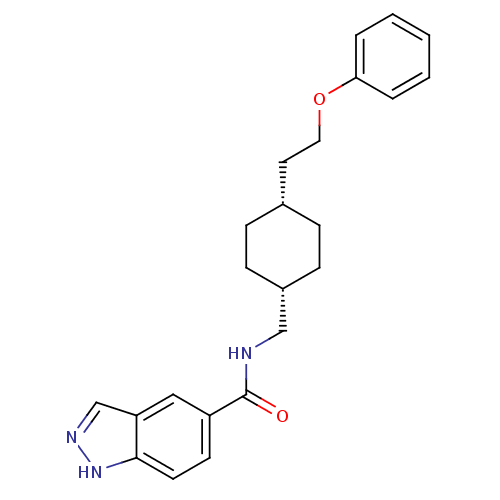
(CHEMBL398356 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccc2[nH]ncc2c1 |wU:4.3,7.7,(-1.62,4.65,;-1.62,3.11,;-.28,2.35,;1.05,3.12,;2.39,2.36,;2.39,.82,;3.72,.05,;5.05,.82,;6.38,.04,;7.71,.81,;9.05,.04,;10.38,.81,;11.71,.03,;13.05,.8,;13.05,2.34,;11.71,3.11,;10.38,2.34,;5.05,2.36,;3.72,3.13,;-2.95,2.34,;-2.94,.81,;-4.26,.03,;-5.61,.8,;-7.08,.33,;-7.99,1.58,;-7.07,2.83,;-5.6,2.35,;-4.28,3.11,)| Show InChI InChI=1S/C23H27N3O2/c27-23(19-10-11-22-20(14-19)16-25-26-22)24-15-18-8-6-17(7-9-18)12-13-28-21-4-2-1-3-5-21/h1-5,10-11,14,16-18H,6-9,12-13,15H2,(H,24,27)(H,25,26)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260674
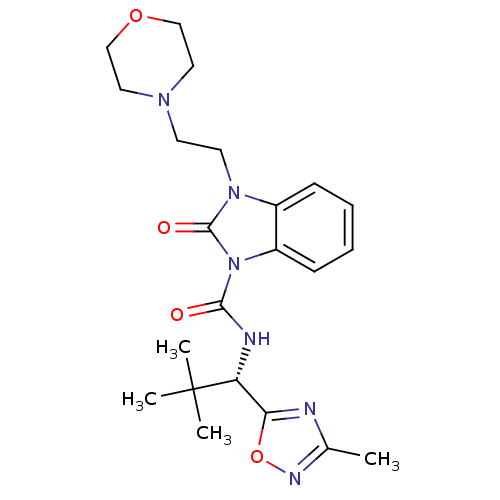
((S)-N-(2,2-dimethyl-1-(3-methyl-1,2,4-oxadiazol-5-...)Show SMILES Cc1noc(n1)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C22H30N6O4/c1-15-23-19(32-25-15)18(22(2,3)4)24-20(29)28-17-8-6-5-7-16(17)27(21(28)30)10-9-26-11-13-31-14-12-26/h5-8,18H,9-14H2,1-4H3,(H,24,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260514
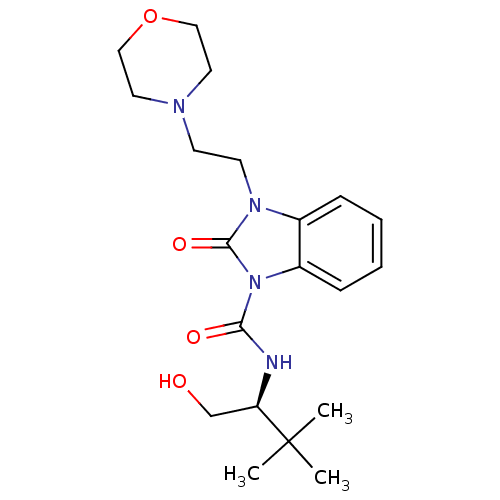
((S)-N-(1-hydroxy-3,3-dimethylbutan-2-yl)-3-(2-morp...)Show SMILES CC(C)(C)[C@@H](CO)NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O |r| Show InChI InChI=1S/C20H30N4O4/c1-20(2,3)17(14-25)21-18(26)24-16-7-5-4-6-15(16)23(19(24)27)9-8-22-10-12-28-13-11-22/h4-7,17,25H,8-14H2,1-3H3,(H,21,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220607
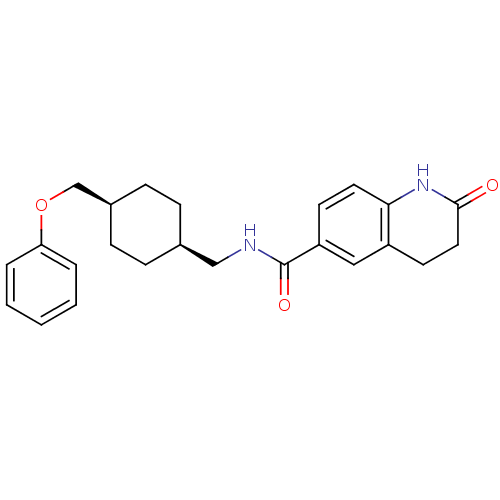
(2-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1ccc2NC(=O)CCc2c1 |wU:4.3,7.7,(-2.62,-24.66,;-2.61,-26.2,;-1.28,-26.97,;.06,-26.19,;1.39,-26.96,;2.72,-26.18,;4.05,-26.96,;4.05,-28.5,;5.38,-29.27,;6.72,-28.5,;8.05,-29.27,;8.04,-30.81,;9.37,-31.58,;10.71,-30.81,;10.71,-29.27,;9.38,-28.5,;2.72,-29.26,;1.39,-28.5,;-3.94,-26.97,;-3.93,-28.5,;-5.26,-29.28,;-6.6,-28.52,;-7.93,-29.3,;-9.27,-28.53,;-10.6,-29.31,;-9.28,-26.99,;-7.94,-26.22,;-6.61,-26.97,;-5.28,-26.2,)| Show InChI InChI=1S/C24H28N2O3/c27-23-13-11-19-14-20(10-12-22(19)26-23)24(28)25-15-17-6-8-18(9-7-17)16-29-21-4-2-1-3-5-21/h1-5,10,12,14,17-18H,6-9,11,13,15-16H2,(H,25,28)(H,26,27)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM86680

(CAS_4042 | Mpa | NSC_4042)Show SMILES CC1CC2C3CCC(OC(C)=O)(C(C)=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260626
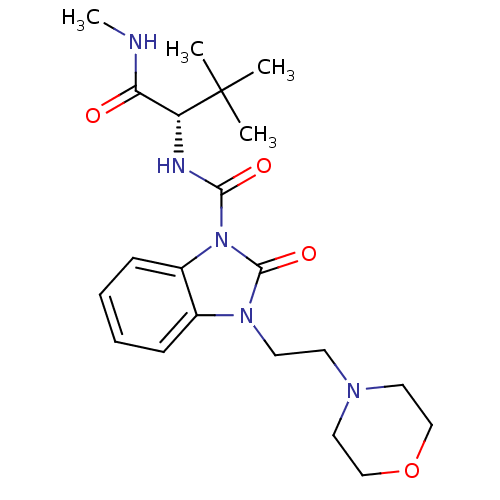
((S)-N'-(3,3-dimethyl-1-(methylamino)-1-oxobutan-2-...)Show SMILES CNC(=O)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C21H31N5O4/c1-21(2,3)17(18(27)22-4)23-19(28)26-16-8-6-5-7-15(16)25(20(26)29)10-9-24-11-13-30-14-12-24/h5-8,17H,9-14H2,1-4H3,(H,22,27)(H,23,28)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220590
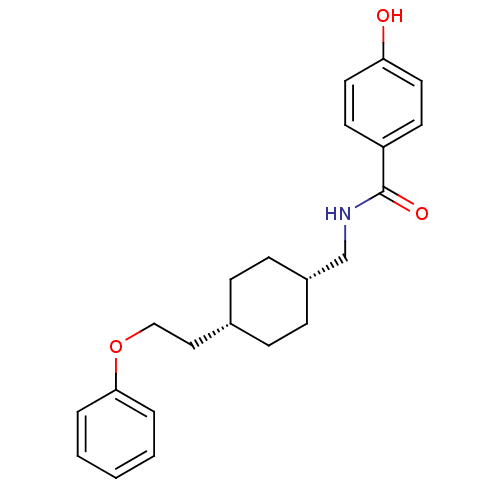
(4-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...)Show SMILES Oc1ccc(cc1)C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:11.11,14.15,(12.9,-11.25,;14.23,-10.47,;14.23,-8.93,;15.56,-8.16,;16.89,-8.93,;16.9,-10.46,;15.58,-11.24,;18.23,-8.16,;18.22,-6.62,;19.56,-8.92,;20.89,-8.15,;22.23,-8.91,;22.23,-10.45,;23.56,-11.22,;24.89,-10.45,;26.22,-11.23,;27.55,-10.46,;28.89,-11.23,;30.22,-10.46,;31.55,-11.24,;32.89,-10.47,;32.89,-8.93,;31.55,-8.16,;30.22,-8.93,;24.89,-8.91,;23.56,-8.14,)| Show InChI InChI=1S/C22H27NO3/c24-20-12-10-19(11-13-20)22(25)23-16-18-8-6-17(7-9-18)14-15-26-21-4-2-1-3-5-21/h1-5,10-13,17-18,24H,6-9,14-16H2,(H,23,25)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM86678

(CP8816)Show SMILES COC1C=C2C=C3N(C)C(=O)C(C)=C3[C@@H](C)[C@]2(C)CC1OC(=O)N(C)C |r,c:12,t:3,5| Show InChI InChI=1S/C20H28N2O4/c1-11-17-12(2)20(3)10-16(26-19(24)21(4)5)15(25-7)9-13(20)8-14(17)22(6)18(11)23/h8-9,12,15-16H,10H2,1-7H3/t12-,15?,16?,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260538
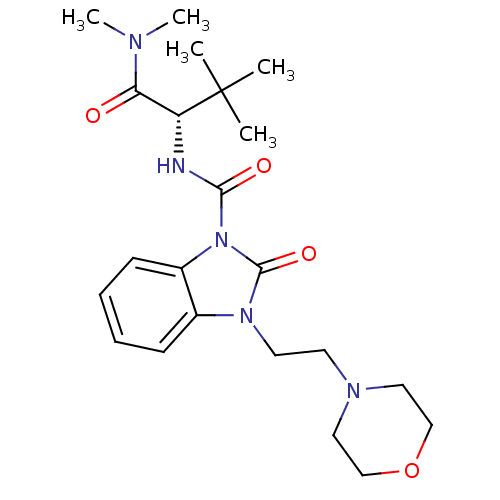
((S)-N-(1-(dimethylamino)-3,3-dimethyl-1-oxobutan-2...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(C)(C)C |r| Show InChI InChI=1S/C22H33N5O4/c1-22(2,3)18(19(28)24(4)5)23-20(29)27-17-9-7-6-8-16(17)26(21(27)30)11-10-25-12-14-31-15-13-25/h6-9,18H,10-15H2,1-5H3,(H,23,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM86679
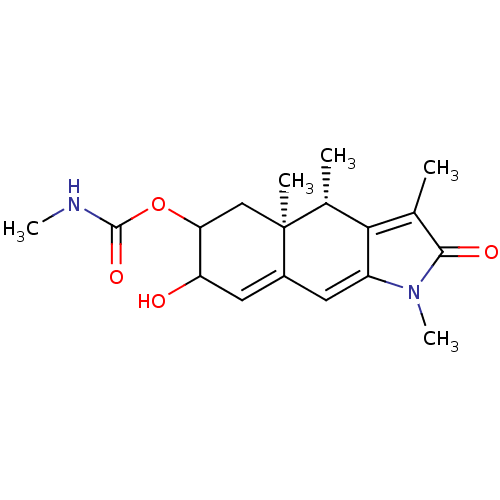
(CP8863)Show SMILES CNC(=O)OC1C[C@@]2(C)[C@H](C)C3=C(C)C(=O)N(C)C3=CC2=CC1O |r,c:11,19,22| Show InChI InChI=1S/C18H24N2O4/c1-9-15-10(2)18(3)8-14(24-17(23)19-4)13(21)7-11(18)6-12(15)20(5)16(9)22/h6-7,10,13-14,21H,8H2,1-5H3,(H,19,23)/t10-,13?,14?,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261053
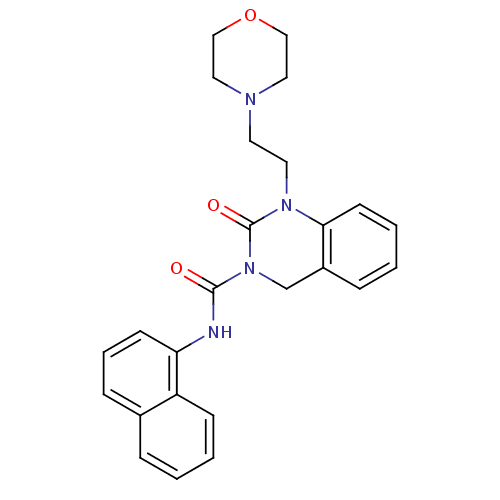
(1-(2-morpholinoethyl)-N-(naphthalen-1-yl)-2-oxo-1,...)Show SMILES O=C(Nc1cccc2ccccc12)N1Cc2ccccc2N(CCN2CCOCC2)C1=O Show InChI InChI=1S/C25H26N4O3/c30-24(26-22-10-5-8-19-6-1-3-9-21(19)22)29-18-20-7-2-4-11-23(20)28(25(29)31)13-12-27-14-16-32-17-15-27/h1-11H,12-18H2,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM86680

(CAS_4042 | Mpa | NSC_4042)Show SMILES CC1CC2C3CCC(OC(C)=O)(C(C)=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260512
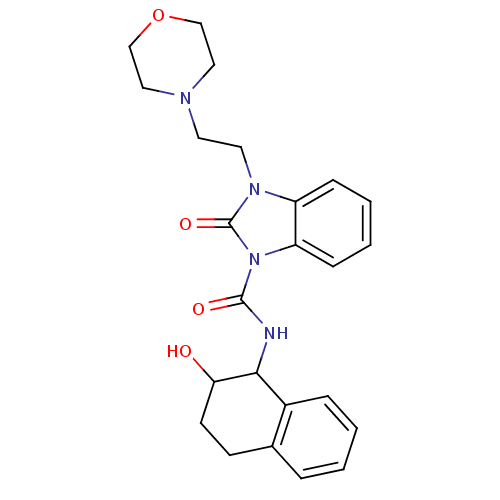
(CHEMBL494563 | N-(2-hydroxy-1,2,3,4-tetrahydronaph...)Show SMILES OC1CCc2ccccc2C1NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O Show InChI InChI=1S/C24H28N4O4/c29-21-10-9-17-5-1-2-6-18(17)22(21)25-23(30)28-20-8-4-3-7-19(20)27(24(28)31)12-11-26-13-15-32-16-14-26/h1-8,21-22,29H,9-16H2,(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50261100
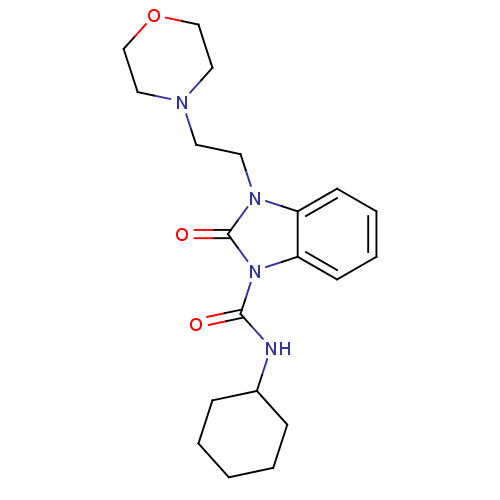
(CHEMBL494627 | N-cyclohexyl-3-(2-morpholinoethyl)-...)Show InChI InChI=1S/C20H28N4O3/c25-19(21-16-6-2-1-3-7-16)24-18-9-5-4-8-17(18)23(20(24)26)11-10-22-12-14-27-15-13-22/h4-5,8-9,16H,1-3,6-7,10-15H2,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260628
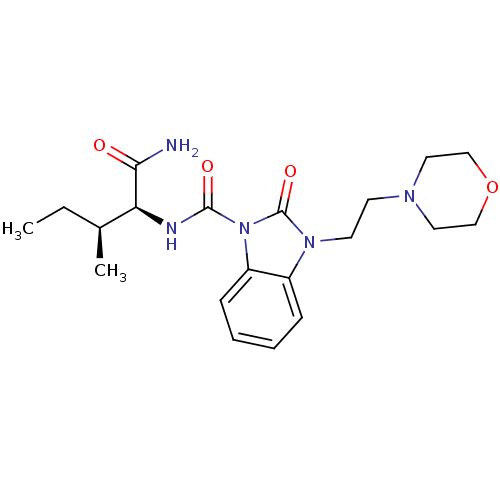
(CHEMBL495391 | N-((2S,3S)-1-amino-3-methyl-1-oxope...)Show SMILES CC[C@H](C)[C@H](NC(=O)n1c2ccccc2n(CCN2CCOCC2)c1=O)C(N)=O |r| Show InChI InChI=1S/C20H29N5O4/c1-3-14(2)17(18(21)26)22-19(27)25-16-7-5-4-6-15(16)24(20(25)28)9-8-23-10-12-29-13-11-23/h4-7,14,17H,3,8-13H2,1-2H3,(H2,21,26)(H,22,27)/t14-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260712

((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-iso...)Show SMILES CC(C)CCn1c2ccccc2n(C(=O)N[C@H](C(N)=O)C(C)(C)C)c1=O |r| Show InChI InChI=1S/C19H28N4O3/c1-12(2)10-11-22-13-8-6-7-9-14(13)23(18(22)26)17(25)21-15(16(20)24)19(3,4)5/h6-9,12,15H,10-11H2,1-5H3,(H2,20,24)(H,21,25)/t15-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260511
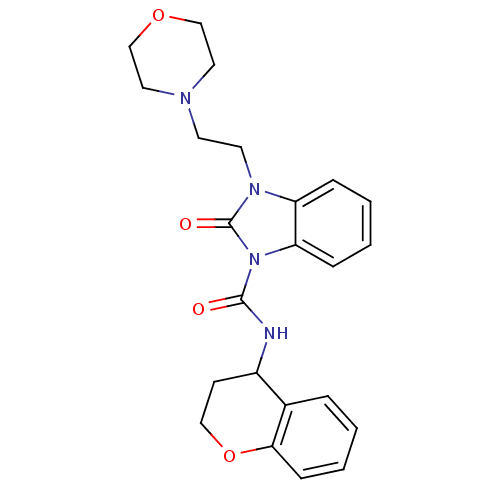
(CHEMBL495407 | N-(chroman-4-yl)-3-(2-morpholinoeth...)Show SMILES O=C(NC1CCOc2ccccc12)n1c2ccccc2n(CCN2CCOCC2)c1=O Show InChI InChI=1S/C23H26N4O4/c28-22(24-18-9-14-31-21-8-4-1-5-17(18)21)27-20-7-3-2-6-19(20)26(23(27)29)11-10-25-12-15-30-16-13-25/h1-8,18H,9-16H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220600
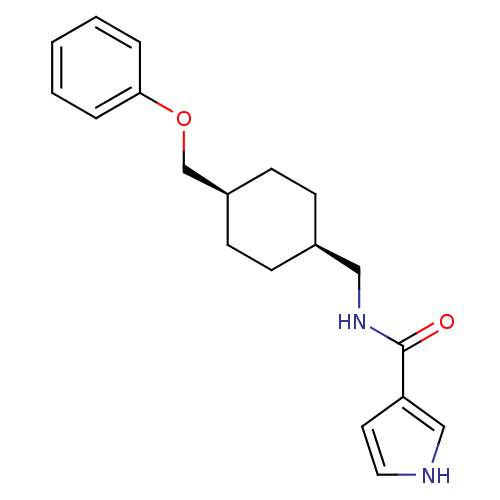
(CHEMBL398357 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1cc[nH]c1 |wU:4.3,7.7,(-3.78,-5.13,;-3.78,-6.67,;-2.44,-7.44,;-1.11,-6.66,;.23,-7.43,;1.55,-6.65,;2.88,-7.43,;2.88,-8.97,;4.22,-9.74,;5.55,-8.97,;6.88,-9.74,;6.88,-11.28,;8.21,-12.05,;9.54,-11.28,;9.54,-9.74,;8.21,-8.97,;1.55,-9.73,;.23,-8.97,;-5.12,-7.44,;-6.52,-6.82,;-7.55,-7.97,;-6.78,-9.3,;-5.27,-8.97,)| Show InChI InChI=1S/C19H24N2O2/c22-19(17-10-11-20-13-17)21-12-15-6-8-16(9-7-15)14-23-18-4-2-1-3-5-18/h1-5,10-11,13,15-16,20H,6-9,12,14H2,(H,21,22)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50260750
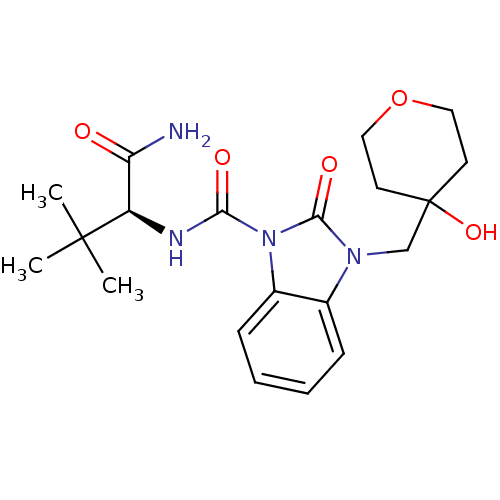
((S)-N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-3-((4...)Show SMILES CC(C)(C)[C@H](NC(=O)n1c2ccccc2n(CC2(O)CCOCC2)c1=O)C(N)=O |r| Show InChI InChI=1S/C20H28N4O5/c1-19(2,3)15(16(21)25)22-17(26)24-14-7-5-4-6-13(14)23(18(24)27)12-20(28)8-10-29-11-9-20/h4-7,15,28H,8-12H2,1-3H3,(H2,21,25)(H,22,26)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3310-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.032
BindingDB Entry DOI: 10.7270/Q2VD70C3 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220594

(3,5-dimethyl-N-(((1s,4s)-4-(phenoxymethyl)cyclohex...)Show SMILES Cc1n[nH]c(C)c1C(=O)NC[C@H]1CC[C@@H](COc2ccccc2)CC1 |wU:11.11,14.15,(-5.39,-42.57,;-5.07,-44.07,;-6.1,-45.22,;-5.32,-46.55,;-3.82,-46.23,;-2.67,-47.25,;-3.66,-44.7,;-2.32,-43.93,;-2.33,-42.39,;-.99,-44.69,;.34,-43.92,;1.68,-44.68,;3.01,-43.91,;4.34,-44.68,;4.34,-46.22,;5.67,-47,;7.01,-46.23,;8.34,-47,;8.33,-48.54,;9.66,-49.31,;11,-48.54,;11,-46.99,;9.66,-46.23,;3.01,-46.99,;1.68,-46.22,)| Show InChI InChI=1S/C20H27N3O2/c1-14-19(15(2)23-22-14)20(24)21-12-16-8-10-17(11-9-16)13-25-18-6-4-3-5-7-18/h3-7,16-17H,8-13H2,1-2H3,(H,21,24)(H,22,23)/t16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220609
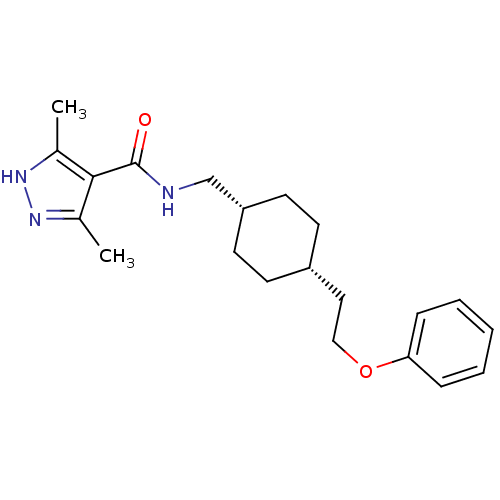
(3,5-dimethyl-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohe...)Show SMILES Cc1n[nH]c(C)c1C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:11.11,14.15,(19.66,-44.61,;19.98,-46.12,;18.95,-47.26,;19.73,-48.59,;21.23,-48.27,;22.38,-49.3,;21.39,-46.74,;22.72,-45.96,;22.72,-44.42,;24.06,-46.73,;25.39,-45.96,;26.72,-46.72,;26.72,-48.26,;28.05,-49.02,;29.38,-48.26,;30.72,-49.03,;32.05,-48.26,;33.38,-49.04,;34.72,-48.27,;36.05,-49.04,;37.38,-48.28,;37.39,-46.74,;36.05,-45.96,;34.72,-46.74,;29.38,-46.72,;28.05,-45.94,)| Show InChI InChI=1S/C21H29N3O2/c1-15-20(16(2)24-23-15)21(25)22-14-18-10-8-17(9-11-18)12-13-26-19-6-4-3-5-7-19/h3-7,17-18H,8-14H2,1-2H3,(H,22,25)(H,23,24)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220604

(CHEMBL249297 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1ccn[nH]1 |wU:4.3,7.7,(-3.28,-24.38,;-3.28,-25.92,;-1.94,-26.69,;-.61,-25.91,;.72,-26.68,;2.05,-25.9,;3.38,-26.68,;3.38,-28.22,;4.71,-28.99,;6.05,-28.22,;7.38,-28.99,;7.37,-30.53,;8.7,-31.3,;10.04,-30.53,;10.04,-28.99,;8.71,-28.22,;2.05,-28.98,;.72,-28.22,;-4.62,-26.69,;-6.03,-26.07,;-7.05,-27.22,;-6.28,-28.55,;-4.77,-28.22,)| Show InChI InChI=1S/C18H23N3O2/c22-18(17-10-11-20-21-17)19-12-14-6-8-15(9-7-14)13-23-16-4-2-1-3-5-16/h1-5,10-11,14-15H,6-9,12-13H2,(H,19,22)(H,20,21)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220605

(6-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)C1NNC(=O)C=C1 |w:18.19,wU:4.3,7.7,c:25,(-3.75,-42.93,;-3.75,-44.47,;-2.41,-45.24,;-1.08,-44.46,;.26,-45.23,;1.59,-44.45,;2.91,-45.23,;2.91,-46.77,;4.25,-47.54,;5.58,-46.77,;6.91,-47.54,;6.91,-49.08,;8.24,-49.85,;9.57,-49.08,;9.57,-47.54,;8.24,-46.77,;1.59,-47.53,;.26,-46.77,;-5.08,-45.25,;-5.07,-46.79,;-6.41,-47.56,;-7.75,-46.79,;-9.08,-47.56,;-7.74,-45.25,;-6.42,-44.48,)| Show InChI InChI=1S/C19H25N3O3/c23-18-11-10-17(21-22-18)19(24)20-12-14-6-8-15(9-7-14)13-25-16-4-2-1-3-5-16/h1-5,10-11,14-15,17,21H,6-9,12-13H2,(H,20,24)(H,22,23)/t14-,15+,17? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data