

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Mus musculus) | BDBM50280299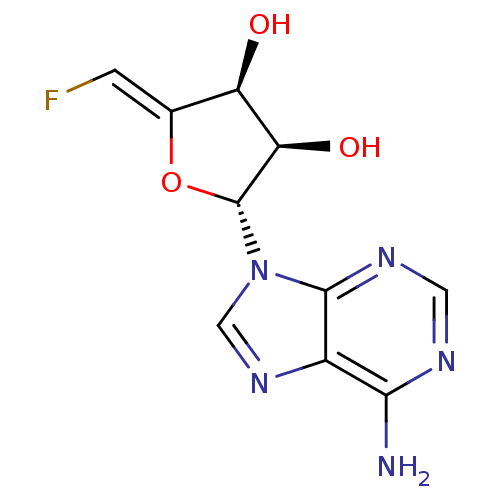 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50280301 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280298 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for binding affinity of compound against S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 3: 165-168 (1993) Article DOI: 10.1016/S0960-894X(01)80869-6 BindingDB Entry DOI: 10.7270/Q2DR2VDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368169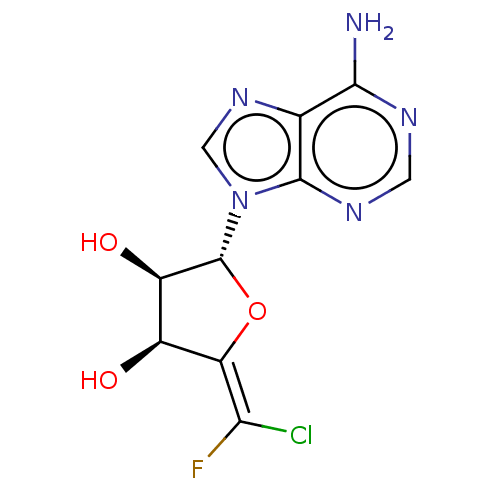 (CHEMBL2368687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368167 (CHEMBL3349334 | CHEMBL611905) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280301 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368170 (CHEMBL2368677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50406477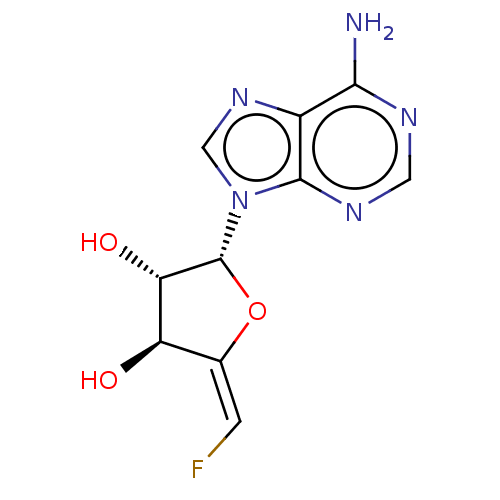 (CHEMBL2051968 | CHEMBL2069133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280299 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280300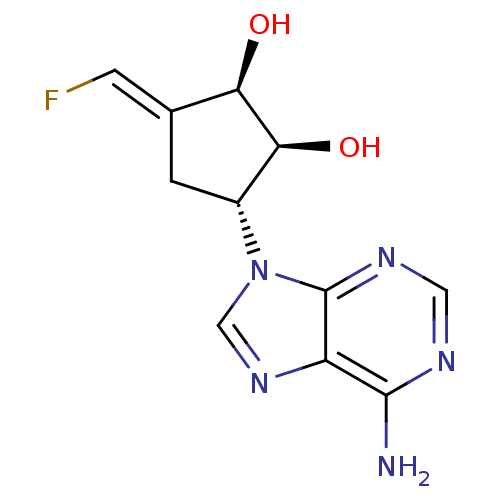 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for binding affinity of compound against S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 3: 165-168 (1993) Article DOI: 10.1016/S0960-894X(01)80869-6 BindingDB Entry DOI: 10.7270/Q2DR2VDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368168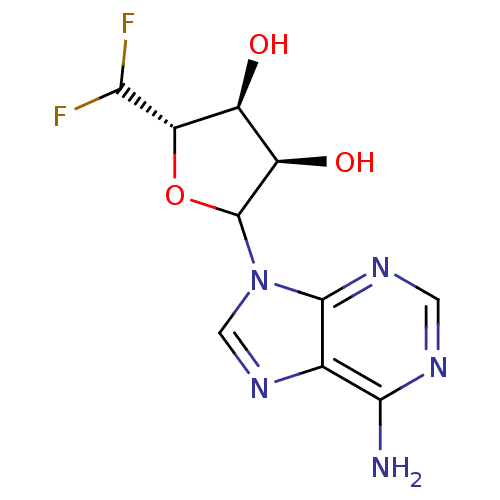 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-adenosyl-L-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368168 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50368168 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50229043 (CHEMBL2051969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50144936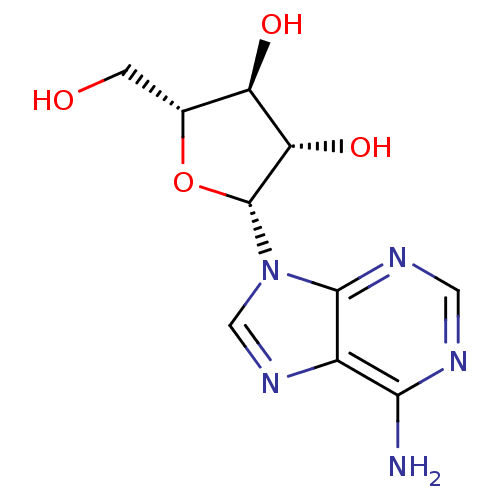 (CHEMBL1090 | VIDARABINE | adenine arabinoside) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in mouse liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50011352 (5-(6-Amino-purin-9-yl)-2-fluoromethylene-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50030823 (CHEMBL3342553) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50030822 (CHEMBL3342554) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50030823 (CHEMBL3342553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50030822 (CHEMBL3342554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195711 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)-2-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195708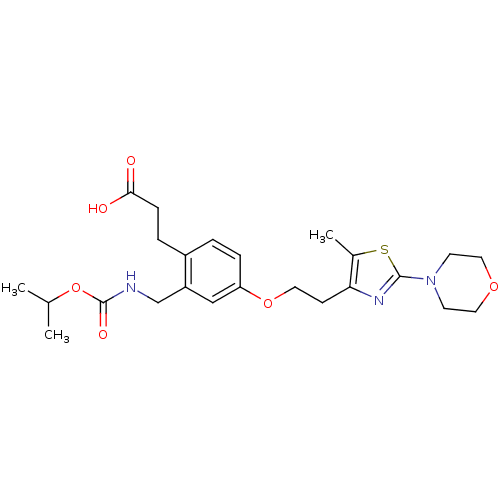 (3-(2-((isopropoxycarbonyl)methyl)-4-(2-(5-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195714 (3-(2-((isopropoxycarbonyl)methyl)-4-(2-(5-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195716 (3-{4-[2-(2-biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195702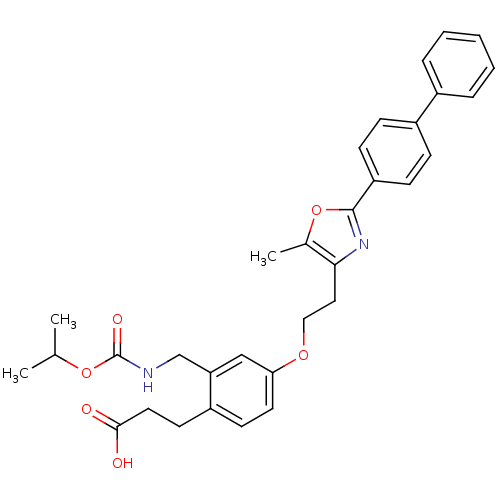 (3-[4-[2-(2-biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195715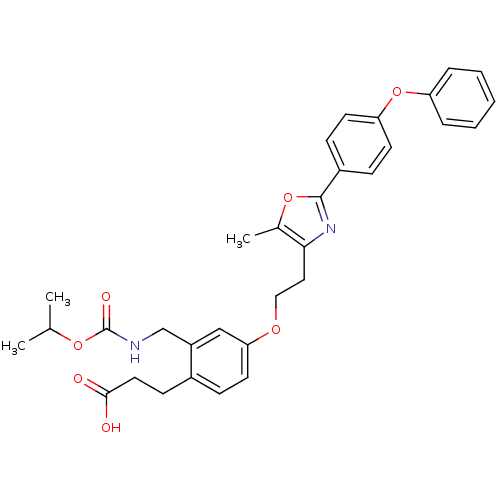 (3-(2-((isopropoxycarbonyl)methyl)-4-(2-(5-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195713 (3-(2-((isopropoxycarbonyl)methyl)-4-(2-(5-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50195711 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)-2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalpha | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195707 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)-2-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195710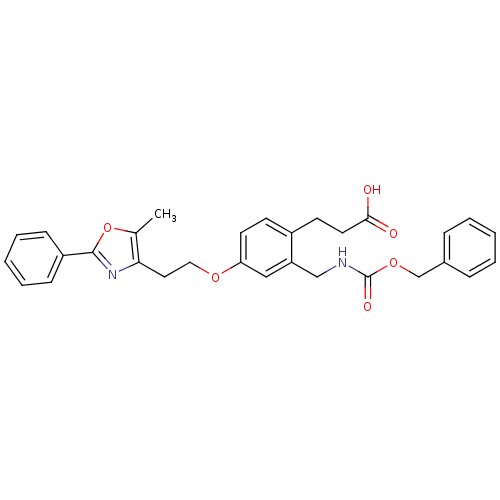 (3-(2-((benzyloxycarbonyl)methyl)-4-(2-(5-methyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50015622 (1-(2-Thiophen-2-yl-ethyl)-1,3-dihydro-imidazole-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH) | J Med Chem 33: 1866-73 (1990) BindingDB Entry DOI: 10.7270/Q2W094XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50015620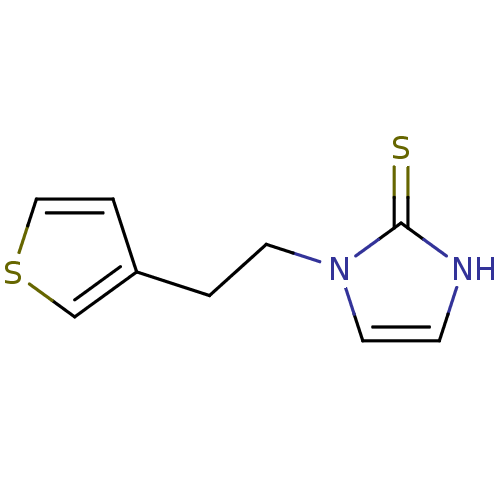 (1-(2-Thiophen-3-yl-ethyl)-1,3-dihydro-imidazole-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH) | J Med Chem 33: 1866-73 (1990) BindingDB Entry DOI: 10.7270/Q2W094XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50195714 (3-(2-((isopropoxycarbonyl)methyl)-4-(2-(5-methyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalpha | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195712 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)-2-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014983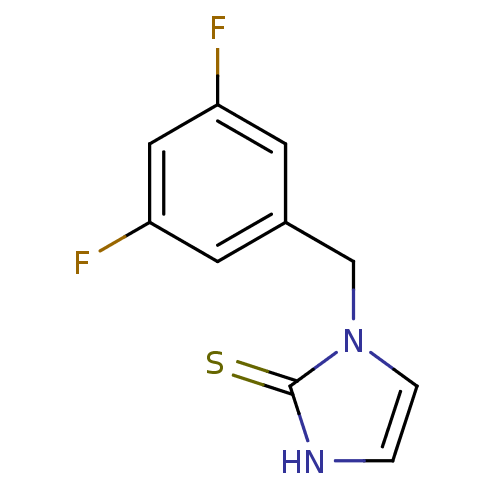 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH) | J Med Chem 33: 1866-73 (1990) BindingDB Entry DOI: 10.7270/Q2W094XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50195710 (3-(2-((benzyloxycarbonyl)methyl)-4-(2-(5-methyl-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalpha | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50195716 (3-{4-[2-(2-biphenyl-4-yl-5-methyl-oxazol-4-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 449 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalpha | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50015617 (1-[2-(5-Methyl-thiophen-2-yl)-ethyl]-1,3-dihydro-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 491 | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH) | J Med Chem 33: 1866-73 (1990) BindingDB Entry DOI: 10.7270/Q2W094XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50195715 (3-(2-((isopropoxycarbonyl)methyl)-4-(2-(5-methyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 516 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalpha | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50195709 (3-(2-((3-benzylureido)methyl)-4-(2-(5-methyl-2-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 706 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-methyl-2-(4-{3-propyl-(5-pyridin-2yl-thiophene-2-sulfonyl)-amino]-pro-pyl}-phenoxy)-propionic acid from human PPARgamma | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50015621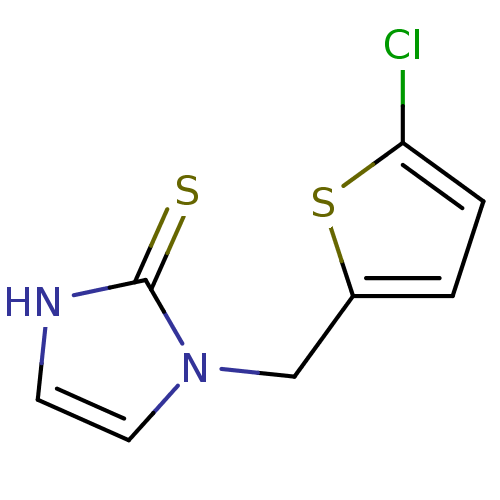 (1-(5-Chloro-thiophen-2-ylmethyl)-1,3-dihydro-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH) | J Med Chem 33: 1866-73 (1990) BindingDB Entry DOI: 10.7270/Q2W094XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50030816 (CHEMBL3342550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50030817 (CHEMBL3342551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50195708 (3-(2-((isopropoxycarbonyl)methyl)-4-(2-(5-methyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalpha | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50030816 (CHEMBL3342550) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50015619 (1-(3-Thiophen-2-yl-propyl)-1,3-dihydro-imidazole-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine adrenal dopamine beta-hydroxylase(DBH) | J Med Chem 33: 1866-73 (1990) BindingDB Entry DOI: 10.7270/Q2W094XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50030817 (CHEMBL3342551) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay | ACS Med Chem Lett 5: 1138-42 (2014) Article DOI: 10.1021/ml500283g BindingDB Entry DOI: 10.7270/Q2765GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50241379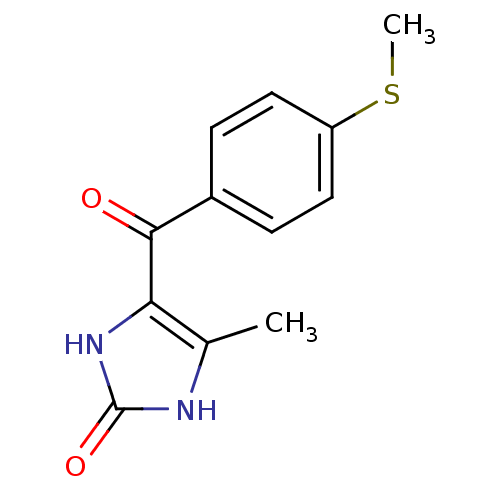 ((enoximone)4-Methyl-5-(4-methylsulfanyl-benzoyl)-1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Inhibition of canine heart Phosphodiesterase 4 | J Med Chem 33: 317-27 (1990) BindingDB Entry DOI: 10.7270/Q27S7R0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50195707 (3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)-2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from hPPARalpha | Bioorg Med Chem Lett 16: 6328-33 (2006) Article DOI: 10.1016/j.bmcl.2006.09.011 BindingDB Entry DOI: 10.7270/Q2PK0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |