

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50000066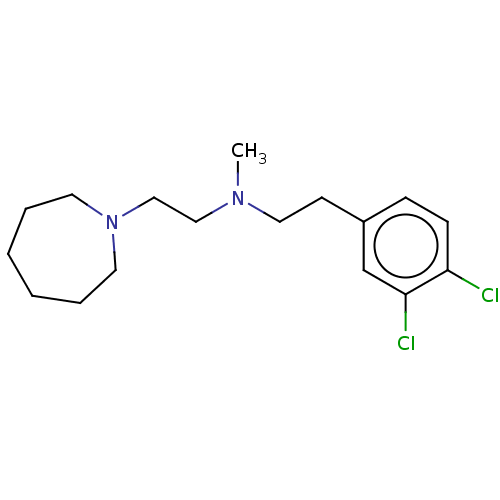 ((2-Azepan-1-yl-ethyl)-[2-(3,4-dichloro-phenyl)-eth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607-7061 Curated by ChEMBL | Assay Description Inhibition of [3H]-NANM binding to sigma receptor obtained from tissue homogenate preparation of fresh whole rat brain minus cerebellum | J Med Chem 41: 468-77 (1998) Article DOI: 10.1021/jm970059p BindingDB Entry DOI: 10.7270/Q27W6CW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50041240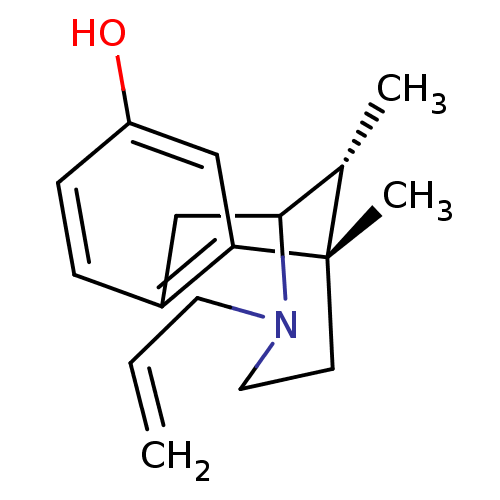 ((6S,11R)-3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50000069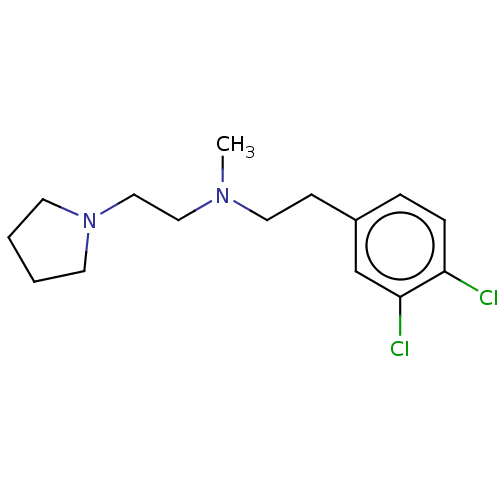 (CHEMBL20377 | [2-(3,4-Dichloro-phenyl)-ethyl]-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Tested for its binding affinity towards sigma-1 site in rat brain, using [3H](+)-3-PPP as radioligand | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50095694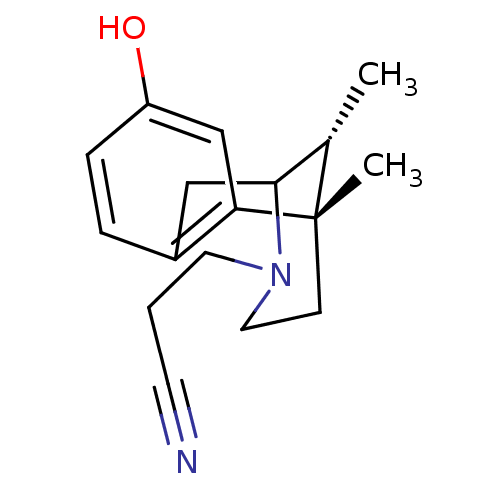 (3-(8-Hydroxy-6,11-dimethyl-1,2,5,6-tetrahydro-4H-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50095706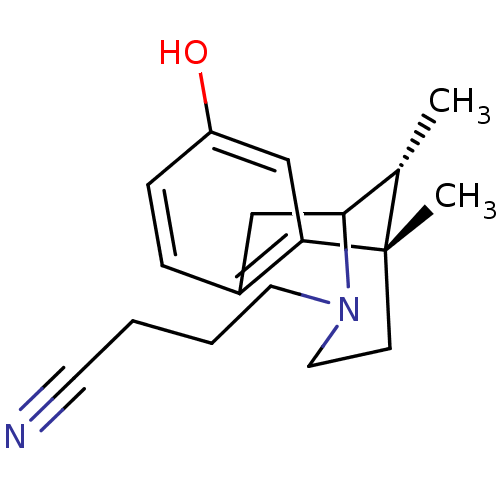 (4-(8-Hydroxy-6,11-dimethyl-1,2,5,6-tetrahydro-4H-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50037492 (CHEMBL113995 | [(S)-2-(2-{(S)-2-[(S)-2-Amino-3-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against opioid receptor by displacing radioligand [3H]DAMGO | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50095705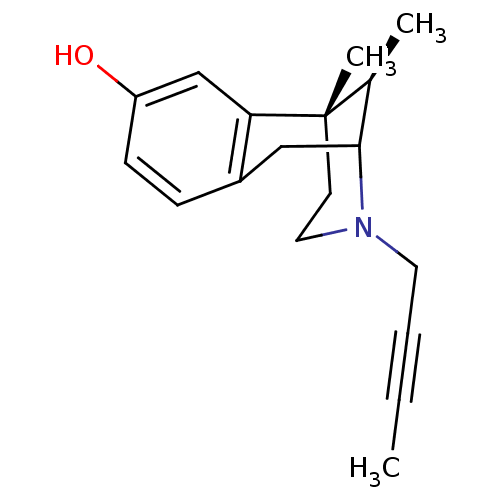 (3-But-2-ynyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor mu 1 using [3H]-DAMGO at the Kd concentration 0.57 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50041240 ((6S,11R)-3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor mu 1 using [3H]-DAMGO at the Kd concentration 0.57 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity towards muscarinic m2 receptor | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50095699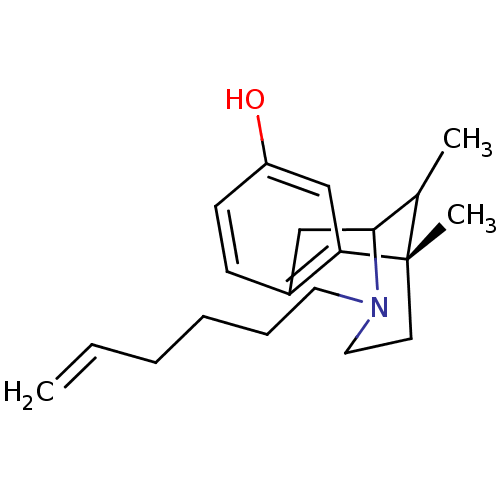 (3-Hex-5-enyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Sigma opioid receptor type 1 using [(+)-[3H]pentazocaine at the Kd concentration 2nM . | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50381677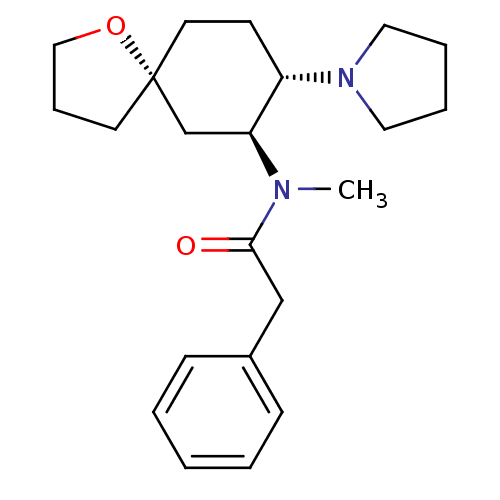 (CHEMBL1256748 | U-69593) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against opioid receptor by displacing radioligand [3HlU69,593 | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50095703 (3-But-3-enyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50095707 (3-But-3-ynyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50037478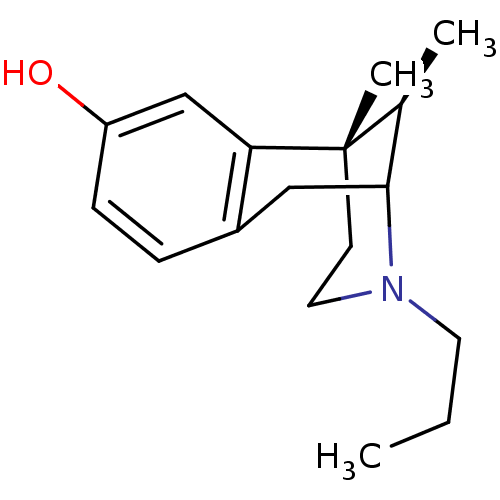 ((6S,11R)-6,11-Dimethyl-3-propyl-1,2,3,4,5,6-hexahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against mu receptor from displacement studies using 0.5 nM [3H]-DAMGO in rhesus monkey cortex membrane | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50029792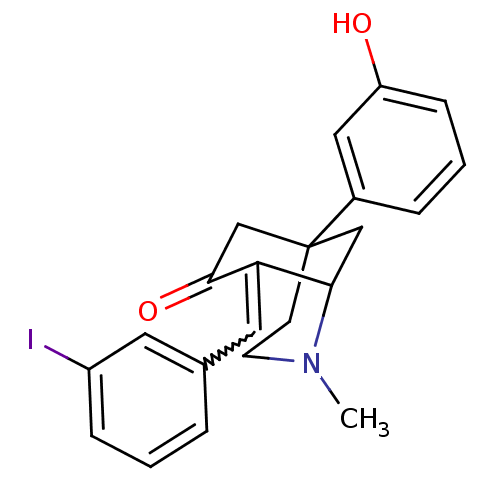 (5-(3-Hydroxy-phenyl)-8-[1-(3-iodo-phenyl)-meth-(E)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-(+)-pentazocine binding to guinea pig brain Sigma receptor type 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50029787 (5-(3-Hydroxy-phenyl)-8-[1-(4-iodo-phenyl)-meth-(E)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-(+)-pentazocine binding to guinea pig brain Sigma receptor type 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50029786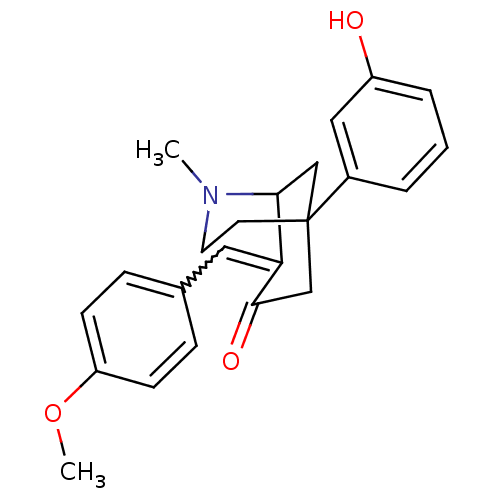 (5-(3-Hydroxy-phenyl)-8-[1-(4-methoxy-phenyl)-meth-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-(+)-pentazocine binding to guinea pig brain Sigma receptor type 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50037489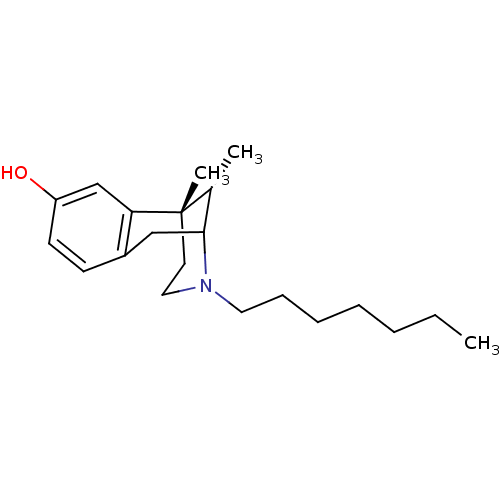 ((6S,11S)-3-Heptyl-6,11-dimethyl-1,2,3,4,5,6-hexahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against sigma receptor using displacement of 3 nM [3H]pentazocine in homogenate of guinea pig brain cerebellum | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50039200 ((4-Phenyl-butyl)-(5-phenyl-pentyl)-amine | CHEMBL2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Tested for its binding affinity towards sigma-1 site in rat brain, using [3H]DTG as radioligand | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity towards muscarinic m1 receptor | J Med Chem 37: 2285-91 (1994) BindingDB Entry DOI: 10.7270/Q29S1RPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50037478 ((6S,11R)-6,11-Dimethyl-3-propyl-1,2,3,4,5,6-hexahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against kappa receptor from displacement studies using 1.5 nM [3H]U-69593 in rhesus monkey cortex membrane | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50367061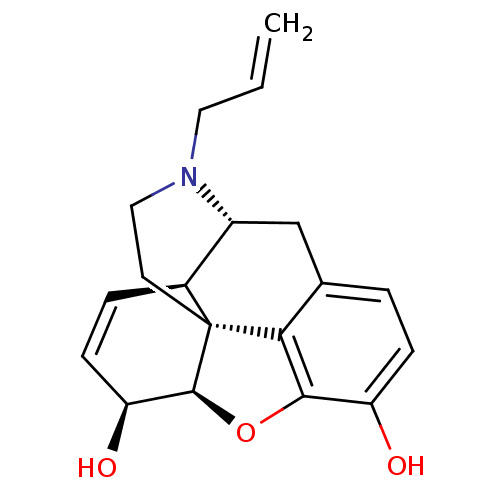 (NALORPHINE | NALORPHINE HYDROCHLORIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound administered subcutaneously was evaluated for inhibition constant measured as the displacement of [3H]DAGO from rat brain | J Med Chem 30: 947-50 (1987) BindingDB Entry DOI: 10.7270/Q2TD9XXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50095703 (3-But-3-enyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor mu 1 using [3H]-DAMGO at the Kd concentration 0.57 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50095711 (6,11-Dimethyl-3-pent-4-enyl-1,2,3,4,5,6-hexahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Sigma opioid receptor type 1 using [(+)-[3H]pentazocaine at the Kd concentration 2nM . | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50029789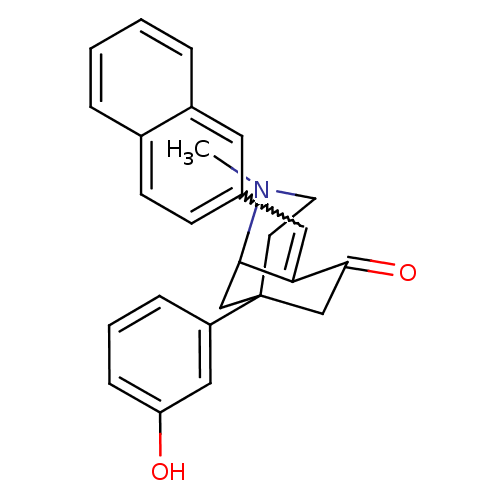 (5-(3-Hydroxy-phenyl)-2-methyl-8-[1-naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-DAMGO binding to rat brain Opioid receptor mu 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50037476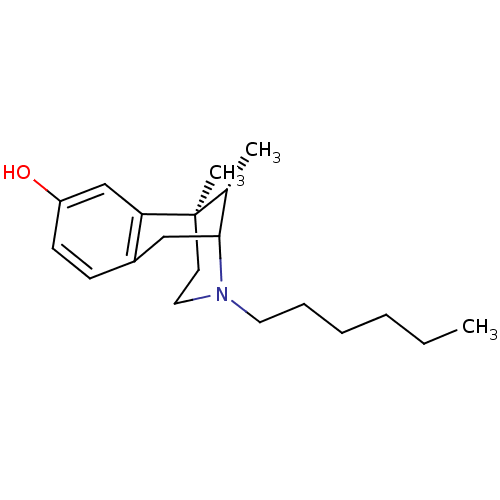 ((6R,11S)-3-Hexyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against sigma receptor using displacement of 3 nM [3H]pentazocine in homogenate of guinea pig brain cerebellum | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50037484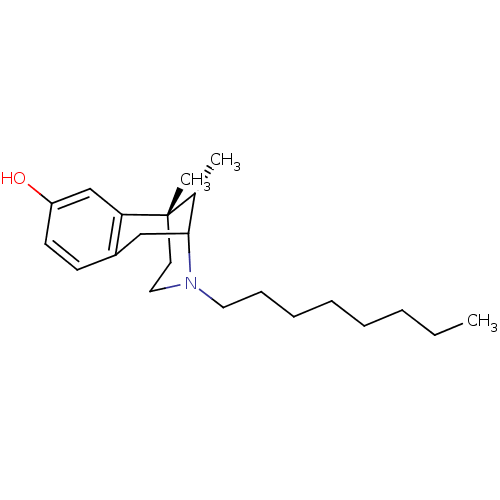 ((6S,11S)-6,11-Dimethyl-3-octyl-1,2,3,4,5,6-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against sigma receptor using displacement of 3 nM [3H]pentazocine in homogenate of guinea pig brain cerebellum | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against opioid receptor by displacing radioligand [3H]DPDPE | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50029791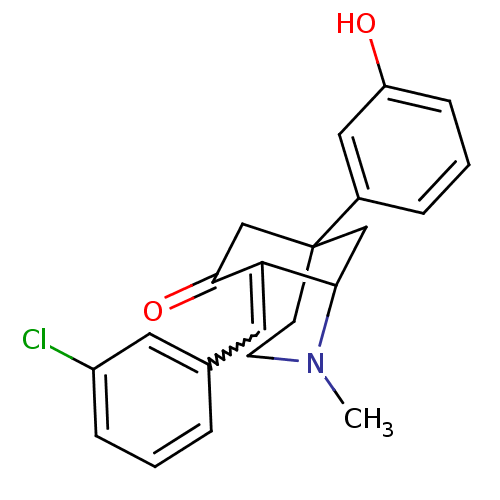 (8-[1-(3-Chloro-phenyl)-meth-(E)-ylidene]-5-(3-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-(+)-pentazocine binding to guinea pig brain Sigma receptor type 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50037482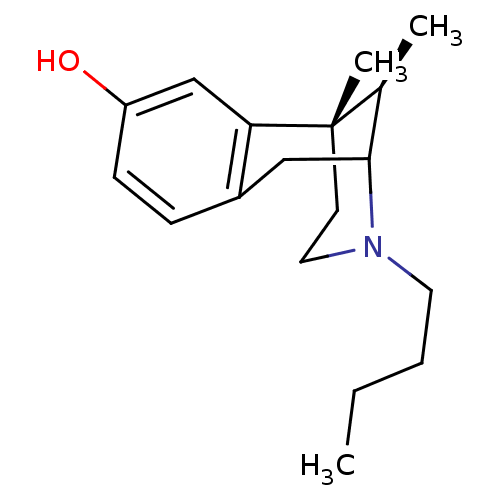 ((6S,11R)-3-Butyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against mu receptor from displacement studies using 0.5 nM [3H]-DAMGO in rhesus monkey cortex membrane | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50037480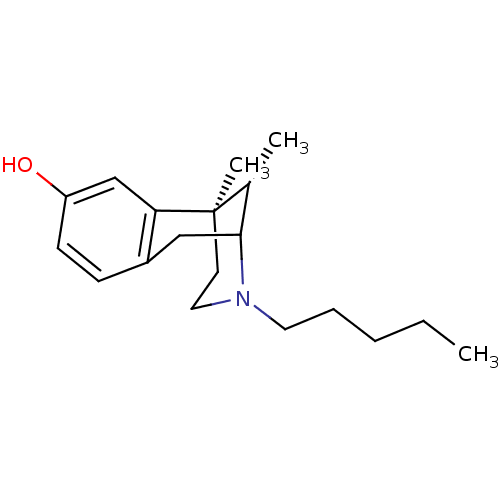 ((6R,11S)-6,11-Dimethyl-3-pentyl-1,2,3,4,5,6-hexahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against sigma receptor using displacement of 3 nM [3H]pentazocine in homogenate of guinea pig brain cerebellum | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50001028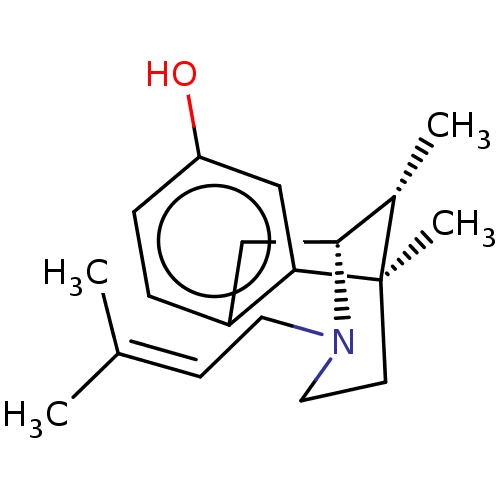 ((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of [3H](+)-pentazocine binding to sigma receptor of guinea pig brain homogenates | J Med Chem 36: 1188-93 (1993) BindingDB Entry DOI: 10.7270/Q23F4Q98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50029786 (5-(3-Hydroxy-phenyl)-8-[1-(4-methoxy-phenyl)-meth-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-DAMGO binding to rat brain Opioid receptor mu 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50029786 (5-(3-Hydroxy-phenyl)-8-[1-(4-methoxy-phenyl)-meth-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-DAMGO binding to rat brain Opioid receptor mu 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50095705 (3-But-2-ynyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50037469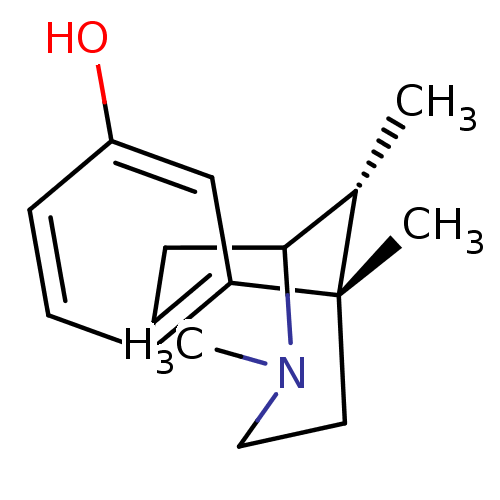 ((6S,11R)-3,6,11-Trimethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against mu receptor from displacement studies using 0.5 nM [3H]-DAMGO in rhesus monkey cortex membrane | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50095694 (3-(8-Hydroxy-6,11-dimethyl-1,2,5,6-tetrahydro-4H-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor mu 1 using [3H]-DAMGO at the Kd concentration 0.57 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50035131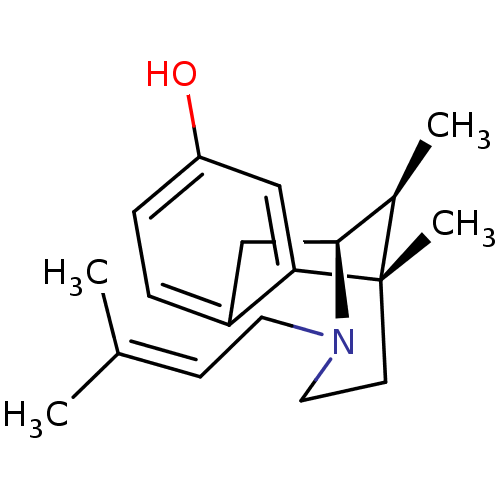 ((+)-(6R,11S)-6,11-dimethyl-3-(3-methyl-but-2-enyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor was determined | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50407214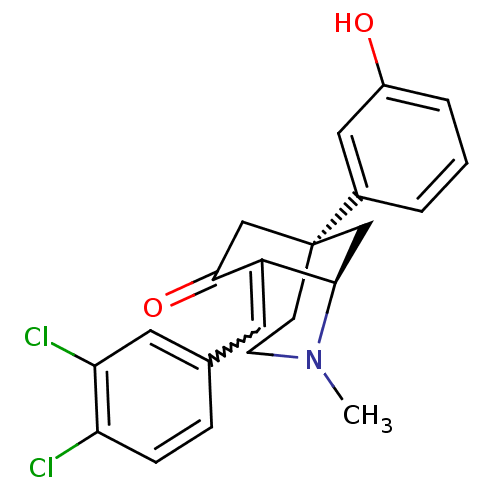 (CHEMBL2008608) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]-DAMGO binding to rat brain mu receptor | J Med Chem 37: 3163-70 (1994) BindingDB Entry DOI: 10.7270/Q2F18XSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50095707 (3-But-3-ynyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor mu 1 using [3H]-DAMGO at the Kd concentration 0.57 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037475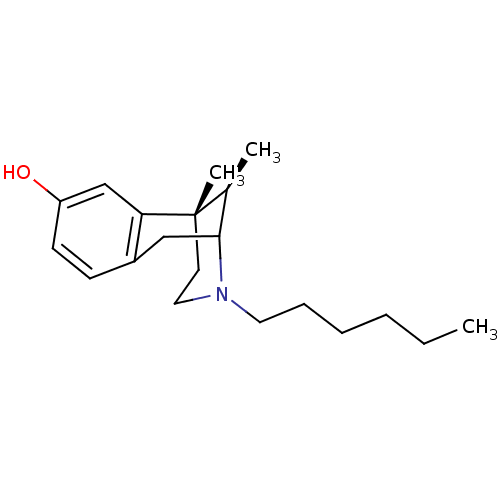 ((6S,11R)-3-Hexyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against mu receptor using [3H]DAMGO in homogenate of rat brain cerebellum | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50037482 ((6S,11R)-3-Butyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against kappa receptor from displacement studies using 1.5 nM [3H]U-69593 in rhesus monkey cortex membrane | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50041240 ((6S,11R)-3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor delta 1 using [3H]-DPDPE at the Kd concentration 2.1 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50095706 (4-(8-Hydroxy-6,11-dimethyl-1,2,5,6-tetrahydro-4H-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor mu 1 using [3H]-DAMGO at the Kd concentration 0.57 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50095708 (3-Hex-5-enyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50095712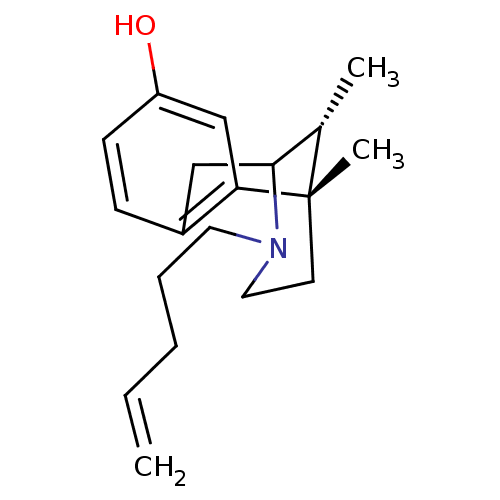 (6,11-Dimethyl-3-pent-4-enyl-1,2,3,4,5,6-hexahydro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Ki value determined against Opioid receptor kappa 1 using [3H]U69, 593 at the Kd concentration 0.95 nM | J Med Chem 43: 5030-6 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50037472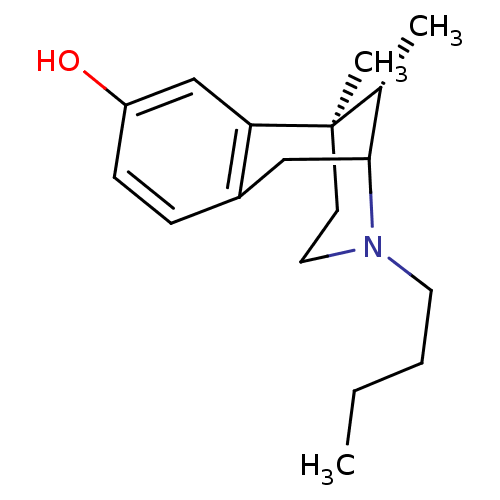 ((6R,11S)-3-Butyl-6,11-dimethyl-1,2,3,4,5,6-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition against sigma receptor using displacement of 3 nM [3H]pentazocine in homogenate of guinea pig brain cerebellum | J Med Chem 37: 3408-18 (1994) BindingDB Entry DOI: 10.7270/Q2M32WD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50015743 (Acetic acid 3-[1-(3,6-dihydro-2H-pyridin-1-yl)-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]- TCP from PCP receptor in tissue homogenate preparation of fresh whole rat brain minus cerebellum. | J Med Chem 33: 2211-5 (1990) BindingDB Entry DOI: 10.7270/Q2BZ6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50029788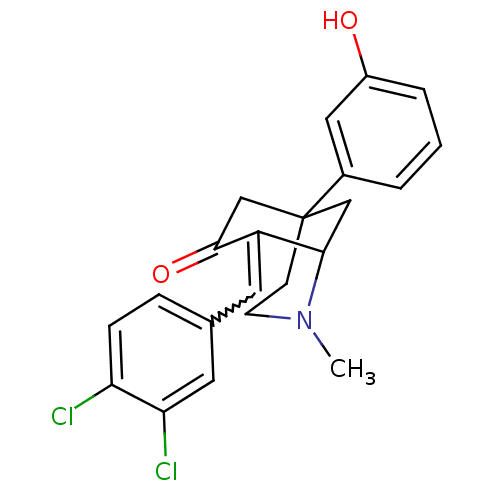 (8-[1-(3,4-Dichloro-phenyl)-meth-(E)-ylidene]-5-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-DAMGO binding to rat brain Opioid receptor mu 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50029788 (8-[1-(3,4-Dichloro-phenyl)-meth-(E)-ylidene]-5-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Ability to inhibit [3H]-DAMGO binding to rat brain Opioid receptor mu 1 | J Med Chem 38: 4776-85 (1996) BindingDB Entry DOI: 10.7270/Q2XW4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 591 total ) | Next | Last >> |