

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919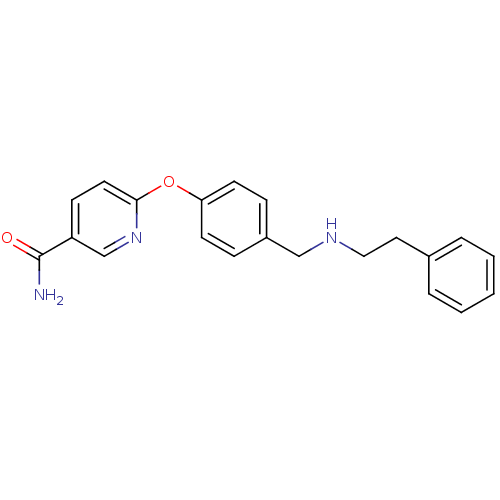 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219921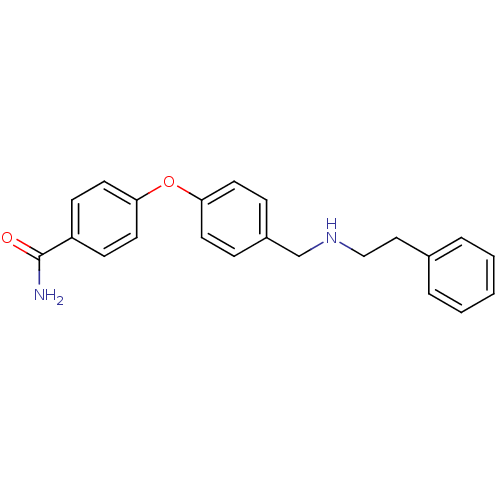 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219918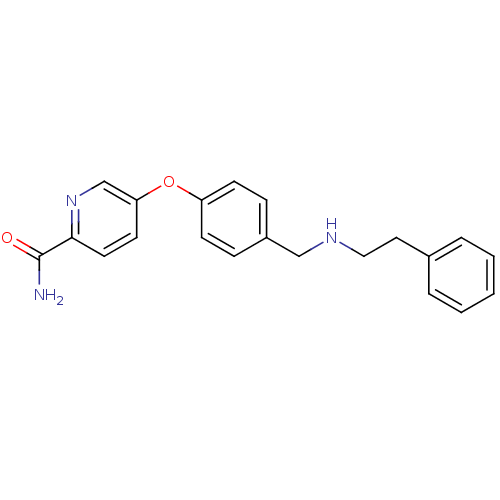 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219918 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219925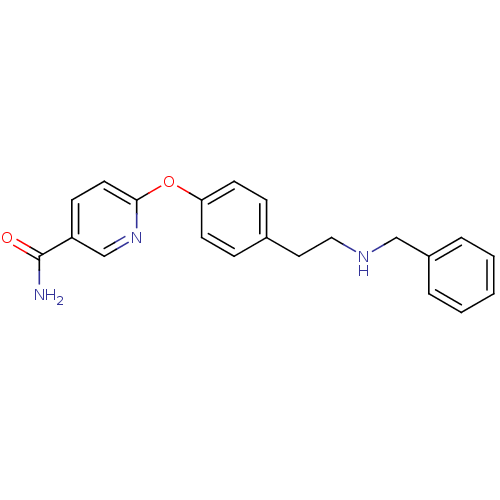 (6-(4-(2-(benzylamino)ethyl)phenoxy)nicotinamide | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219916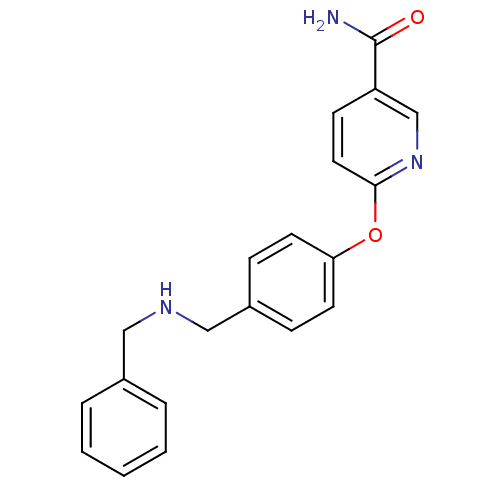 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219918 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219916 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219925 (6-(4-(2-(benzylamino)ethyl)phenoxy)nicotinamide | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219918 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219918 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219915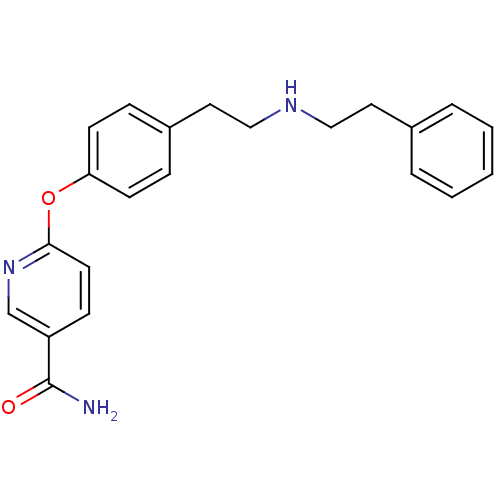 (6-(4-(2-(phenethylamino)ethyl)phenoxy)nicotinamide...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219911 (6-(4-(2-(3-phenylpropylamino)ethyl)phenoxy)nicotin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219925 (6-(4-(2-(benzylamino)ethyl)phenoxy)nicotinamide | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219924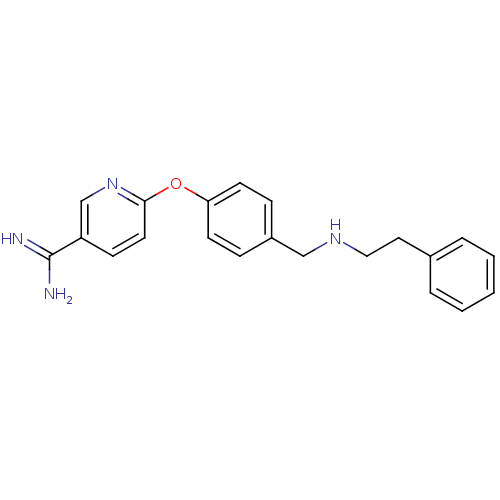 (6-[4-(phenethylamino-methyl)-phenoxy]-nicotinamidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219917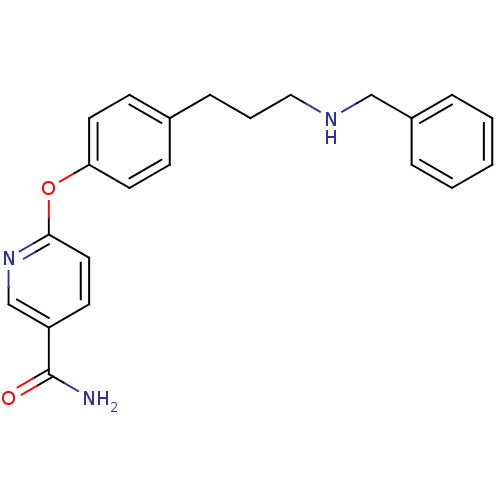 (6-(4-(3-(benzylamino)propyl)phenoxy)nicotinamide |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219925 (6-(4-(2-(benzylamino)ethyl)phenoxy)nicotinamide | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219915 (6-(4-(2-(phenethylamino)ethyl)phenoxy)nicotinamide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219911 (6-(4-(2-(3-phenylpropylamino)ethyl)phenoxy)nicotin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219917 (6-(4-(3-(benzylamino)propyl)phenoxy)nicotinamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219922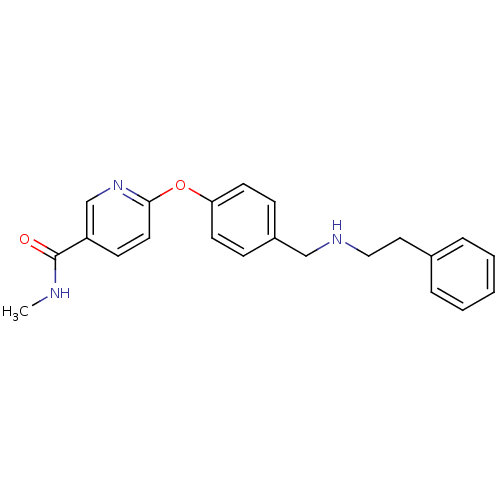 (CHEMBL396392 | N-methyl-6-(4-((phenethylamino)meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219933 (CHEMBL238305 | N-isopropyl-6-(4-((phenethylamino)m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219916 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219930 (CHEMBL238283 | N-ethyl-6-(4-((phenethylamino)methy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219918 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219925 (6-(4-(2-(benzylamino)ethyl)phenoxy)nicotinamide | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219915 (6-(4-(2-(phenethylamino)ethyl)phenoxy)nicotinamide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219925 (6-(4-(2-(benzylamino)ethyl)phenoxy)nicotinamide | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 157 total ) | Next | Last >> |