

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15797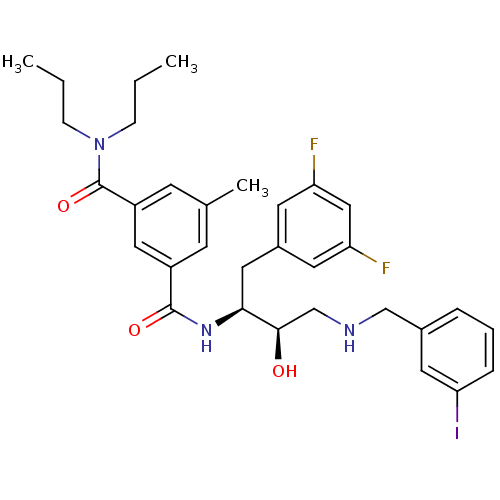 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech Curated by ChEMBL | Assay Description Inhibition of BACE1 by cell-free FRET assay | Bioorg Med Chem Lett 21: 3992-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.007 BindingDB Entry DOI: 10.7270/Q22Z15W0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16034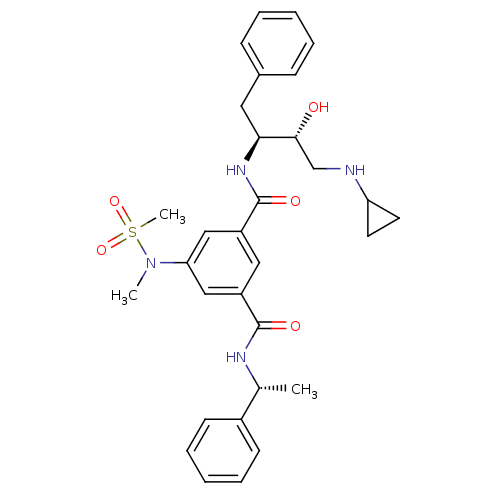 (1-N-[(2S,3R)-4-(cyclopropylamino)-3-hydroxy-1-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech Curated by ChEMBL | Assay Description Inhibition of BACE1 by cell-free FRET assay | Bioorg Med Chem Lett 21: 3992-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.007 BindingDB Entry DOI: 10.7270/Q22Z15W0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006369 ((4R)-6'-chloro-3'-methyl-1'H,2H,5H-spiro[imidazoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006362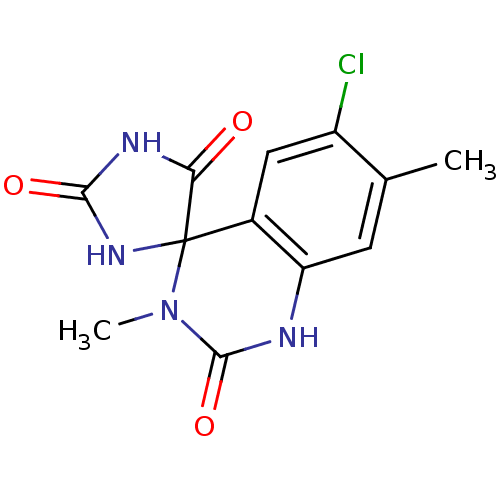 (6'-chloro-3',7'-dimethyl-1'H,2H,5H-spiro[imidazoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006372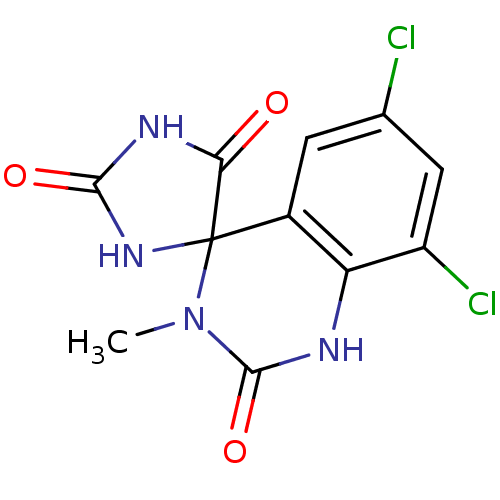 (6',8'-dichloro-3'-methyl-1'H,2H,5H-spiro[imidazoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483944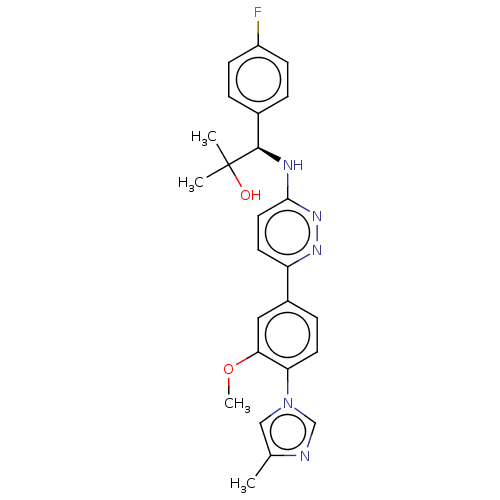 (CHEMBL1797105) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006358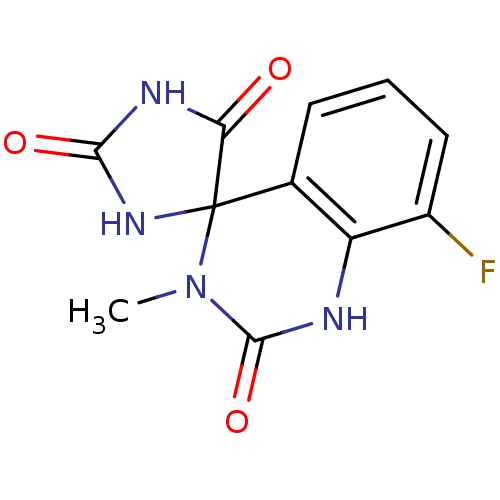 (8'-fluoro-3'-methyl-1'H,2H,5H-spiro[imidazolidine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006404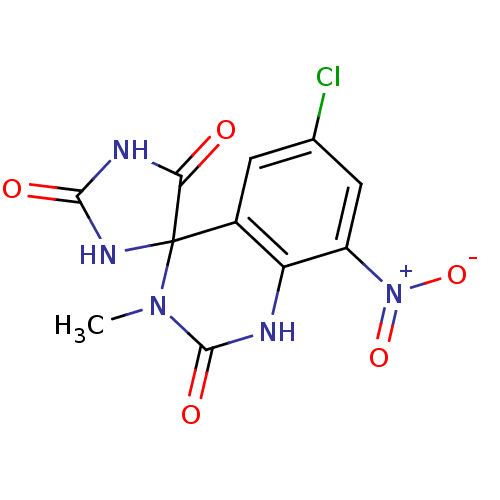 (6'-chloro-3'-methyl-8'-nitro-1'H,2H,5H-spiro[imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483946 (CHEMBL1797111) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483943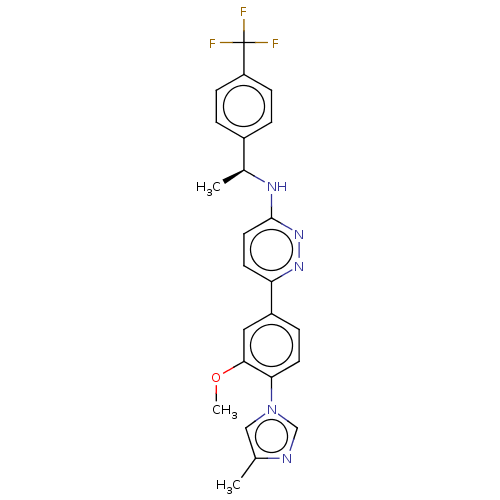 (CHEMBL1797102) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483955 (CHEMBL1797103) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483942 (CHEMBL1797100) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006403 (6'-bromo-3'-methyl-1'H,2H,5H-spiro[imidazolidine-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006391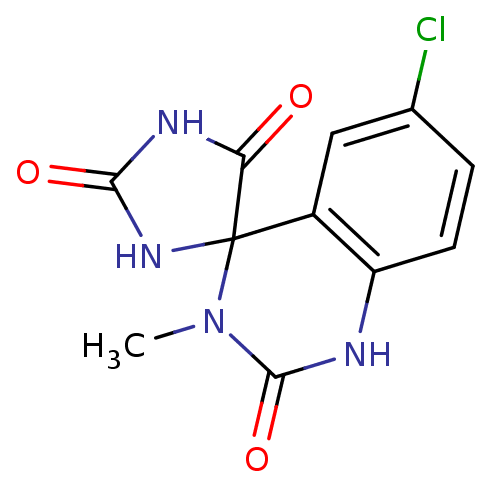 (6'-chloro-3'-methyl-1'H,2H,5H-spiro[imidazolidine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006375 (6'-chloro-7'-methoxy-3'-methyl-1'H,2H,5H-spiro[imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483954 (CHEMBL1797110) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483956 (CHEMBL1797099) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483951 (CHEMBL1797108) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483951 (CHEMBL1797108) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing human recombinant Swedish variant of APP K595N/M596L assessed as reduction of amyloid ... | Bioorg Med Chem Lett 21: 4832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.06.042 BindingDB Entry DOI: 10.7270/Q25T3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483940 (CHEMBL1797097) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing human recombinant Swedish variant of APP K595N/M596L assessed as reduction of amyloid ... | Bioorg Med Chem Lett 21: 4832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.06.042 BindingDB Entry DOI: 10.7270/Q25T3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483940 (CHEMBL1797097) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006396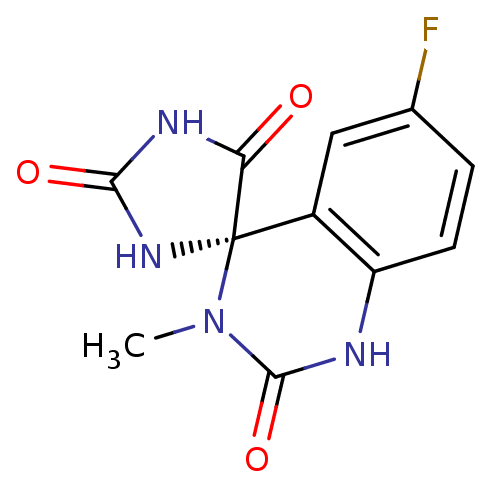 ((4R)-6'-fluoro-3'-methyl-1'H,2H,5H-spiro[imidazoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006392 (6',8'-dichloro-7'-methoxy-3'-methyl-1'H,2H,5H-spir...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006354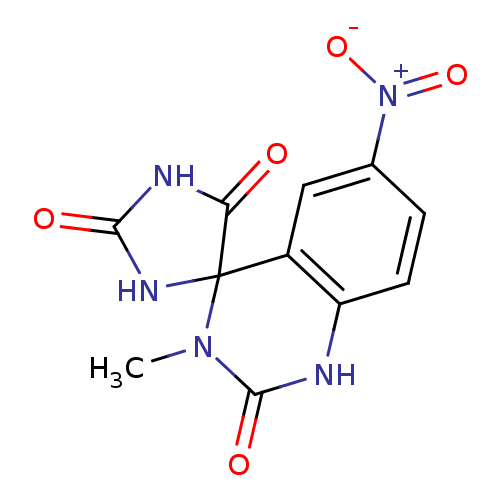 (3'-methyl-6'-nitro-1'H,2H,5H-spiro[imidazolidine-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006370 (6',7'-dichloro-3'-methyl-1'H,2H,5H-spiro[imidazoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483942 (CHEMBL1797100) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484070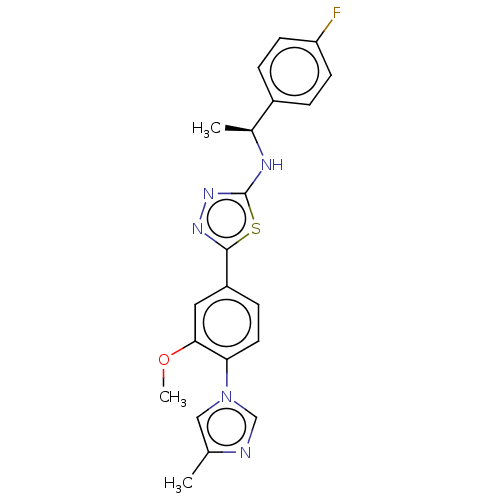 (CHEMBL1806519) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing human recombinant Swedish variant of APP K595N/M596L assessed as reduction of amyloid ... | Bioorg Med Chem Lett 21: 4832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.06.042 BindingDB Entry DOI: 10.7270/Q25T3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483941 (CHEMBL1797098) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006389 (7'-chloro-3'-methyl-1'H,2H,5H-spiro[imidazolidine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483946 (CHEMBL1797111) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006361 (6'-fluoro-3'-methyl-1'H,2H,5H-spiro[imidazolidine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483947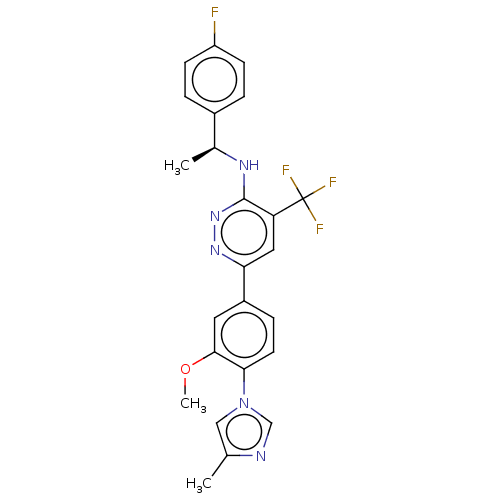 (CHEMBL1797112) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483939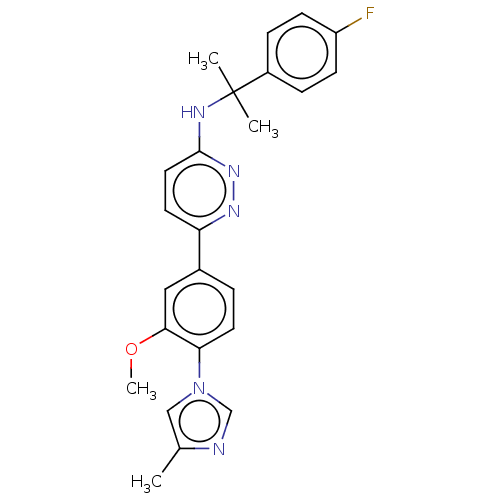 (CHEMBL1797095) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006353 (7'-chloro-3'-methyl-6'-nitro-1'H,2H,5H-spiro[imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483956 (CHEMBL1797099) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483955 (CHEMBL1797103) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483957 (CHEMBL1797109) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50484076 (CHEMBL1808601) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing human recombinant Swedish variant of APP K595N/M596L assessed as reduction of amyloid ... | Bioorg Med Chem Lett 21: 4832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.06.042 BindingDB Entry DOI: 10.7270/Q25T3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483943 (CHEMBL1797102) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006377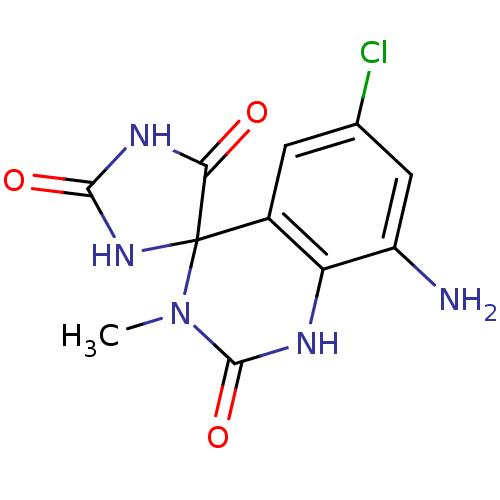 (8'-amino-6'-chloro-3'-methyl-1'H,2H,5H-spiro[imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50006357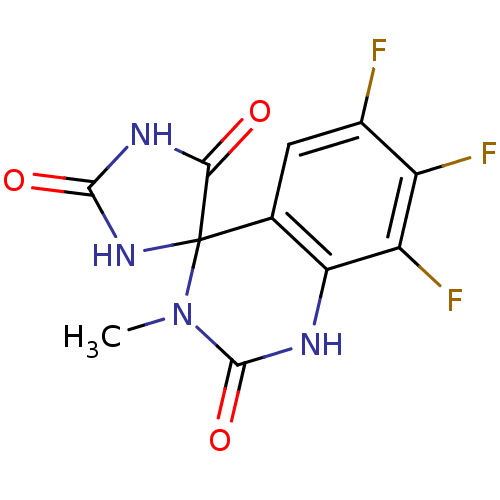 (6',7',8'-trifluoro-3'-methyl-1'H,2H,5H-spiro[imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Tested in vitro for inhibitory activity against partially purified aldose reductase from male rabbit lens | J Med Chem 35: 2085-94 (1992) BindingDB Entry DOI: 10.7270/Q2V123RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483953 (CHEMBL1797104) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483944 (CHEMBL1797105) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483941 (CHEMBL1797098) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483949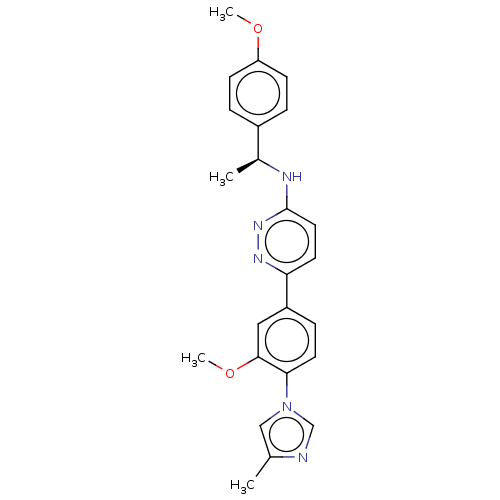 (CHEMBL1797101) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483954 (CHEMBL1797110) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483949 (CHEMBL1797101) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483951 (CHEMBL1797108) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 40 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483951 (CHEMBL1797108) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing human recombinant Swedish variant of APP K595N/M596L assessed as reduction of amyloid ... | Bioorg Med Chem Lett 21: 4832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.06.042 BindingDB Entry DOI: 10.7270/Q25T3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50483945 (CHEMBL1797107) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SHSY5Y cells expressing Swedish variant of APP K595N/M596L assessed as reduction of amyloid beta 42 formation ... | Bioorg Med Chem Lett 21: 4016-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.143 BindingDB Entry DOI: 10.7270/Q2VH5RPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 171 total ) | Next | Last >> |