

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121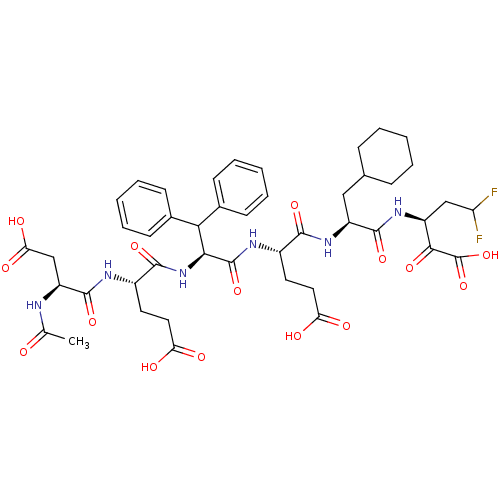 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227363 (CHEMBL299837) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227366 (CHEMBL48927) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227364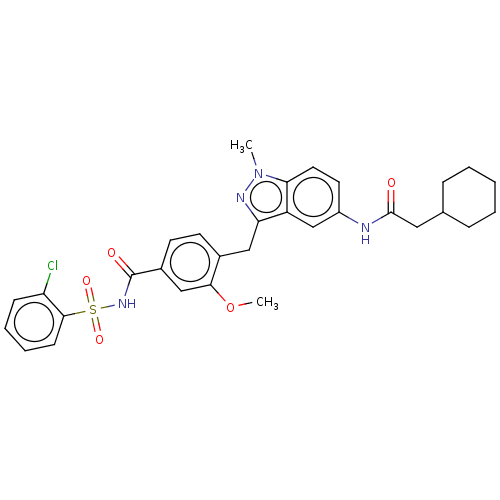 (CHEMBL299093) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227361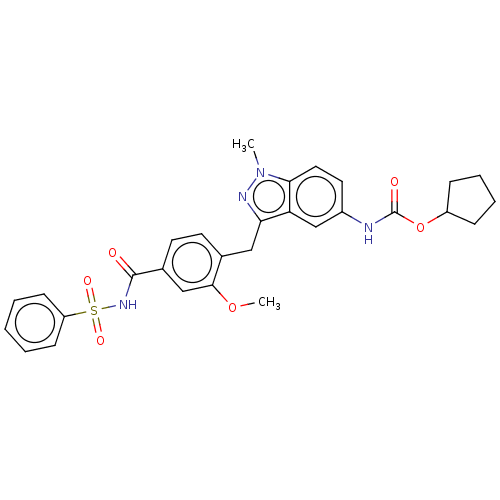 (CHEMBL51585) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227358 (CHEMBL412056) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227365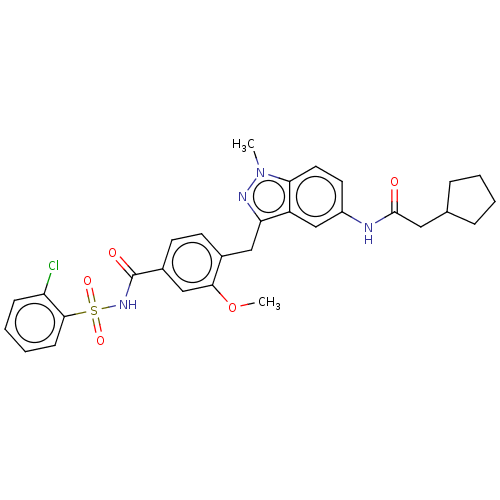 (CHEMBL300096) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227640 (CHEMBL297952) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015528 (CHEMBL50370 | N-[3-(4-Benzenesulfonylaminocarbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227356 (CHEMBL49944) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227355 (CHEMBL296821) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227642 (CHEMBL50562) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50000819 (2-Cyclopentyl-N-{3-[2-methoxy-4-(toluene-2-sulfony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015523 (CHEMBL52492 | N-{4-[5-(3-Cyclopentyl-ureido)-1-pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227390 (CHEMBL301616) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227389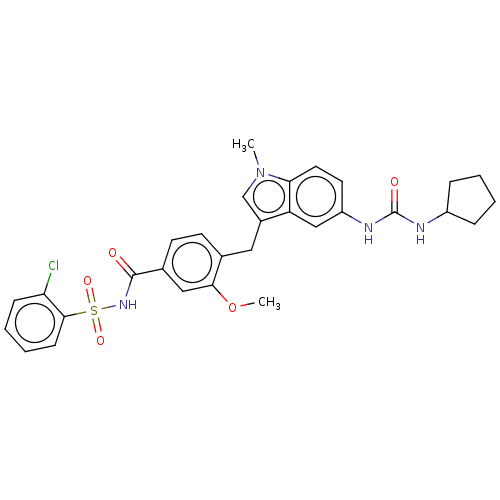 (CHEMBL51580) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227367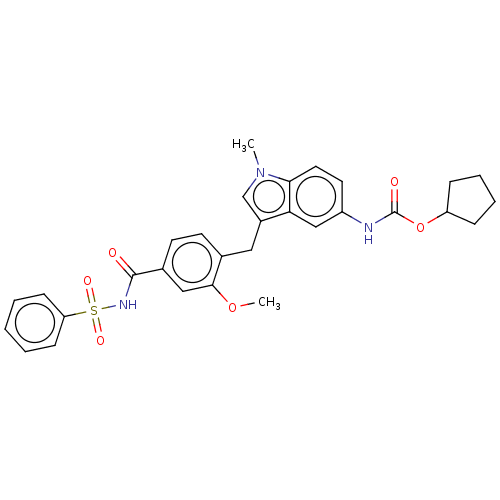 (CHEMBL48435) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015551 (CHEMBL301498 | N-{4-[5-(3-Cyclopentyl-ureido)-1-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015517 (2-Cyclopentyl-N-{3-[2-methoxy-4-(toluene-2-sulfony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015540 (CHEMBL431348 | N-{4-[5-(3-Cyclopentyl-ureido)-1-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227638 (CHEMBL49566) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110122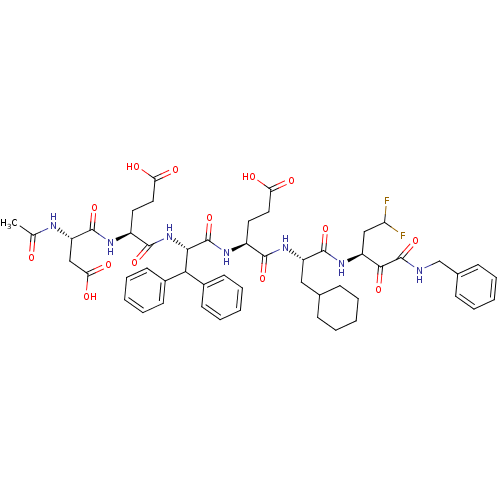 ((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110120 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009070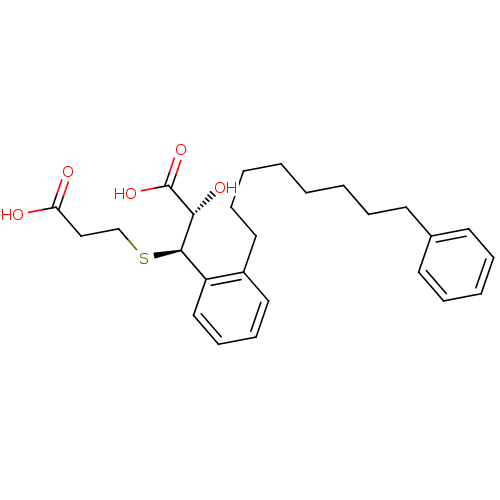 ((2S,3R)-3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displasement of [3H]-LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227354 (CHEMBL51289) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227353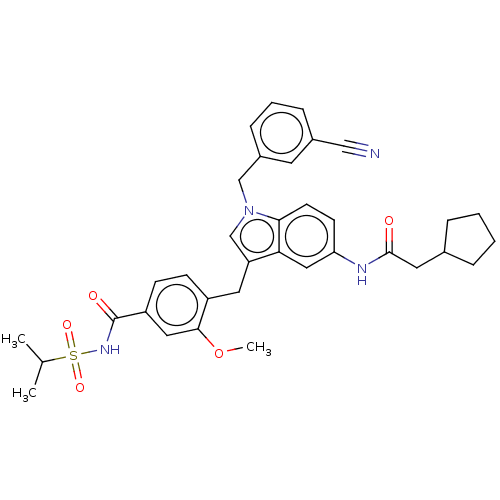 (CHEMBL51239) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110126 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227359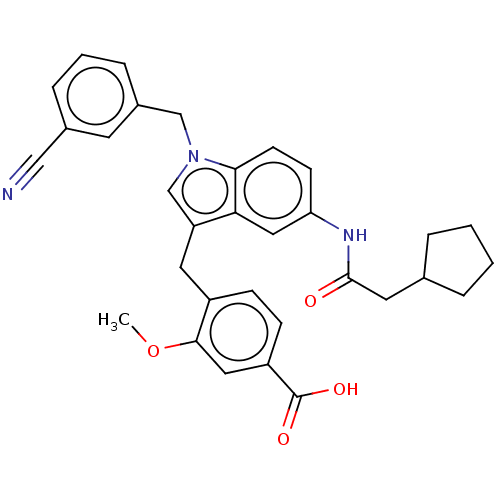 (CHEMBL51061) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150997 (4-{2-[(S)-4,4-Difluoro-2-({(2S,4R)-1-((S)-2-isobut...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150993 (4-[2-(4,4-Difluoro-2-{[(S)-(R)-1-(2-isobutoxycarbo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50084685 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015542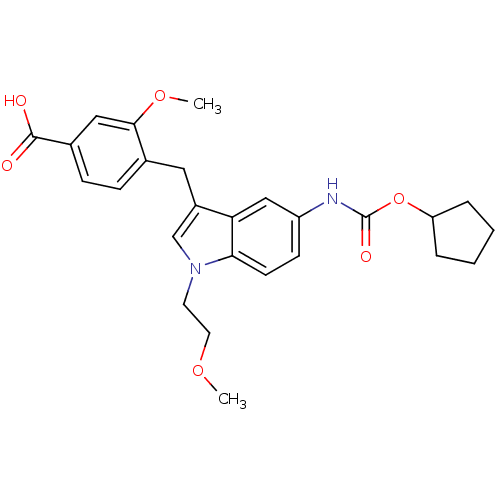 (4-[5-Cyclopentyloxycarbonylamino-1-(2-methoxy-ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015516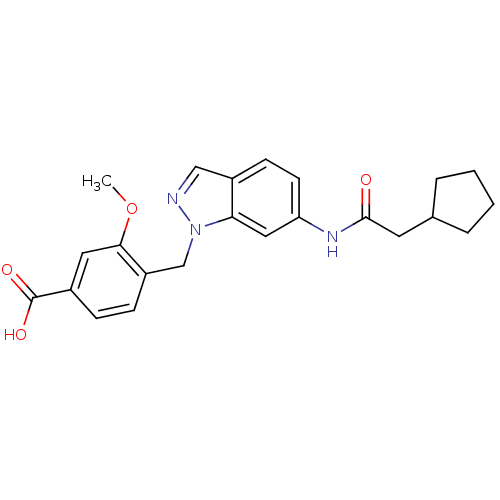 (4-[6-(2-Cyclopentyl-acetylamino)-indazol-1-ylmethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227357 (CHEMBL49981) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227360 (CHEMBL50364) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015539 (4-(5-Cyclopentyloxycarbonylamino-1-propyl-1H-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227391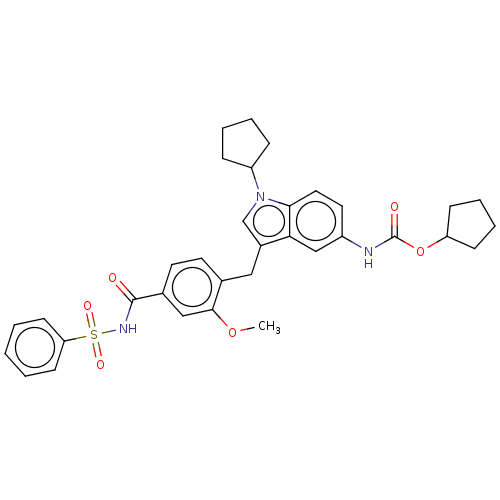 (CHEMBL430967) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227362 (CHEMBL300943) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227641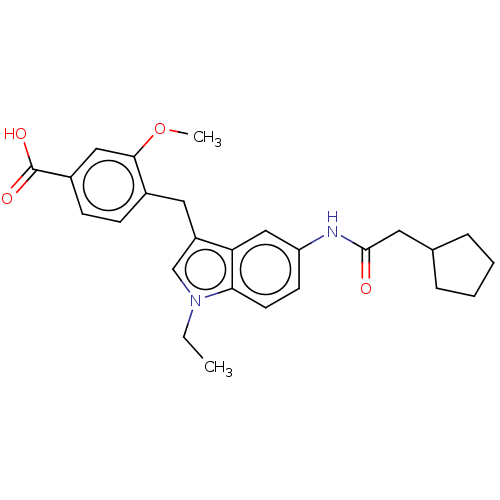 (CHEMBL297014) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015521 (4-[5-(2-Cyclopentyl-acetylamino)-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50015544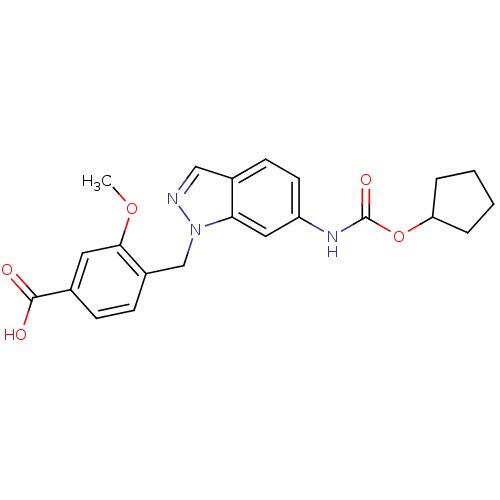 (4-(6-Cyclopentyloxycarbonylamino-indazol-1-ylmethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Displacement of [3H]-LTD4 on guinea pig lung parenchymal membranes | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1/2 (Homo sapiens (Human)) | BDBM50227639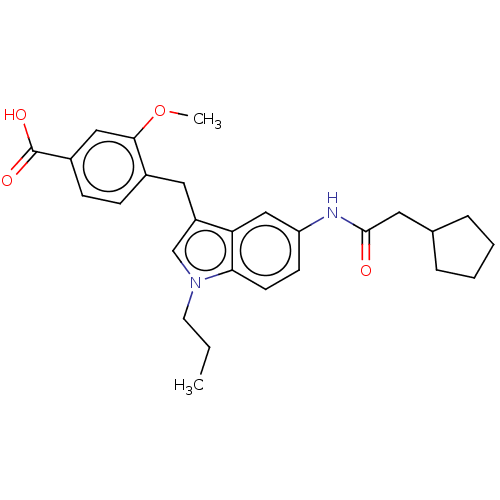 (CHEMBL52260) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Group Curated by ChEMBL | Assay Description Inhibition constant for displacement of [3H]LTD4 on guinea pig lung parenchymal membranes. | J Med Chem 33: 1781-90 (1990) BindingDB Entry DOI: 10.7270/Q2862FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110123 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150992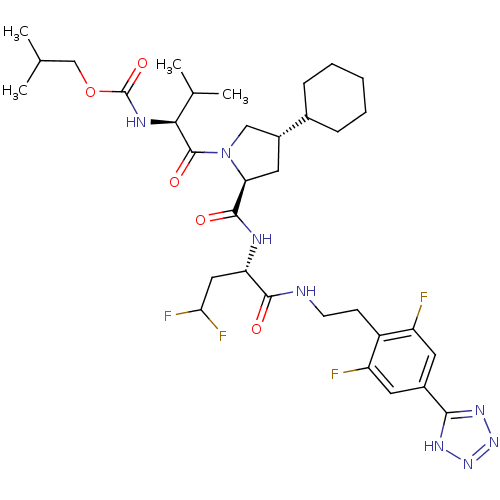 (CHEMBL366279 | {(S)-1-[(2S,4S)-4-Cyclohexyl-2-((S)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1483 total ) | Next | Last >> |