 Found 710 hits with Last Name = 'mathieu' and Initial = 's'
Found 710 hits with Last Name = 'mathieu' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363990
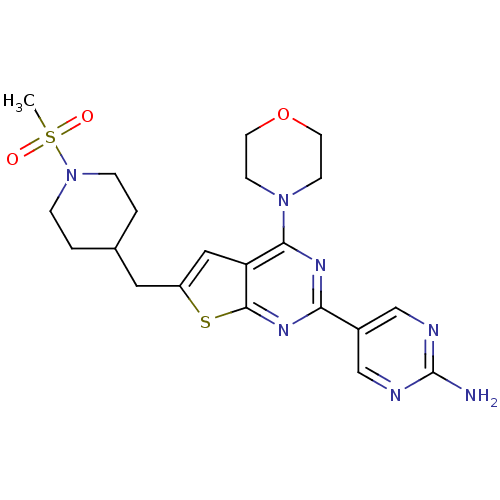
(CHEMBL1949915)Show SMILES CS(=O)(=O)N1CCC(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-4-2-14(3-5-28)10-16-11-17-19(27-6-8-31-9-7-27)25-18(26-20(17)32-16)15-12-23-21(22)24-13-15/h11-14H,2-10H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419769
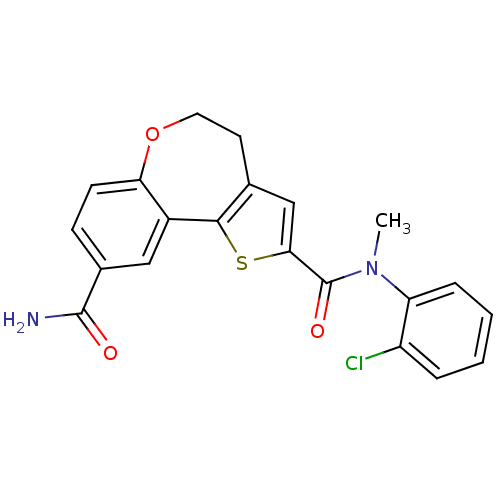
(CHEMBL1950034)Show SMILES CN(C(=O)c1cc2CCOc3ccc(cc3-c2s1)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C21H17ClN2O3S/c1-24(16-5-3-2-4-15(16)22)21(26)18-11-12-8-9-27-17-7-6-13(20(23)25)10-14(17)19(12)28-18/h2-7,10-11H,8-9H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419754
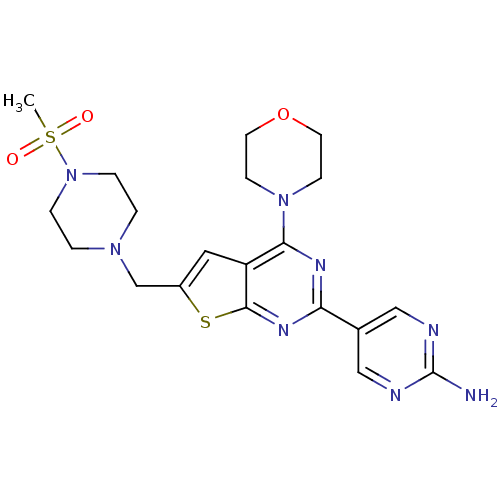
(CHEMBL1949910)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-18(27-6-8-31-9-7-27)24-17(25-19(16)32-15)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347090
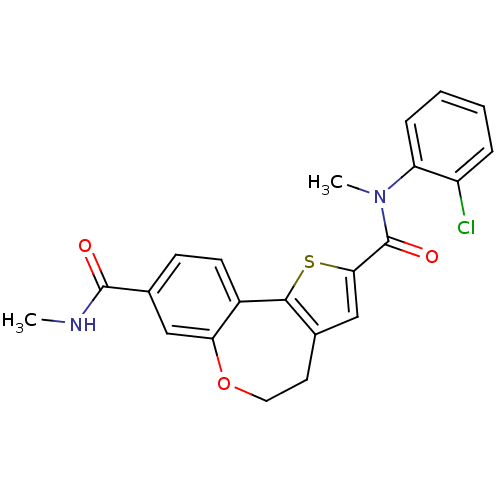
(CHEMBL1796276)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-24-21(26)14-7-8-15-18(11-14)28-10-9-13-12-19(29-20(13)15)22(27)25(2)17-6-4-3-5-16(17)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419759
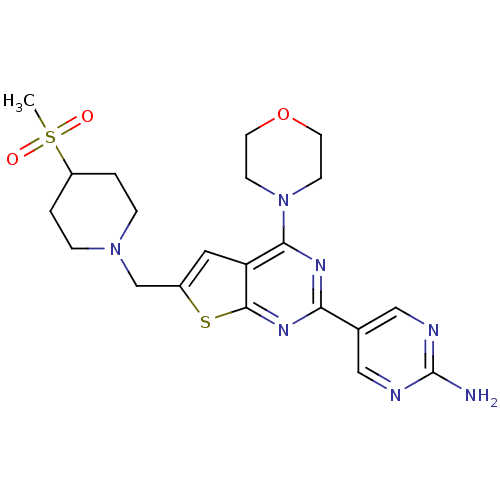
(CHEMBL1949916)Show SMILES CS(=O)(=O)C1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)16-2-4-27(5-3-16)13-15-10-17-19(28-6-8-31-9-7-28)25-18(26-20(17)32-15)14-11-23-21(22)24-12-14/h10-12,16H,2-9,13H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419770
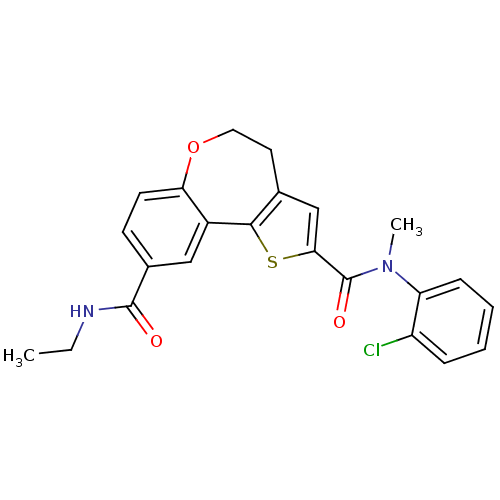
(CHEMBL1950035)Show SMILES CCNC(=O)c1ccc2OCCc3cc(sc3-c2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C23H21ClN2O3S/c1-3-25-22(27)15-8-9-19-16(12-15)21-14(10-11-29-19)13-20(30-21)23(28)26(2)18-7-5-4-6-17(18)24/h4-9,12-13H,3,10-11H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347087
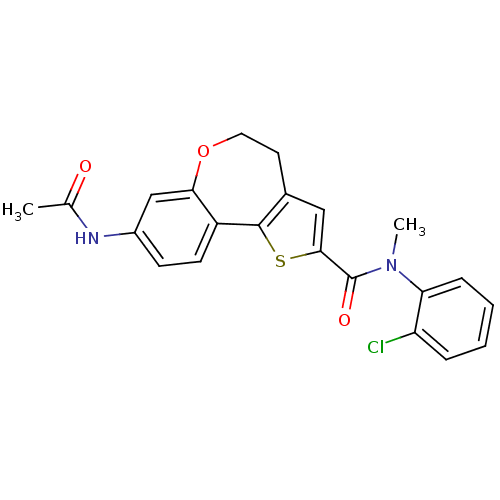
(CHEMBL1796273)Show SMILES CN(C(=O)c1cc2CCOc3cc(NC(C)=O)ccc3-c2s1)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-13(26)24-15-7-8-16-19(12-15)28-10-9-14-11-20(29-21(14)16)22(27)25(2)18-6-4-3-5-17(18)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419761

(CHEMBL1949919)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O3S2/c1-32(28,29)15-4-2-3-13(9-15)17-10-16-19(27-5-7-30-8-6-27)25-18(26-20(16)31-17)14-11-23-21(22)24-12-14/h2-4,9-12H,5-8H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419773
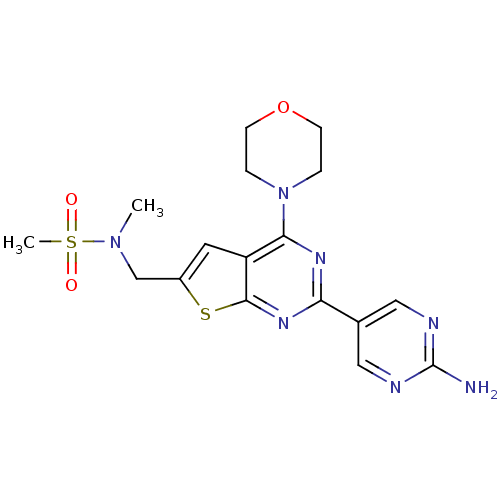
(CHEMBL1949917)Show SMILES CN(Cc1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1)S(C)(=O)=O Show InChI InChI=1S/C17H21N7O3S2/c1-23(29(2,25)26)10-12-7-13-15(24-3-5-27-6-4-24)21-14(22-16(13)28-12)11-8-19-17(18)20-9-11/h7-9H,3-6,10H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419760
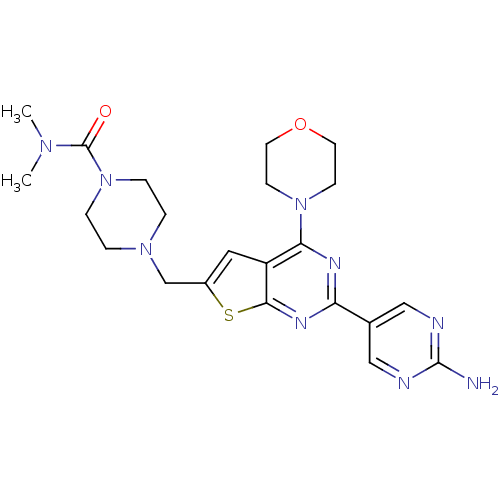
(CHEMBL1949918)Show SMILES CN(C)C(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C22H29N9O2S/c1-28(2)22(32)31-5-3-29(4-6-31)14-16-11-17-19(30-7-9-33-10-8-30)26-18(27-20(17)34-16)15-12-24-21(23)25-13-15/h11-13H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50097131

(CHEMBL3580667)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2N)c1nc(Nc2cc([nH]n2)C2CC2)c2sccc2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PAK4 |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419758

(CHEMBL1949914)Show SMILES Nc1ncc(cn1)-c1nc(N2CCOCC2)c2cc(CC3CCN(CC(F)(F)F)CC3)sc2n1 Show InChI InChI=1S/C22H26F3N7OS/c23-22(24,25)13-31-3-1-14(2-4-31)9-16-10-17-19(32-5-7-33-8-6-32)29-18(30-20(17)34-16)15-11-27-21(26)28-12-15/h10-12,14H,1-9,13H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358203
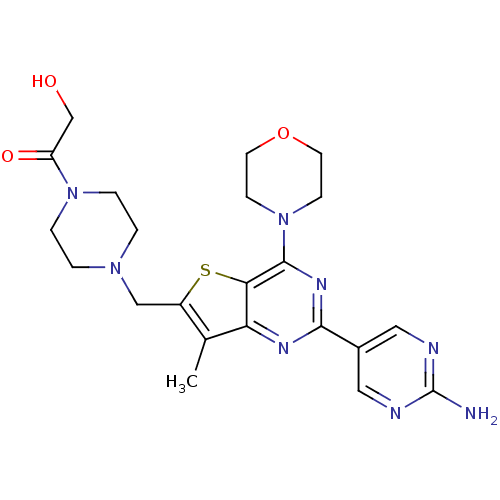
(CHEMBL1922093)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CO)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3S/c1-14-16(12-28-2-4-29(5-3-28)17(32)13-31)34-19-18(14)26-20(15-10-24-22(23)25-11-15)27-21(19)30-6-8-33-9-7-30/h10-11,31H,2-9,12-13H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419762

(CHEMBL1949920)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)-c1cc[nH]n1)c1ccccc1Cl Show InChI InChI=1S/C23H18ClN3O2S/c1-27(19-5-3-2-4-17(19)24)23(28)21-13-15-9-11-29-20-12-14(18-8-10-25-26-18)6-7-16(20)22(15)30-21/h2-8,10,12-13H,9,11H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50096231
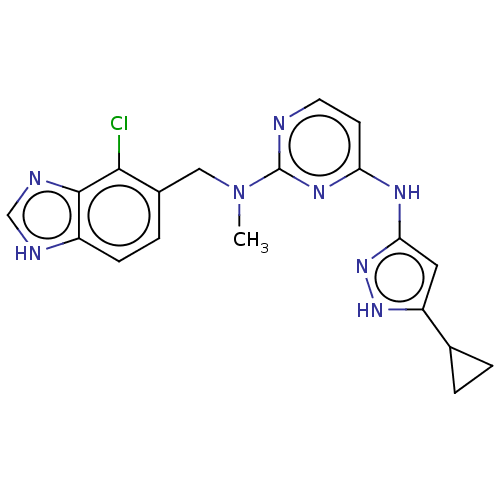
(CHEMBL3580975)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C30H50N4O6/c1-7-19(3)25-29(40)34-17-13-12-16-23(34)28(39)31-21(15-11-9-10-14-20(35)8-2)26(37)32-22(27(38)33-25)18-24(36)30(4,5)6/h19,21-23,25H,7-18H2,1-6H3,(H,31,39)(H,32,37)(H,33,38)/t19?,21-,22-,23+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363991
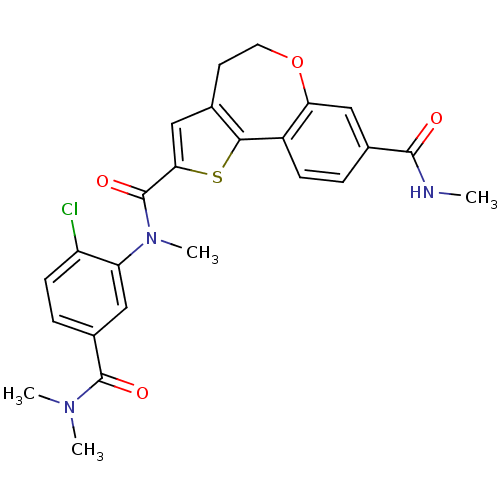
(CHEMBL1796763)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1cc(ccc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-11-16(6-8-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419771
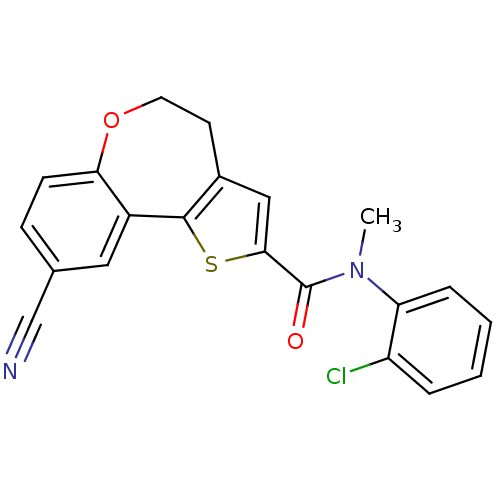
(CHEMBL1950036)Show SMILES CN(C(=O)c1cc2CCOc3ccc(cc3-c2s1)C#N)c1ccccc1Cl Show InChI InChI=1S/C21H15ClN2O2S/c1-24(17-5-3-2-4-16(17)22)21(25)19-11-14-8-9-26-18-7-6-13(12-23)10-15(18)20(14)27-19/h2-7,10-11H,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315914

((3-(2-(2-aminopyrimidin-5-yl)-7-methyl-4-morpholin...)Show SMILES CN1CCN(CC1)C(=O)c1cccc(c1)-c1sc2c(nc(nc2c1C)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C27H30N8O2S/c1-17-21-23(25(34-10-12-37-13-11-34)32-24(31-21)20-15-29-27(28)30-16-20)38-22(17)18-4-3-5-19(14-18)26(36)35-8-6-33(2)7-9-35/h3-5,14-16H,6-13H2,1-2H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315915
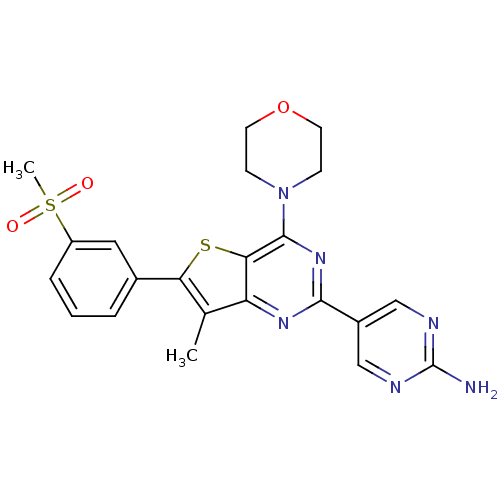
(5-(7-methyl-6-(3-(methylsulfonyl)phenyl)-4-morphol...)Show SMILES Cc1c(sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C22H22N6O3S2/c1-13-17-19(32-18(13)14-4-3-5-16(10-14)33(2,29)30)21(28-6-8-31-9-7-28)27-20(26-17)15-11-24-22(23)25-12-15/h3-5,10-12H,6-9H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419764

(CHEMBL1949922)Show SMILES CCNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C23H21ClN2O3S/c1-3-25-22(27)15-8-9-16-19(12-15)29-11-10-14-13-20(30-21(14)16)23(28)26(2)18-7-5-4-6-17(18)24/h4-9,12-13H,3,10-11H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50185825
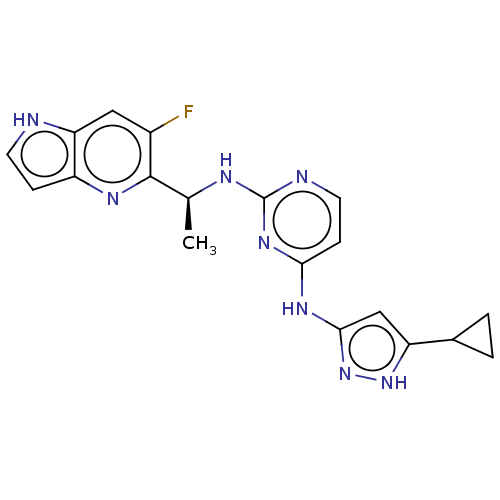
(CHEMBL3824224)Show SMILES C[C@H](Nc1nccc(Nc2cc([nH]n2)C2CC2)n1)c1nc2cc[nH]c2cc1F |r| Show InChI InChI=1S/C19H19FN8/c1-10(18-12(20)8-15-13(24-18)4-6-21-15)23-19-22-7-5-16(26-19)25-17-9-14(27-28-17)11-2-3-11/h4-11,21H,2-3H2,1H3,(H3,22,23,25,26,27,28)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged PAK1 kinase domain expressed in baculovirus expression system assessed as suppression of phosphorylation o... |
Bioorg Med Chem Lett 26: 3518-24 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.031
BindingDB Entry DOI: 10.7270/Q2KK9DQJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096231

(CHEMBL3580975)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C30H50N4O6/c1-7-19(3)25-29(40)34-17-13-12-16-23(34)28(39)31-21(15-11-9-10-14-20(35)8-2)26(37)32-22(27(38)33-25)18-24(36)30(4,5)6/h19,21-23,25H,7-18H2,1-6H3,(H,31,39)(H,32,37)(H,33,38)/t19?,21-,22-,23+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate assessed as substrate phosphorylation... |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50097144
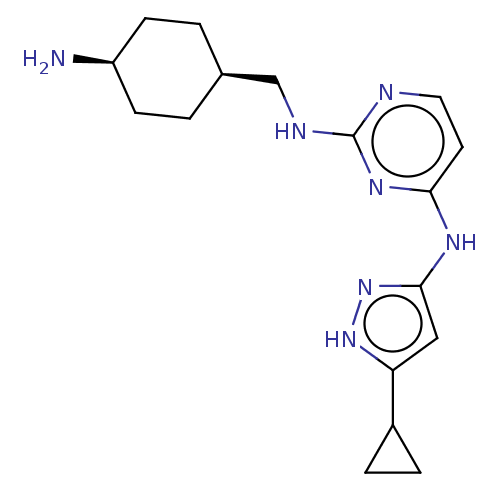
(CHEMBL3580963)Show SMILES N[C@H]1CC[C@@H](CNc2nccc(Nc3cc([nH]n3)C3CC3)n2)CC1 |r,wU:4.4,1.0,(-1.34,-9.7,;-1.34,-8.47,;-2.68,-7.7,;-2.68,-6.16,;-1.34,-5.39,;-1.34,-3.85,;-0,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-2.66,3.08,;-3.91,3.97,;-3.43,5.43,;-1.89,5.43,;-1.42,3.96,;-4.33,6.68,;-5.62,7.36,;-4.29,8.14,;-1.33,-.77,;-.01,-6.16,;-.01,-7.7,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK4 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate assessed as substrate phosphorylation... |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
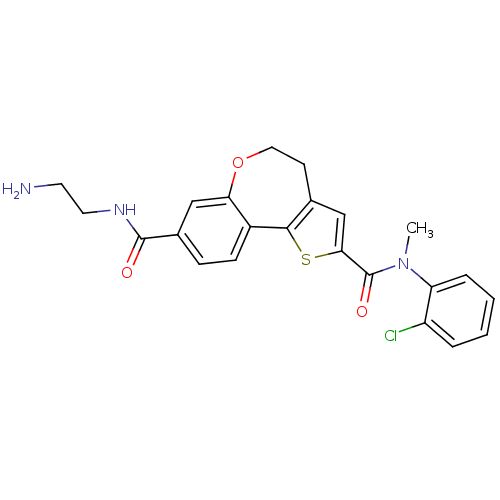
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419766
(CHEMBL1949924)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)C(=O)NCCN)c1ccccc1Cl Show InChI InChI=1S/C23H22ClN3O3S/c1-27(18-5-3-2-4-17(18)24)23(29)20-13-14-8-11-30-19-12-15(22(28)26-10-9-25)6-7-16(19)21(14)31-20/h2-7,12-13H,8-11,25H2,1H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419765
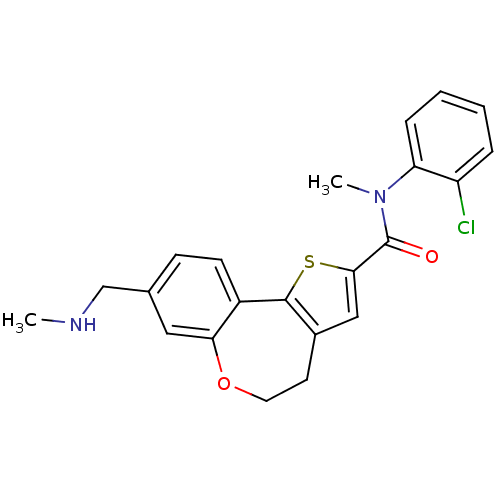
(CHEMBL1949923)Show SMILES CNCc1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H21ClN2O2S/c1-24-13-14-7-8-16-19(11-14)27-10-9-15-12-20(28-21(15)16)22(26)25(2)18-6-4-3-5-17(18)23/h3-8,11-12,24H,9-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419757

(CHEMBL1949913)Show SMILES CN1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C20H26N8OS/c1-26-2-4-27(5-3-26)13-15-10-16-18(28-6-8-29-9-7-28)24-17(25-19(16)30-15)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096231

(CHEMBL3580975)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C30H50N4O6/c1-7-19(3)25-29(40)34-17-13-12-16-23(34)28(39)31-21(15-11-9-10-14-20(35)8-2)26(37)32-22(27(38)33-25)18-24(36)30(4,5)6/h19,21-23,25H,7-18H2,1-6H3,(H,31,39)(H,32,37)(H,33,38)/t19?,21-,22-,23+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419772
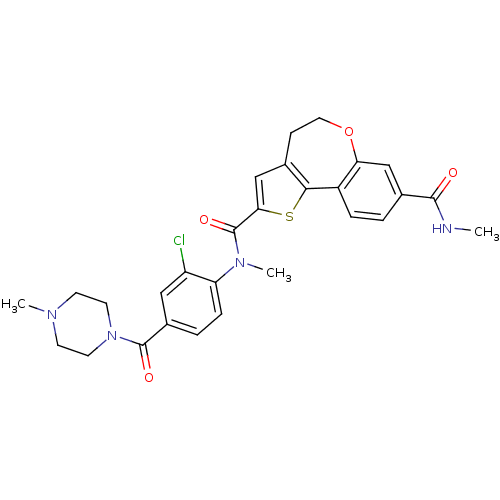
(CHEMBL1950037)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N1CCN(C)CC1 Show InChI InChI=1S/C28H29ClN4O4S/c1-30-26(34)18-4-6-20-23(15-18)37-13-8-17-16-24(38-25(17)20)28(36)32(3)22-7-5-19(14-21(22)29)27(35)33-11-9-31(2)10-12-33/h4-7,14-16H,8-13H2,1-3H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315911
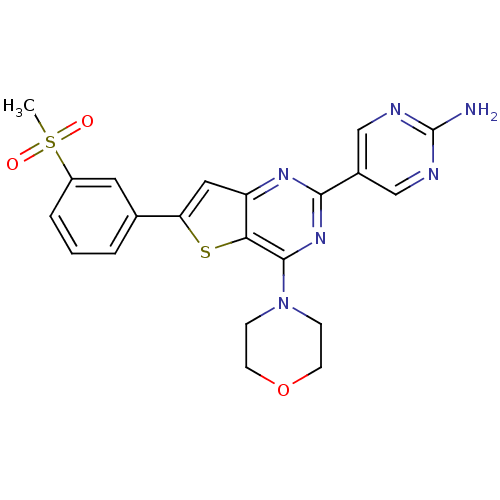
(5-(6-(3-(methylsulfonyl)phenyl)-4-morpholinothieno...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C21H20N6O3S2/c1-32(28,29)15-4-2-3-13(9-15)17-10-16-18(31-17)20(27-5-7-30-8-6-27)26-19(25-16)14-11-23-21(22)24-12-14/h2-4,9-12H,5-8H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50185823

(CHEMBL3823612)Show SMILES C[C@H](Nc1nccc(Nc2cc([nH]n2)C2CC2)n1)c1cc2cc[nH]c2cn1 |r| Show InChI InChI=1S/C19H20N8/c1-11(14-8-13-4-6-20-16(13)10-22-14)23-19-21-7-5-17(25-19)24-18-9-15(26-27-18)12-2-3-12/h4-12,20H,2-3H2,1H3,(H3,21,23,24,25,26,27)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged PAK1 kinase domain expressed in baculovirus expression system assessed as suppression of phosphorylation o... |
Bioorg Med Chem Lett 26: 3518-24 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.031
BindingDB Entry DOI: 10.7270/Q2KK9DQJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50097128

(CHEMBL3580976)Show SMILES CCN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C20H21ClN8/c1-2-29(10-13-5-6-14-19(18(13)21)24-11-23-14)20-22-8-7-16(26-20)25-17-9-15(27-28-17)12-3-4-12/h5-9,11-12H,2-4,10H2,1H3,(H,23,24)(H2,22,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate assessed as substrate phosphorylation... |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50097135
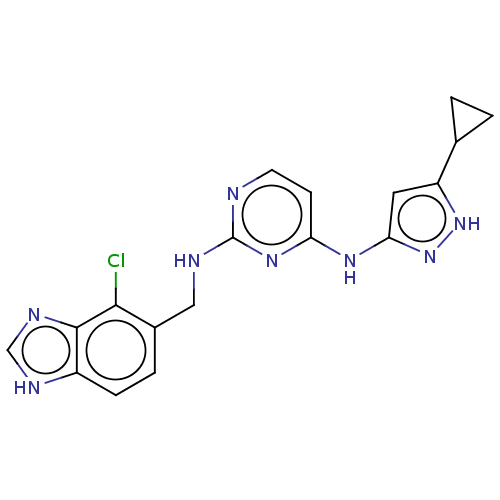
(CHEMBL3580974)Show SMILES Clc1c(CNc2nccc(Nc3cc([nH]n3)C3CC3)n2)ccc2[nH]cnc12 Show InChI InChI=1S/C18H17ClN8/c19-16-11(3-4-12-17(16)23-9-22-12)8-21-18-20-6-5-14(25-18)24-15-7-13(26-27-15)10-1-2-10/h3-7,9-10H,1-2,8H2,(H,22,23)(H3,20,21,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate assessed as substrate phosphorylation... |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50097128

(CHEMBL3580976)Show SMILES CCN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C20H21ClN8/c1-2-29(10-13-5-6-14-19(18(13)21)24-11-23-14)20-22-8-7-16(26-20)25-17-9-15(27-28-17)12-3-4-12/h5-9,11-12H,2-4,10H2,1H3,(H,23,24)(H2,22,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora A (unknown origin) |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50185827
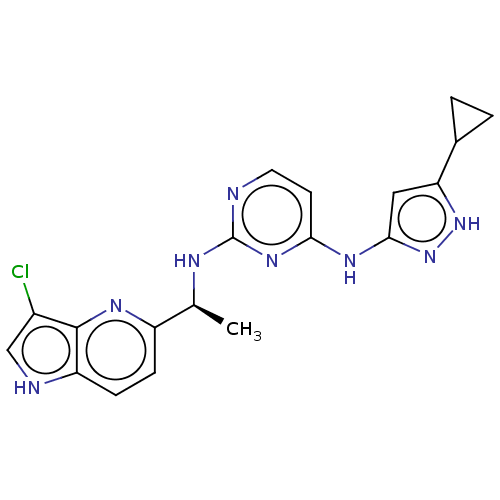
(CHEMBL3823523)Show SMILES C[C@H](Nc1nccc(Nc2cc([nH]n2)C2CC2)n1)c1ccc2[nH]cc(Cl)c2n1 |r| Show InChI InChI=1S/C19H19ClN8/c1-10(13-4-5-14-18(24-13)12(20)9-22-14)23-19-21-7-6-16(26-19)25-17-8-15(27-28-17)11-2-3-11/h4-11,22H,2-3H2,1H3,(H3,21,23,25,26,27,28)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged PAK1 kinase domain expressed in baculovirus expression system assessed as suppression of phosphorylation o... |
Bioorg Med Chem Lett 26: 3518-24 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.031
BindingDB Entry DOI: 10.7270/Q2KK9DQJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358206
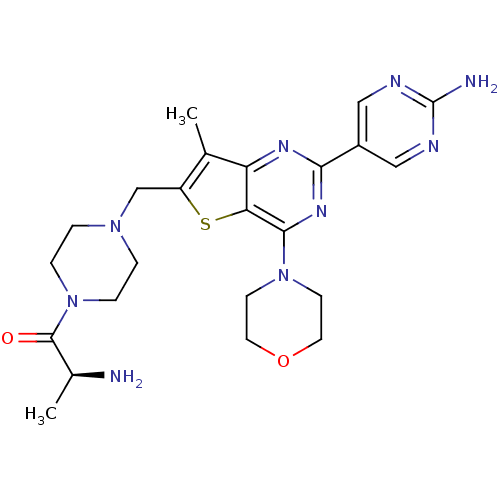
(CHEMBL1922091)Show SMILES C[C@H](N)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H31N9O2S/c1-14-17(13-30-3-5-32(6-4-30)22(33)15(2)24)35-19-18(14)28-20(16-11-26-23(25)27-12-16)29-21(19)31-7-9-34-10-8-31/h11-12,15H,3-10,13,24H2,1-2H3,(H2,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50419757

(CHEMBL1949913)Show SMILES CN1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C20H26N8OS/c1-26-2-4-27(5-3-26)13-15-10-16-18(28-6-8-29-9-7-28)24-17(25-19(16)30-15)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kbeta using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419756
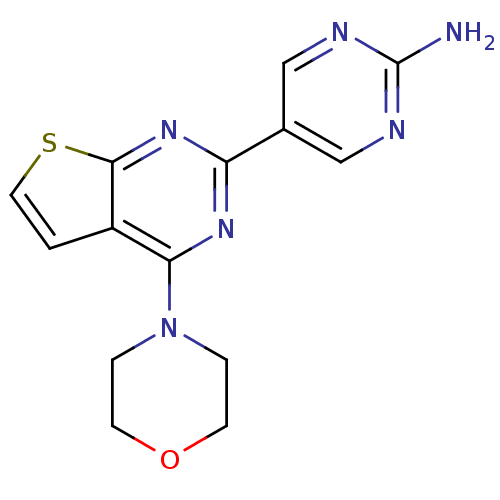
(CHEMBL1949912)Show InChI InChI=1S/C14H14N6OS/c15-14-16-7-9(8-17-14)11-18-12(20-2-4-21-5-3-20)10-1-6-22-13(10)19-11/h1,6-8H,2-5H2,(H2,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315913

((3-(2-(2-aminopyrimidin-5-yl)-4-morpholinothieno[3...)Show SMILES CN1CCN(CC1)C(=O)c1cccc(c1)-c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C26H28N8O2S/c1-32-5-7-34(8-6-32)25(35)18-4-2-3-17(13-18)21-14-20-22(37-21)24(33-9-11-36-12-10-33)31-23(30-20)19-15-28-26(27)29-16-19/h2-4,13-16H,5-12H2,1H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315908

(CHEMBL1090598 | N-((2-(2-aminopyrimidin-5-yl)-4-mo...)Show SMILES CN(Cc1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C17H21N7O3S2/c1-23(29(2,25)26)10-12-7-13-14(28-12)16(24-3-5-27-6-4-24)22-15(21-13)11-8-19-17(18)20-9-11/h7-9H,3-6,10H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50185828
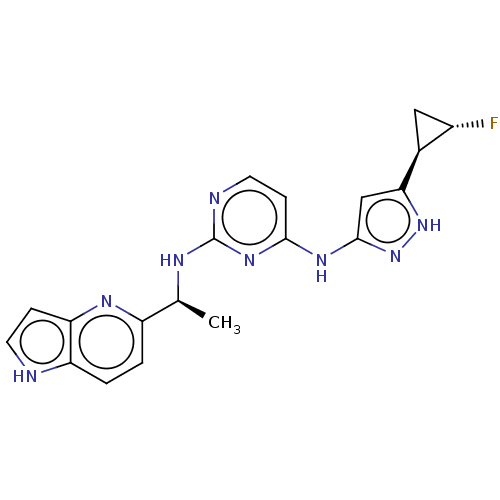
(CHEMBL3824061)Show SMILES C[C@H](Nc1nccc(Nc2cc([nH]n2)[C@H]2C[C@@H]2F)n1)c1ccc2[nH]ccc2n1 |r| Show InChI InChI=1S/C19H19FN8/c1-10(13-2-3-14-15(24-13)4-6-21-14)23-19-22-7-5-17(26-19)25-18-9-16(27-28-18)11-8-12(11)20/h2-7,9-12,21H,8H2,1H3,(H3,22,23,25,26,27,28)/t10-,11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged PAK1 kinase domain expressed in baculovirus expression system assessed as suppression of phosphorylation o... |
Bioorg Med Chem Lett 26: 3518-24 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.031
BindingDB Entry DOI: 10.7270/Q2KK9DQJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419763

(CHEMBL1949921)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)C#N)c1ccccc1Cl Show InChI InChI=1S/C21H15ClN2O2S/c1-24(17-5-3-2-4-16(17)22)21(25)19-11-14-8-9-26-18-10-13(12-23)6-7-15(18)20(14)27-19/h2-7,10-11H,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358205
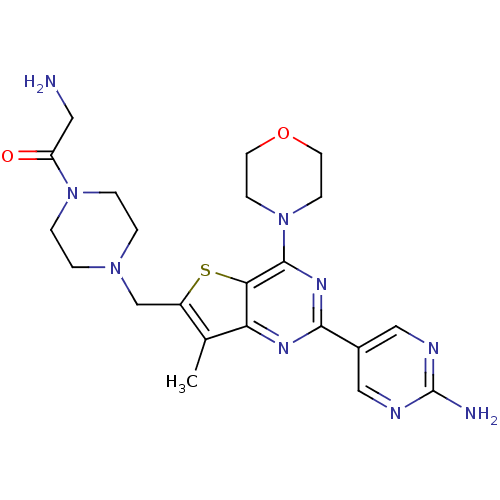
(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50097134
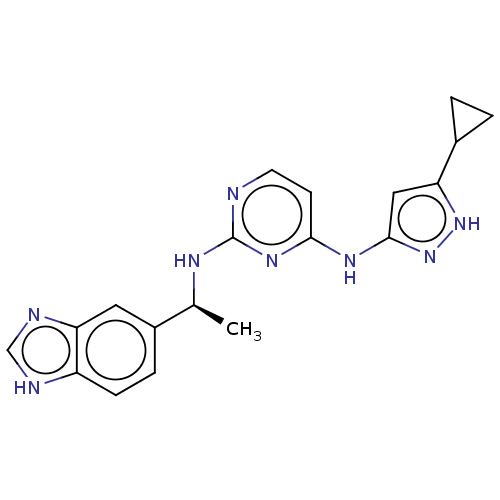
(CHEMBL3580972)Show SMILES C[C@H](Nc1nccc(Nc2cc([nH]n2)C2CC2)n1)c1ccc2[nH]cnc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK1 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate assessed as substrate phosphorylation... |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50097127
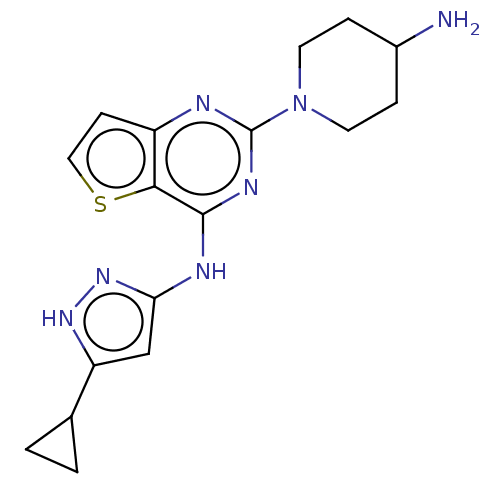
(CHEMBL3580949)Show SMILES NC1CCN(CC1)c1nc(Nc2cc([nH]n2)C2CC2)c2sccc2n1 Show InChI InChI=1S/C17H21N7S/c18-11-3-6-24(7-4-11)17-19-12-5-8-25-15(12)16(21-17)20-14-9-13(22-23-14)10-1-2-10/h5,8-11H,1-4,6-7,18H2,(H2,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PAK4 kinase domain using coumarin/fluorescein-labeled FRET peptide as substrate assessed as substrate phosphorylation... |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358209

(CHEMBL1922095)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H32N8O3S/c1-15-17(14-30-4-6-32(7-5-30)22(33)24(2,3)34)36-19-18(15)28-20(16-12-26-23(25)27-13-16)29-21(19)31-8-10-35-11-9-31/h12-13,34H,4-11,14H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM101618
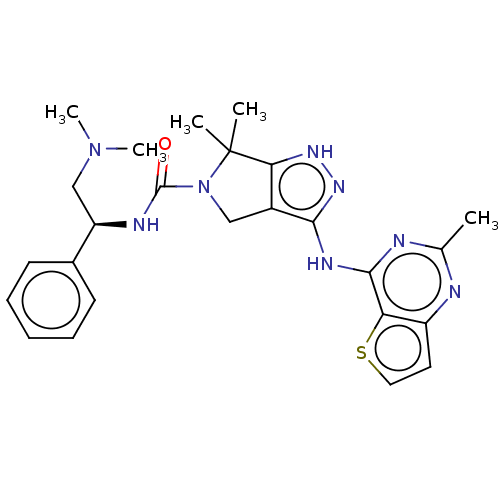
(US8530652, 114)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)n[nH]c2C1(C)C)c1ccccc1 Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Chempartner Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human recombinant PAK4 (300 to 591 amino acids) using peptide-7 substrate by pyruvate kinase and lactate dehydro... |
J Med Chem 58: 5121-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00572
BindingDB Entry DOI: 10.7270/Q2DV1MN9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096233
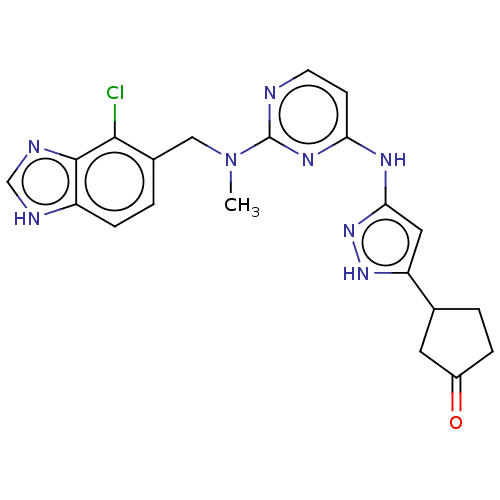
(CHEMBL3593824)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CCC(=O)C2)n1 Show InChI InChI=1S/C34H50BrN5O6/c1-5-21(3)30-34(45)39-17-11-10-14-29(39)33(44)36-26(13-9-7-8-12-24(41)6-2)31(42)37-27(32(43)38-30)18-22-20-40(46-4)28-16-15-23(35)19-25(22)28/h15-16,19,21-22,26-27,29-30H,5-14,17-18,20H2,1-4H3,(H,36,44)(H,37,42)(H,38,43)/t21?,22?,26-,27-,29+,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347085

(CHEMBL1796271)Show InChI InChI=1S/C20H16ClNO2S/c1-22(16-8-4-3-7-15(16)21)20(23)18-12-13-10-11-24-17-9-5-2-6-14(17)19(13)25-18/h2-9,12H,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data