 Found 51 hits with Last Name = 'may' and Initial = 'ew'
Found 51 hits with Last Name = 'may' and Initial = 'ew' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50555575
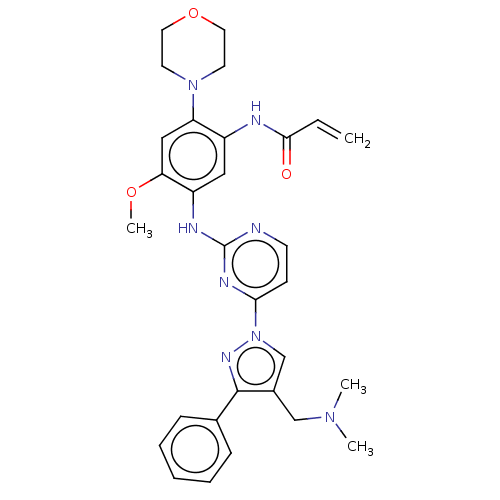
(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50555575

(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 434 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50555575

(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437357
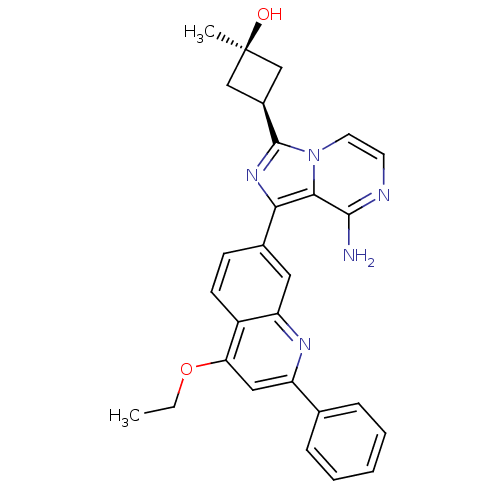
(CHEMBL3037909)Show SMILES CCOc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.21,16.17,wD:18.20,(12.1,-.5,;13.44,-1.27,;13.44,-2.81,;14.77,-3.58,;16.1,-2.81,;17.44,-3.57,;17.45,-5.13,;16.11,-5.9,;16.11,-7.43,;14.79,-8.21,;13.44,-7.44,;13.44,-5.9,;14.77,-5.13,;14.8,-9.75,;16.05,-10.65,;15.58,-12.12,;16.49,-13.36,;18.01,-13.59,;17.77,-15.11,;19.26,-15.51,;18.17,-16.6,;16.26,-14.88,;14.04,-12.13,;13.02,-13.27,;11.51,-12.95,;11.04,-11.49,;12.07,-10.35,;11.59,-8.88,;13.56,-10.67,;18.77,-2.8,;20.11,-3.57,;21.44,-2.8,;21.43,-1.25,;20.09,-.49,;18.76,-1.27,)| Show InChI InChI=1S/C28H27N5O2/c1-3-35-23-14-21(17-7-5-4-6-8-17)31-22-13-18(9-10-20(22)23)24-25-26(29)30-11-12-33(25)27(32-24)19-15-28(2,34)16-19/h4-14,19,34H,3,15-16H2,1-2H3,(H2,29,30)/t19-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437358

(CHEMBL3037908)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.20,15.16,wD:17.19,(2.3,-.85,;2.3,-2.39,;3.63,-3.16,;4.96,-2.39,;6.3,-3.15,;6.31,-4.71,;4.97,-5.48,;4.97,-7.01,;3.65,-7.79,;2.3,-7.02,;2.3,-5.48,;3.63,-4.71,;3.66,-9.33,;4.91,-10.23,;4.44,-11.7,;5.35,-12.94,;6.87,-13.17,;6.63,-14.69,;8.12,-15.09,;7.03,-16.18,;5.12,-14.46,;2.9,-11.71,;1.88,-12.85,;.37,-12.53,;-.11,-11.07,;.93,-9.93,;.45,-8.46,;2.42,-10.25,;7.63,-2.38,;8.97,-3.15,;10.3,-2.38,;10.29,-.83,;8.94,-.07,;7.62,-.85,)| Show InChI InChI=1S/C27H25N5O2/c1-27(33)14-18(15-27)26-31-23(24-25(28)29-10-11-32(24)26)17-8-9-19-21(12-17)30-20(13-22(19)34-2)16-6-4-3-5-7-16/h3-13,18,33H,14-15H2,1-2H3,(H2,28,29)/t18-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437358

(CHEMBL3037908)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.20,15.16,wD:17.19,(2.3,-.85,;2.3,-2.39,;3.63,-3.16,;4.96,-2.39,;6.3,-3.15,;6.31,-4.71,;4.97,-5.48,;4.97,-7.01,;3.65,-7.79,;2.3,-7.02,;2.3,-5.48,;3.63,-4.71,;3.66,-9.33,;4.91,-10.23,;4.44,-11.7,;5.35,-12.94,;6.87,-13.17,;6.63,-14.69,;8.12,-15.09,;7.03,-16.18,;5.12,-14.46,;2.9,-11.71,;1.88,-12.85,;.37,-12.53,;-.11,-11.07,;.93,-9.93,;.45,-8.46,;2.42,-10.25,;7.63,-2.38,;8.97,-3.15,;10.3,-2.38,;10.29,-.83,;8.94,-.07,;7.62,-.85,)| Show InChI InChI=1S/C27H25N5O2/c1-27(33)14-18(15-27)26-31-23(24-25(28)29-10-11-32(24)26)17-8-9-19-21(12-17)30-20(13-22(19)34-2)16-6-4-3-5-7-16/h3-13,18,33H,14-15H2,1-2H3,(H2,28,29)/t18-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437357

(CHEMBL3037909)Show SMILES CCOc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.21,16.17,wD:18.20,(12.1,-.5,;13.44,-1.27,;13.44,-2.81,;14.77,-3.58,;16.1,-2.81,;17.44,-3.57,;17.45,-5.13,;16.11,-5.9,;16.11,-7.43,;14.79,-8.21,;13.44,-7.44,;13.44,-5.9,;14.77,-5.13,;14.8,-9.75,;16.05,-10.65,;15.58,-12.12,;16.49,-13.36,;18.01,-13.59,;17.77,-15.11,;19.26,-15.51,;18.17,-16.6,;16.26,-14.88,;14.04,-12.13,;13.02,-13.27,;11.51,-12.95,;11.04,-11.49,;12.07,-10.35,;11.59,-8.88,;13.56,-10.67,;18.77,-2.8,;20.11,-3.57,;21.44,-2.8,;21.43,-1.25,;20.09,-.49,;18.76,-1.27,)| Show InChI InChI=1S/C28H27N5O2/c1-3-35-23-14-21(17-7-5-4-6-8-17)31-22-13-18(9-10-20(22)23)24-25-26(29)30-11-12-33(25)27(32-24)19-15-28(2,34)16-19/h4-14,19,34H,3,15-16H2,1-2H3,(H2,29,30)/t19-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437370
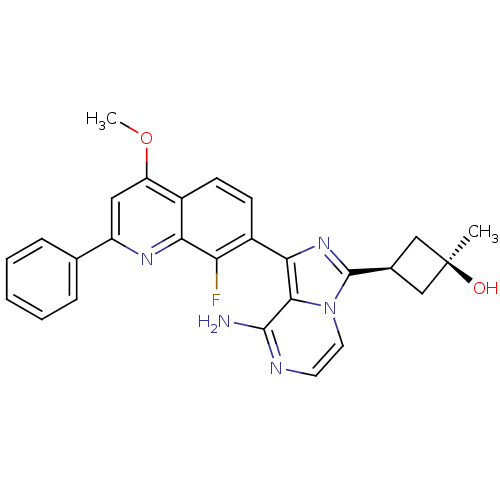
(CHEMBL3037912)Show SMILES COc1cc(nc2c(F)c(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.21,16.17,wD:18.20,(47.77,-1.38,;47.77,-2.92,;49.1,-3.69,;50.43,-2.92,;51.77,-3.68,;51.78,-5.24,;50.44,-6.01,;50.44,-7.54,;51.78,-8.31,;49.12,-8.32,;47.77,-7.55,;47.77,-6.01,;49.1,-5.24,;49.13,-9.86,;50.38,-10.76,;49.91,-12.23,;50.82,-13.47,;52.34,-13.7,;52.1,-15.22,;53.59,-15.62,;52.5,-16.71,;50.59,-14.99,;48.37,-12.24,;47.35,-13.38,;45.84,-13.06,;45.37,-11.6,;46.4,-10.46,;45.92,-8.99,;47.89,-10.77,;53.1,-2.91,;54.44,-3.68,;55.77,-2.9,;55.76,-1.36,;54.41,-.6,;53.09,-1.37,)| Show InChI InChI=1S/C27H24FN5O2/c1-27(34)13-16(14-27)26-32-23(24-25(29)30-10-11-33(24)26)18-9-8-17-20(35-2)12-19(31-22(17)21(18)28)15-6-4-3-5-7-15/h3-12,16,34H,13-14H2,1-2H3,(H2,29,30)/t16-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437369
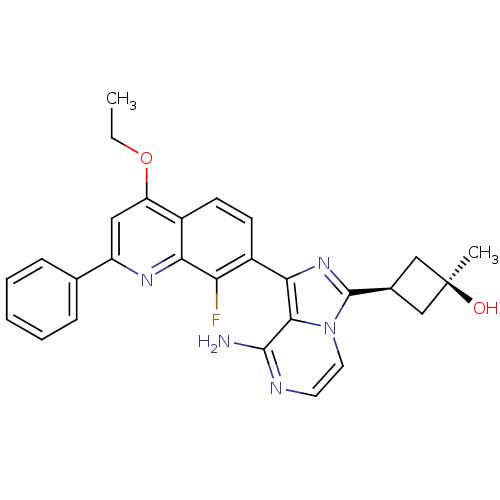
(CHEMBL3037913)Show SMILES CCOc1cc(nc2c(F)c(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:19.22,17.18,wD:19.21,(23.32,-.37,;24.65,-1.14,;24.65,-2.69,;25.99,-3.46,;27.32,-2.69,;28.65,-3.45,;28.66,-5,;27.32,-5.77,;27.32,-7.31,;28.66,-8.07,;26,-8.08,;24.65,-7.32,;24.65,-5.77,;25.98,-5,;26.01,-9.63,;27.26,-10.53,;26.79,-11.99,;27.7,-13.24,;29.23,-13.47,;28.99,-14.99,;30.47,-15.38,;29.39,-16.47,;27.47,-14.76,;25.25,-12,;24.23,-13.15,;22.72,-12.82,;22.25,-11.36,;23.28,-10.22,;22.8,-8.76,;24.77,-10.54,;29.98,-2.68,;31.32,-3.45,;32.65,-2.67,;32.64,-1.13,;31.3,-.36,;29.97,-1.14,)| Show InChI InChI=1S/C28H26FN5O2/c1-3-36-21-13-20(16-7-5-4-6-8-16)32-23-18(21)9-10-19(22(23)29)24-25-26(30)31-11-12-34(25)27(33-24)17-14-28(2,35)15-17/h4-13,17,35H,3,14-15H2,1-2H3,(H2,30,31)/t17-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437358

(CHEMBL3037908)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.20,15.16,wD:17.19,(2.3,-.85,;2.3,-2.39,;3.63,-3.16,;4.96,-2.39,;6.3,-3.15,;6.31,-4.71,;4.97,-5.48,;4.97,-7.01,;3.65,-7.79,;2.3,-7.02,;2.3,-5.48,;3.63,-4.71,;3.66,-9.33,;4.91,-10.23,;4.44,-11.7,;5.35,-12.94,;6.87,-13.17,;6.63,-14.69,;8.12,-15.09,;7.03,-16.18,;5.12,-14.46,;2.9,-11.71,;1.88,-12.85,;.37,-12.53,;-.11,-11.07,;.93,-9.93,;.45,-8.46,;2.42,-10.25,;7.63,-2.38,;8.97,-3.15,;10.3,-2.38,;10.29,-.83,;8.94,-.07,;7.62,-.85,)| Show InChI InChI=1S/C27H25N5O2/c1-27(33)14-18(15-27)26-31-23(24-25(28)29-10-11-32(24)26)17-8-9-19-21(12-17)30-20(13-22(19)34-2)16-6-4-3-5-7-16/h3-13,18,33H,14-15H2,1-2H3,(H2,28,29)/t18-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339062
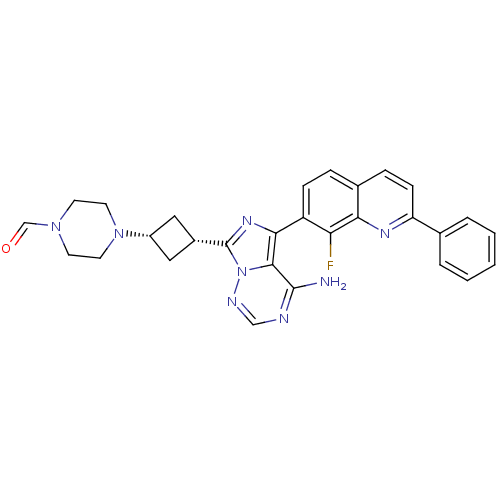
(4-(3-(4-amino-5-(8-fluoro-2-phenylquinolin-7-yl)im...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:27.31,29.36,(5.56,-13.24,;5.57,-14.78,;4.24,-15.55,;4.24,-17.09,;5.57,-17.86,;6.9,-17.08,;8.37,-17.56,;9.28,-16.31,;8.37,-15.06,;9.12,-13.72,;8.34,-12.4,;9.1,-11.06,;10.64,-11.05,;11.4,-9.71,;12.94,-9.7,;13.72,-11.04,;12.95,-12.37,;11.41,-12.38,;10.67,-13.71,;11.45,-15.04,;15.25,-11.03,;16.03,-12.36,;17.57,-12.35,;18.33,-11.01,;17.55,-9.68,;16.01,-9.69,;6.9,-15.54,;8.84,-19.01,;8.14,-20.39,;9.52,-21.09,;10.21,-19.71,;10,-22.55,;8.96,-23.7,;9.43,-25.15,;10.94,-25.48,;11.97,-24.34,;11.5,-22.87,;11.41,-26.95,;10.37,-28.09,)| Show InChI InChI=1S/C29H27FN8O/c30-24-22(8-6-19-7-9-23(34-25(19)24)18-4-2-1-3-5-18)26-27-28(31)32-16-33-38(27)29(35-26)20-14-21(15-20)37-12-10-36(17-39)11-13-37/h1-9,16-17,20-21H,10-15H2,(H2,31,32,33)/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437367
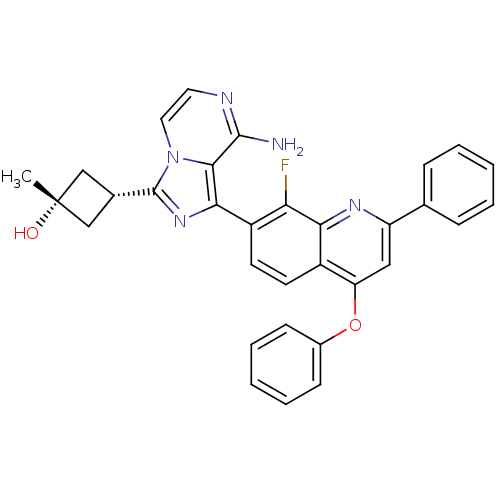
(CHEMBL3037915)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3c(Oc4ccccc4)cc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.16,-33.71,;16.68,-33.31,;17.08,-34.79,;16.92,-31.79,;15.39,-31.56,;15.16,-33.08,;14.48,-30.32,;14.95,-28.85,;13.7,-27.95,;13.69,-26.4,;12.34,-25.64,;12.34,-24.09,;13.68,-23.32,;13.68,-21.78,;12.34,-21.01,;12.34,-19.47,;13.69,-18.69,;13.69,-17.17,;12.35,-16.4,;11.01,-17.17,;11.02,-18.7,;15.01,-21.01,;16.34,-21.77,;16.35,-23.32,;15.01,-24.09,;15.02,-25.63,;16.35,-26.39,;17.67,-21,;19.01,-21.77,;20.34,-20.99,;20.34,-19.45,;18.99,-18.68,;17.66,-19.46,;12.46,-28.86,;10.97,-28.54,;10.5,-27.08,;9.94,-29.68,;10.41,-31.14,;11.92,-31.47,;12.94,-30.32,)| Show InChI InChI=1S/C32H26FN5O2/c1-32(39)17-20(18-32)31-37-28(29-30(34)35-14-15-38(29)31)23-13-12-22-25(40-21-10-6-3-7-11-21)16-24(36-27(22)26(23)33)19-8-4-2-5-9-19/h2-16,20,39H,17-18H2,1H3,(H2,34,35)/t20-,32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437362
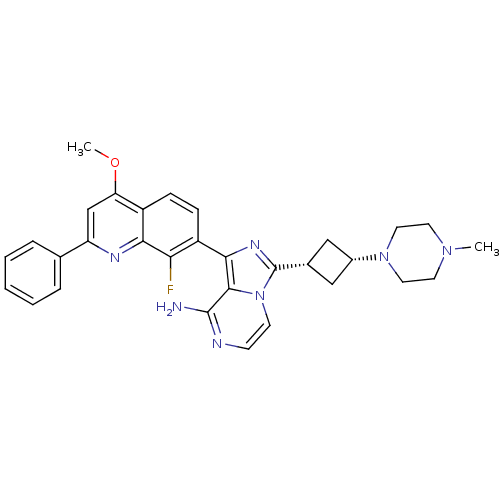
(CHEMBL3037920)Show SMILES COc1cc(nc2c(F)c(ccc12)-c1nc([C@@H]2C[C@@H](C2)N2CCN(C)CC2)n2ccnc(N)c12)-c1ccccc1 |r,wU:16.17,18.22,(3.57,-35.88,;3.58,-37.43,;4.91,-38.2,;6.24,-37.43,;7.58,-38.19,;7.58,-39.74,;6.24,-40.51,;6.25,-42.05,;7.59,-42.81,;4.92,-42.82,;3.58,-42.06,;3.58,-40.51,;4.91,-39.74,;4.93,-44.37,;6.18,-45.27,;5.71,-46.73,;6.63,-47.98,;6.39,-49.5,;7.91,-49.73,;8.15,-48.21,;8.82,-50.97,;8.2,-52.38,;9.1,-53.62,;10.64,-53.46,;11.54,-54.7,;11.26,-52.05,;10.35,-50.79,;4.17,-46.74,;3.15,-47.89,;1.65,-47.56,;1.17,-46.1,;2.2,-44.96,;1.73,-43.5,;3.7,-45.28,;8.9,-37.42,;10.24,-38.19,;11.57,-37.41,;11.57,-35.87,;10.22,-35.1,;8.9,-35.88,)| Show InChI InChI=1S/C31H32FN7O/c1-37-12-14-38(15-13-37)21-16-20(17-21)31-36-28(29-30(33)34-10-11-39(29)31)23-9-8-22-25(40-2)18-24(35-27(22)26(23)32)19-6-4-3-5-7-19/h3-11,18,20-21H,12-17H2,1-2H3,(H2,33,34)/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437361
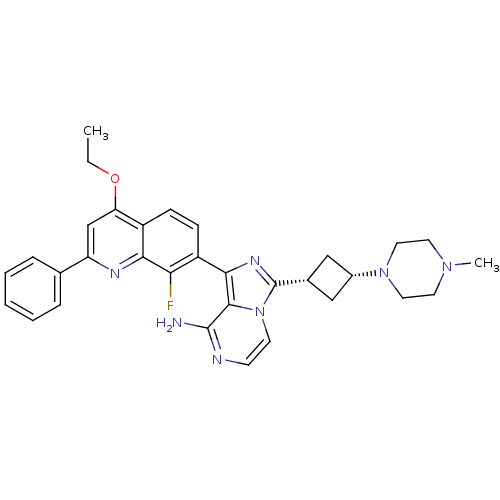
(CHEMBL3037921)Show SMILES CCOc1cc(nc2c(F)c(ccc12)-c1nc([C@@H]2C[C@@H](C2)N2CCN(C)CC2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.18,19.23,(16.88,-36.18,;15.55,-36.95,;15.55,-38.49,;16.88,-39.26,;18.21,-38.49,;19.55,-39.25,;19.56,-40.81,;18.22,-41.58,;18.22,-43.11,;19.56,-43.88,;16.9,-43.89,;15.55,-43.12,;15.55,-41.58,;16.88,-40.81,;16.91,-45.43,;18.16,-46.33,;17.69,-47.8,;18.6,-49.04,;18.37,-50.56,;19.88,-50.79,;20.12,-49.27,;20.79,-52.03,;20.17,-53.44,;21.08,-54.68,;22.61,-54.52,;23.52,-55.77,;23.23,-53.11,;22.33,-51.86,;16.15,-47.81,;15.13,-48.95,;13.62,-48.63,;13.15,-47.17,;14.18,-46.03,;13.7,-44.56,;15.67,-46.34,;20.88,-38.48,;22.21,-39.25,;23.55,-38.48,;23.54,-36.93,;22.19,-36.17,;20.87,-36.95,)| Show InChI InChI=1S/C32H34FN7O/c1-3-41-26-19-25(20-7-5-4-6-8-20)36-28-23(26)9-10-24(27(28)33)29-30-31(34)35-11-12-40(30)32(37-29)21-17-22(18-21)39-15-13-38(2)14-16-39/h4-12,19,21-22H,3,13-18H2,1-2H3,(H2,34,35)/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437372
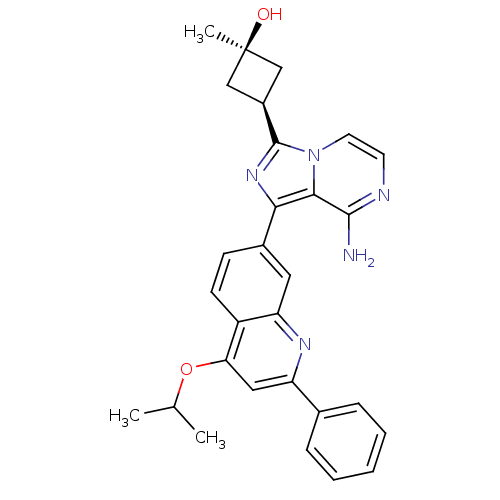
(CHEMBL3037910)Show SMILES CC(C)Oc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:19.22,17.18,wD:19.21,(26.66,-.05,;25.33,-.82,;23.99,-.05,;25.33,-2.36,;26.66,-3.13,;27.99,-2.36,;29.33,-3.12,;29.34,-4.68,;28,-5.45,;28,-6.98,;26.68,-7.76,;25.33,-6.99,;25.33,-5.45,;26.66,-4.68,;26.69,-9.3,;27.94,-10.2,;27.47,-11.67,;28.38,-12.91,;29.9,-13.14,;29.66,-14.66,;31.15,-15.06,;30.06,-16.15,;28.15,-14.43,;25.93,-11.68,;24.91,-12.82,;23.4,-12.5,;22.93,-11.04,;23.96,-9.9,;23.48,-8.43,;25.45,-10.21,;30.66,-2.35,;32,-3.12,;33.33,-2.34,;33.32,-.8,;31.98,-.04,;30.65,-.81,)| Show InChI InChI=1S/C29H29N5O2/c1-17(2)36-24-14-22(18-7-5-4-6-8-18)32-23-13-19(9-10-21(23)24)25-26-27(30)31-11-12-34(26)28(33-25)20-15-29(3,35)16-20/h4-14,17,20,35H,15-16H2,1-3H3,(H2,30,31)/t20-,29+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437368
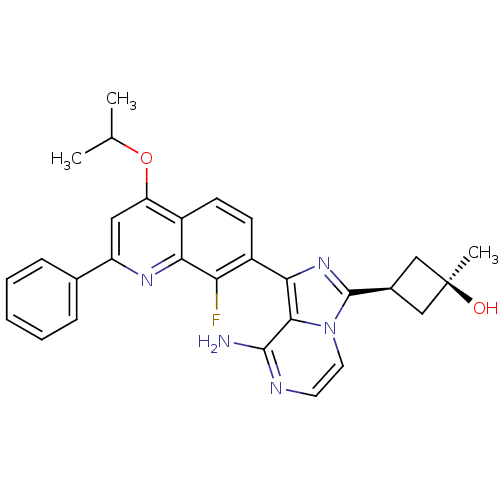
(CHEMBL3037914)Show SMILES CC(C)Oc1cc(nc2c(F)c(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:20.23,18.19,wD:20.22,(3.2,-17.1,;1.87,-17.87,;.53,-17.1,;1.87,-19.41,;3.21,-20.18,;4.54,-19.41,;5.87,-20.17,;5.88,-21.73,;4.54,-22.5,;4.54,-24.03,;5.88,-24.8,;3.22,-24.81,;1.87,-24.04,;1.87,-22.5,;3.2,-21.73,;3.23,-26.35,;4.48,-27.25,;4.01,-28.72,;4.92,-29.96,;6.44,-30.19,;6.21,-31.71,;7.69,-32.11,;6.61,-33.2,;4.69,-31.48,;2.47,-28.73,;1.45,-29.87,;-.07,-29.55,;-.54,-28.09,;.5,-26.95,;.02,-25.48,;1.99,-27.26,;7.2,-19.4,;8.54,-20.17,;9.87,-19.39,;9.86,-17.85,;8.52,-17.09,;7.19,-17.87,)| Show InChI InChI=1S/C29H28FN5O2/c1-16(2)37-22-13-21(17-7-5-4-6-8-17)33-24-19(22)9-10-20(23(24)30)25-26-27(31)32-11-12-35(26)28(34-25)18-14-29(3,36)15-18/h4-13,16,18,36H,14-15H2,1-3H3,(H2,31,32)/t18-,29+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887
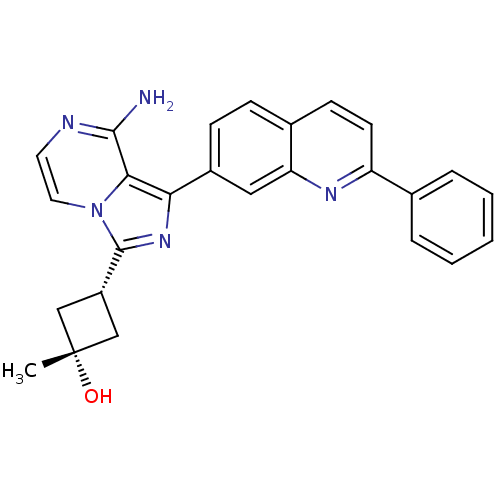
((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339064
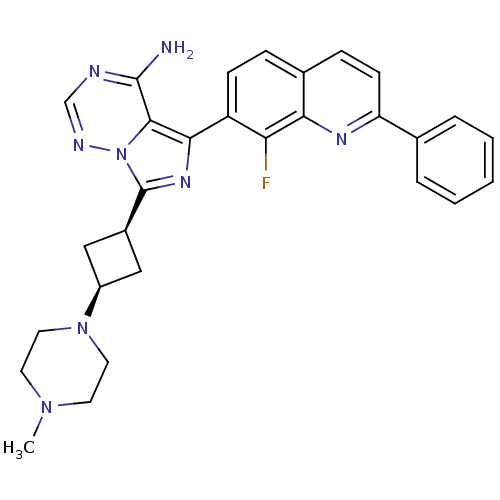
(5-(8-fluoro-2-phenylquinolin-7-yl)-7-(3-(4-methylp...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(40.85,-28.31,;40.38,-26.84,;38.87,-26.51,;38.4,-25.05,;39.44,-23.91,;40.94,-24.23,;41.41,-25.7,;38.96,-22.44,;37.58,-21.74,;38.28,-20.37,;39.66,-21.07,;37.81,-18.92,;38.72,-17.67,;37.81,-16.42,;38.56,-15.08,;37.78,-13.76,;38.54,-12.42,;40.08,-12.4,;40.84,-11.07,;42.38,-11.06,;43.16,-12.39,;42.39,-13.73,;40.85,-13.74,;40.11,-15.07,;40.89,-16.39,;44.69,-12.38,;45.47,-13.72,;47.01,-13.71,;47.77,-12.37,;46.99,-11.04,;45.45,-11.05,;36.34,-16.9,;35.01,-16.13,;35,-14.59,;33.68,-16.9,;33.68,-18.45,;35.01,-19.22,;36.34,-18.44,)| Show InChI InChI=1S/C29H29FN8/c1-36-11-13-37(14-12-36)21-15-20(16-21)29-35-26(27-28(31)32-17-33-38(27)29)22-9-7-19-8-10-23(34-25(19)24(22)30)18-5-3-2-4-6-18/h2-10,17,20-21H,11-16H2,1H3,(H2,31,32,33)/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
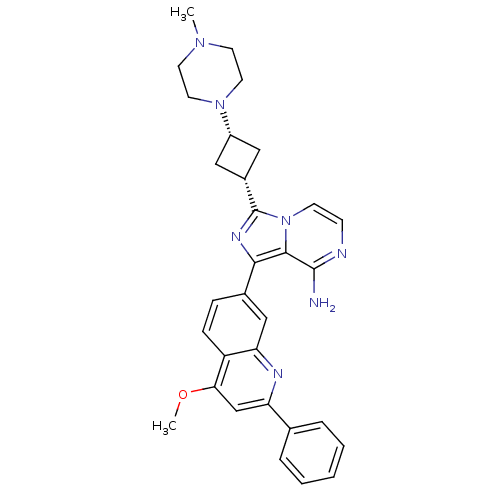
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437366
(CHEMBL3037916)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@@H]2C[C@@H](C2)N2CCN(C)CC2)n2ccnc(N)c12)-c1ccccc1 |r,wU:15.16,17.21,(22.07,-17.53,;22.08,-19.07,;23.41,-19.84,;24.74,-19.07,;26.08,-19.83,;26.08,-21.38,;24.74,-22.16,;24.75,-23.69,;23.42,-24.47,;22.08,-23.7,;22.08,-22.16,;23.41,-21.39,;23.43,-26.01,;24.68,-26.91,;24.22,-28.38,;25.13,-29.62,;24.89,-31.14,;26.41,-31.37,;26.65,-29.85,;27.32,-32.61,;26.7,-34.02,;27.6,-35.26,;29.14,-35.1,;30.04,-36.35,;29.76,-33.69,;28.85,-32.44,;22.67,-28.39,;21.65,-29.53,;20.15,-29.21,;19.67,-27.74,;20.7,-26.61,;20.23,-25.14,;22.2,-26.92,;27.4,-19.06,;28.74,-19.83,;30.07,-19.05,;30.07,-17.51,;28.72,-16.74,;27.4,-17.52,)| Show InChI InChI=1S/C31H33N7O/c1-36-12-14-37(15-13-36)23-16-22(17-23)31-35-28(29-30(32)33-10-11-38(29)31)21-8-9-24-26(18-21)34-25(19-27(24)39-2)20-6-4-3-5-7-20/h3-11,18-19,22-23H,12-17H2,1-2H3,(H2,32,33)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
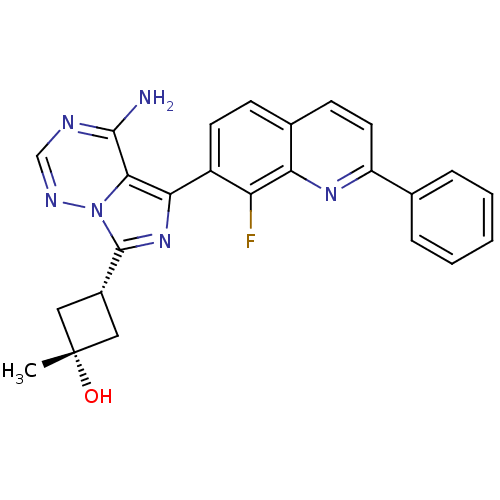
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336317
(CHEMBL1667935 | cis-3-[4-Amino-5-(8-fluoro-2-pheny...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(-2.6,-22.44,;-3.93,-21.67,;-4.34,-23.15,;-3.24,-20.29,;-4.61,-19.61,;-5.31,-20.98,;-5.1,-18.14,;-4.2,-16.89,;-5.11,-15.65,;-4.34,-14.32,;-5.11,-12.99,;-4.34,-11.66,;-2.79,-11.65,;-2.03,-10.33,;-.5,-10.33,;.27,-11.66,;-.5,-12.99,;-2.03,-12.99,;-2.8,-14.32,;-2.03,-15.66,;1.81,-11.66,;2.58,-13,;4.12,-13,;4.89,-11.66,;4.11,-10.32,;2.57,-10.33,;-6.58,-16.13,;-7.91,-15.37,;-7.92,-13.83,;-9.24,-16.14,;-9.25,-17.68,;-7.91,-18.46,;-6.57,-17.68,)| Show InChI InChI=1S/C25H21FN6O/c1-25(33)11-16(12-25)24-31-21(22-23(27)28-13-29-32(22)24)17-9-7-15-8-10-18(30-20(15)19(17)26)14-5-3-2-4-6-14/h2-10,13,16,33H,11-12H2,1H3,(H2,27,28,29)/t16-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
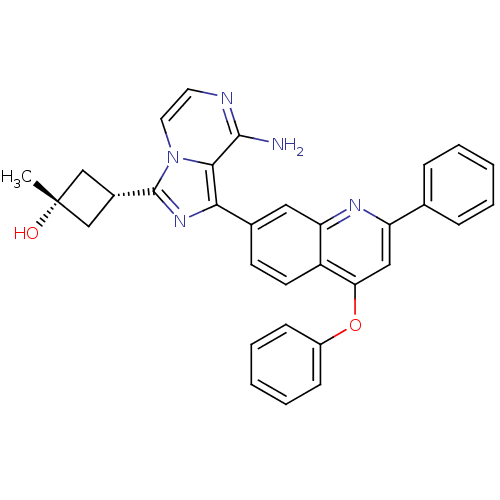
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437371
(CHEMBL3037911)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3c(Oc4ccccc4)cc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(41.6,-16.89,;40.11,-16.49,;40.51,-17.98,;40.35,-14.97,;38.83,-14.74,;38.6,-16.26,;37.92,-13.5,;38.39,-12.03,;37.14,-11.13,;37.13,-9.59,;35.78,-8.82,;35.78,-7.28,;37.11,-6.51,;37.11,-4.96,;35.78,-4.19,;35.78,-2.65,;37.12,-1.88,;37.12,-.35,;35.79,.44,;34.45,-.36,;34.46,-1.88,;38.44,-4.19,;39.78,-4.95,;39.79,-6.5,;38.45,-7.28,;38.45,-8.81,;41.11,-4.18,;42.45,-4.95,;43.78,-4.17,;43.77,-2.63,;42.42,-1.87,;41.1,-2.64,;35.9,-12.04,;34.41,-11.73,;33.93,-10.26,;33.38,-12.87,;33.85,-14.33,;35.36,-14.65,;36.38,-13.51,)| Show InChI InChI=1S/C32H27N5O2/c1-32(38)18-22(19-32)31-36-28(29-30(33)34-14-15-37(29)31)21-12-13-24-26(16-21)35-25(20-8-4-2-5-9-20)17-27(24)39-23-10-6-3-7-11-23/h2-17,22,38H,18-19H2,1H3,(H2,33,34)/t22-,32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
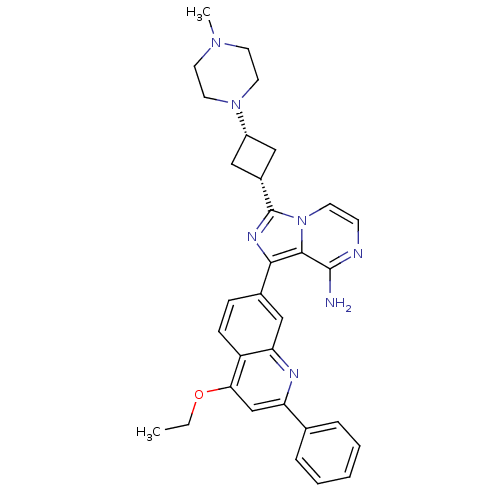
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437365
(CHEMBL3037917)Show SMILES CCOc1cc(nc2cc(ccc12)-c1nc([C@@H]2C[C@@H](C2)N2CCN(C)CC2)n2ccnc(N)c12)-c1ccccc1 |r,wU:16.17,18.22,(33.34,-17.94,;32.01,-18.71,;32.01,-20.25,;33.34,-21.02,;34.67,-20.25,;36.01,-21.01,;36.01,-22.57,;34.68,-23.34,;34.68,-24.87,;33.35,-25.65,;32.01,-24.88,;32.01,-23.34,;33.34,-22.57,;33.36,-27.19,;34.62,-28.09,;34.15,-29.56,;35.06,-30.8,;34.82,-32.32,;36.34,-32.55,;36.58,-31.03,;37.25,-33.79,;36.63,-35.2,;37.54,-36.44,;39.07,-36.28,;39.98,-37.53,;39.69,-34.87,;38.79,-33.62,;32.61,-29.57,;31.59,-30.71,;30.08,-30.39,;29.6,-28.93,;30.64,-27.79,;30.16,-26.32,;32.13,-28.1,;37.34,-20.24,;38.67,-21.01,;40.01,-20.24,;40,-18.69,;38.65,-17.93,;37.33,-18.71,)| Show InChI InChI=1S/C32H35N7O/c1-3-40-28-20-26(21-7-5-4-6-8-21)35-27-19-22(9-10-25(27)28)29-30-31(33)34-11-12-39(30)32(36-29)23-17-24(18-23)38-15-13-37(2)14-16-38/h4-12,19-20,23-24H,3,13-18H2,1-2H3,(H2,33,34)/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
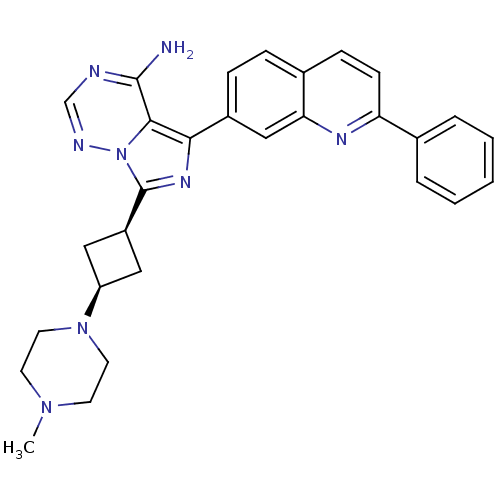
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339063
(7-(3-(4-methylpiperazin-1-yl)cyclobutyl)-5-(2-phen...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(25.86,-27.54,;25.39,-26.08,;23.89,-25.75,;23.42,-24.29,;24.45,-23.15,;25.96,-23.46,;26.43,-24.93,;23.97,-21.68,;22.6,-20.98,;23.3,-19.61,;24.67,-20.31,;22.82,-18.15,;23.73,-16.9,;22.82,-15.66,;23.58,-14.32,;22.79,-13,;23.55,-11.66,;25.09,-11.64,;25.85,-10.31,;27.39,-10.3,;28.17,-11.63,;27.41,-12.97,;25.87,-12.98,;25.12,-14.3,;29.71,-11.62,;30.48,-12.95,;32.02,-12.95,;32.79,-11.61,;32,-10.27,;30.47,-10.29,;21.36,-16.13,;20.02,-15.37,;20.02,-13.83,;18.69,-16.14,;18.69,-17.68,;20.03,-18.45,;21.36,-17.68,)| Show InChI InChI=1S/C29H30N8/c1-35-11-13-36(14-12-35)23-15-22(16-23)29-34-26(27-28(30)31-18-32-37(27)29)21-8-7-20-9-10-24(33-25(20)17-21)19-5-3-2-4-6-19/h2-10,17-18,22-23H,11-16H2,1H3,(H2,30,31,32)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
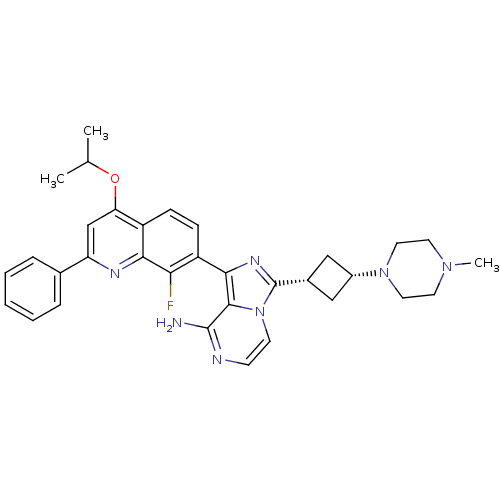
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437360
(CHEMBL3037922)Show SMILES CC(C)Oc1cc(nc2c(F)c(ccc12)-c1nc([C@@H]2C[C@@H](C2)N2CCN(C)CC2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.19,20.24,(26.9,-37.09,;28.24,-37.86,;29.57,-37.09,;28.24,-39.4,;29.57,-40.17,;30.9,-39.4,;32.24,-40.16,;32.24,-41.71,;30.9,-42.49,;30.91,-44.02,;32.25,-44.79,;29.58,-44.8,;28.24,-44.03,;28.24,-42.49,;29.57,-41.71,;29.59,-46.34,;30.84,-47.24,;30.38,-48.71,;31.29,-49.95,;31.05,-51.47,;32.57,-51.7,;32.81,-50.18,;33.48,-52.94,;32.86,-54.35,;33.76,-55.59,;35.3,-55.43,;36.2,-56.67,;35.92,-54.02,;35.01,-52.76,;28.83,-48.71,;27.81,-49.86,;26.31,-49.53,;25.83,-48.07,;26.86,-46.93,;26.39,-45.47,;28.36,-47.25,;33.56,-39.39,;34.9,-40.16,;36.23,-39.38,;36.23,-37.84,;34.88,-37.08,;33.56,-37.85,)| Show InChI InChI=1S/C33H36FN7O/c1-20(2)42-27-19-26(21-7-5-4-6-8-21)37-29-24(27)9-10-25(28(29)34)30-31-32(35)36-11-12-41(31)33(38-30)22-17-23(18-22)40-15-13-39(3)14-16-40/h4-12,19-20,22-23H,13-18H2,1-3H3,(H2,35,36)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
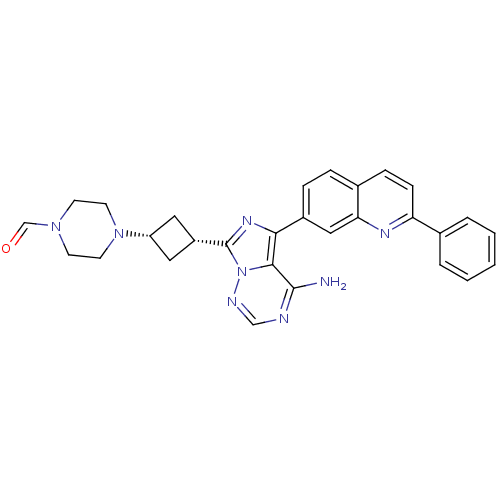
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339061
(4-(3-(4-amino-5-(2-phenylquinolin-7-yl)imidazo[1,5...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:26.30,28.35,(-7.24,-13.78,;-7.23,-15.32,;-8.56,-16.09,;-8.56,-17.63,;-7.23,-18.41,;-5.9,-17.63,;-4.43,-18.1,;-3.52,-16.85,;-4.43,-15.61,;-3.68,-14.27,;-4.46,-12.95,;-3.7,-11.61,;-2.16,-11.59,;-1.4,-10.26,;.14,-10.24,;.92,-11.58,;.15,-12.92,;-1.39,-12.92,;-2.13,-14.25,;2.45,-11.57,;3.23,-12.9,;4.77,-12.9,;5.53,-11.56,;4.75,-10.22,;3.21,-10.24,;-5.9,-16.08,;-3.96,-19.56,;-4.66,-20.93,;-3.28,-21.63,;-2.58,-20.26,;-2.8,-23.1,;-3.84,-24.24,;-3.37,-25.7,;-1.86,-26.03,;-.83,-24.88,;-1.3,-23.41,;-1.39,-27.49,;-2.43,-28.63,)| Show InChI InChI=1S/C29H28N8O/c30-28-27-26(21-7-6-20-8-9-24(33-25(20)16-21)19-4-2-1-3-5-19)34-29(37(27)32-17-31-28)22-14-23(15-22)36-12-10-35(18-38)11-13-36/h1-9,16-18,22-23H,10-15H2,(H2,30,31,32)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
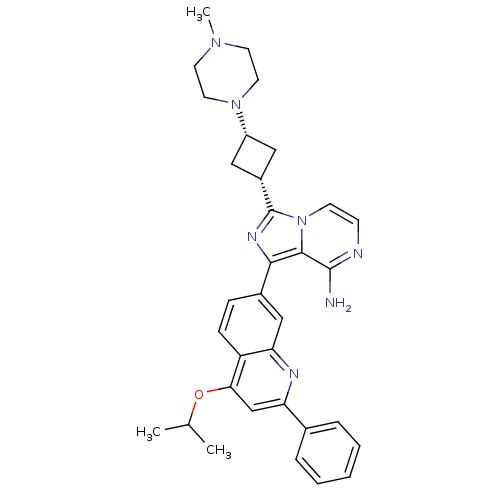
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437364
(CHEMBL3037918)Show SMILES CC(C)Oc1cc(nc2cc(ccc12)-c1nc([C@@H]2C[C@@H](C2)N2CCN(C)CC2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.18,19.23,(44.8,-17.77,;46.14,-18.54,;47.47,-17.77,;46.14,-20.08,;47.47,-20.85,;48.8,-20.08,;50.14,-20.84,;50.14,-22.39,;48.81,-23.17,;48.81,-24.7,;47.48,-25.48,;46.14,-24.71,;46.14,-23.17,;47.47,-22.39,;47.49,-27.02,;48.74,-27.92,;48.28,-29.39,;49.19,-30.63,;48.95,-32.15,;50.47,-32.38,;50.71,-30.86,;51.38,-33.62,;50.76,-35.03,;51.66,-36.27,;53.2,-36.11,;54.1,-37.35,;53.82,-34.7,;52.91,-33.45,;46.73,-29.39,;45.71,-30.54,;44.21,-30.21,;43.73,-28.75,;44.76,-27.61,;44.29,-26.15,;46.26,-27.93,;51.46,-20.07,;52.8,-20.84,;54.13,-20.06,;54.13,-18.52,;52.78,-17.75,;51.46,-18.53,)| Show InChI InChI=1S/C33H37N7O/c1-21(2)41-29-20-27(22-7-5-4-6-8-22)36-28-19-23(9-10-26(28)29)30-31-32(34)35-11-12-40(31)33(37-30)24-17-25(18-24)39-15-13-38(3)14-16-39/h4-12,19-21,24-25H,13-18H2,1-3H3,(H2,34,35)/t24-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339062

(4-(3-(4-amino-5-(8-fluoro-2-phenylquinolin-7-yl)im...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:27.31,29.36,(5.56,-13.24,;5.57,-14.78,;4.24,-15.55,;4.24,-17.09,;5.57,-17.86,;6.9,-17.08,;8.37,-17.56,;9.28,-16.31,;8.37,-15.06,;9.12,-13.72,;8.34,-12.4,;9.1,-11.06,;10.64,-11.05,;11.4,-9.71,;12.94,-9.7,;13.72,-11.04,;12.95,-12.37,;11.41,-12.38,;10.67,-13.71,;11.45,-15.04,;15.25,-11.03,;16.03,-12.36,;17.57,-12.35,;18.33,-11.01,;17.55,-9.68,;16.01,-9.69,;6.9,-15.54,;8.84,-19.01,;8.14,-20.39,;9.52,-21.09,;10.21,-19.71,;10,-22.55,;8.96,-23.7,;9.43,-25.15,;10.94,-25.48,;11.97,-24.34,;11.5,-22.87,;11.41,-26.95,;10.37,-28.09,)| Show InChI InChI=1S/C29H27FN8O/c30-24-22(8-6-19-7-9-23(34-25(19)24)18-4-2-1-3-5-18)26-27-28(31)32-16-33-38(27)29(35-26)20-14-21(15-20)37-12-10-36(17-39)11-13-37/h1-9,16-17,20-21H,10-15H2,(H2,31,32,33)/t20-,21+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339064

(5-(8-fluoro-2-phenylquinolin-7-yl)-7-(3-(4-methylp...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(40.85,-28.31,;40.38,-26.84,;38.87,-26.51,;38.4,-25.05,;39.44,-23.91,;40.94,-24.23,;41.41,-25.7,;38.96,-22.44,;37.58,-21.74,;38.28,-20.37,;39.66,-21.07,;37.81,-18.92,;38.72,-17.67,;37.81,-16.42,;38.56,-15.08,;37.78,-13.76,;38.54,-12.42,;40.08,-12.4,;40.84,-11.07,;42.38,-11.06,;43.16,-12.39,;42.39,-13.73,;40.85,-13.74,;40.11,-15.07,;40.89,-16.39,;44.69,-12.38,;45.47,-13.72,;47.01,-13.71,;47.77,-12.37,;46.99,-11.04,;45.45,-11.05,;36.34,-16.9,;35.01,-16.13,;35,-14.59,;33.68,-16.9,;33.68,-18.45,;35.01,-19.22,;36.34,-18.44,)| Show InChI InChI=1S/C29H29FN8/c1-36-11-13-37(14-12-36)21-15-20(16-21)29-35-26(27-28(31)32-17-33-38(27)29)22-9-7-19-8-10-23(34-25(19)24(22)30)18-5-3-2-4-6-18/h2-10,17,20-21H,11-16H2,1H3,(H2,31,32,33)/t20-,21+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437359

(CHEMBL3037923)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3c(Oc4ccccc4)cc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:9.12,7.7,(52.28,-55.94,;51.37,-54.69,;49.83,-54.85,;48.93,-53.61,;49.55,-52.21,;51.08,-52.03,;51.99,-53.28,;48.64,-50.96,;47.12,-50.73,;47.36,-49.21,;48.88,-49.44,;46.45,-47.97,;46.92,-46.5,;45.66,-45.6,;45.65,-44.06,;44.31,-43.29,;44.31,-41.75,;45.64,-40.98,;45.64,-39.43,;44.31,-38.66,;44.31,-37.12,;45.65,-36.35,;45.65,-34.81,;44.31,-34.04,;42.97,-34.82,;42.98,-36.36,;46.97,-38.66,;48.31,-39.43,;48.31,-40.98,;46.98,-41.75,;46.98,-43.28,;48.32,-44.05,;49.64,-38.65,;50.97,-39.42,;52.3,-38.65,;52.3,-37.1,;50.95,-36.34,;49.63,-37.12,;44.43,-46.52,;42.93,-46.2,;42.46,-44.73,;41.9,-47.34,;42.38,-48.8,;43.89,-49.12,;44.91,-47.98,)| Show InChI InChI=1S/C36H34FN7O/c1-42-16-18-43(19-17-42)25-20-24(21-25)36-41-33(34-35(38)39-14-15-44(34)36)28-13-12-27-30(45-26-10-6-3-7-11-26)22-29(40-32(27)31(28)37)23-8-4-2-5-9-23/h2-15,22,24-25H,16-21H2,1H3,(H2,38,39)/t24-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50336317

(CHEMBL1667935 | cis-3-[4-Amino-5-(8-fluoro-2-pheny...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(-2.6,-22.44,;-3.93,-21.67,;-4.34,-23.15,;-3.24,-20.29,;-4.61,-19.61,;-5.31,-20.98,;-5.1,-18.14,;-4.2,-16.89,;-5.11,-15.65,;-4.34,-14.32,;-5.11,-12.99,;-4.34,-11.66,;-2.79,-11.65,;-2.03,-10.33,;-.5,-10.33,;.27,-11.66,;-.5,-12.99,;-2.03,-12.99,;-2.8,-14.32,;-2.03,-15.66,;1.81,-11.66,;2.58,-13,;4.12,-13,;4.89,-11.66,;4.11,-10.32,;2.57,-10.33,;-6.58,-16.13,;-7.91,-15.37,;-7.92,-13.83,;-9.24,-16.14,;-9.25,-17.68,;-7.91,-18.46,;-6.57,-17.68,)| Show InChI InChI=1S/C25H21FN6O/c1-25(33)11-16(12-25)24-31-21(22-23(27)28-13-29-32(22)24)17-9-7-15-8-10-18(30-20(15)19(17)26)14-5-3-2-4-6-14/h2-10,13,16,33H,11-12H2,1H3,(H2,27,28,29)/t16-,25+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339059
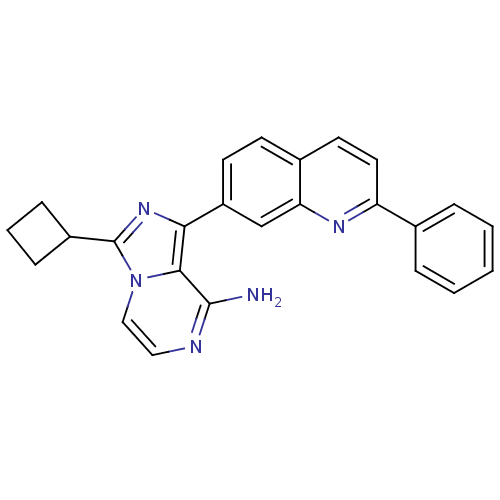
(3-cyclobutyl-1-(2-phenylquinolin-7-yl)-imidazo[1,5...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C25H21N5/c26-24-23-22(29-25(18-7-4-8-18)30(23)14-13-27-24)19-10-9-17-11-12-20(28-21(17)15-19)16-5-2-1-3-6-16/h1-3,5-6,9-15,18H,4,7-8H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R in presence of 100 uM ATP |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339059

(3-cyclobutyl-1-(2-phenylquinolin-7-yl)-imidazo[1,5...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C25H21N5/c26-24-23-22(29-25(18-7-4-8-18)30(23)14-13-27-24)19-10-9-17-11-12-20(28-21(17)15-19)16-5-2-1-3-6-16/h1-3,5-6,9-15,18H,4,7-8H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339060
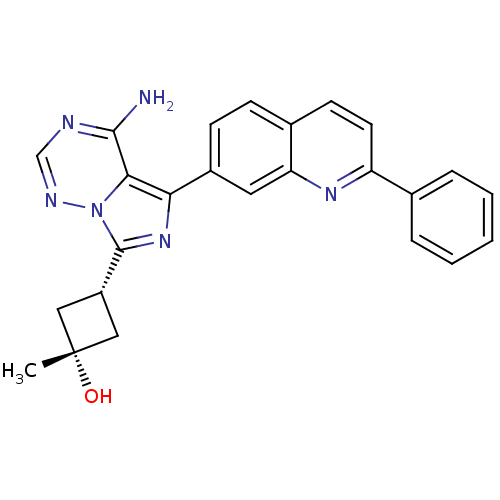
(CHEMBL1688359 | cis-3-(4-amino-5-(2-phenylquinolin...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(26.46,-7.08,;25.39,-6.01,;24.98,-7.48,;26.09,-4.63,;24.71,-3.93,;24.01,-5.31,;24.24,-2.48,;25.15,-1.23,;24.24,.02,;24.99,1.36,;24.21,2.68,;24.97,4.02,;26.51,4.03,;27.27,5.37,;28.81,5.38,;29.59,4.04,;28.82,2.71,;27.29,2.7,;26.54,1.37,;31.12,4.05,;31.9,2.72,;33.44,2.73,;34.2,4.07,;33.42,5.4,;31.88,5.39,;22.77,-.46,;21.44,.31,;21.44,1.85,;20.11,-.46,;20.11,-2.01,;21.44,-2.78,;22.77,-2,)| Show InChI InChI=1S/C25H22N6O/c1-25(32)12-18(13-25)24-30-21(22-23(26)27-14-28-31(22)24)17-8-7-16-9-10-19(29-20(16)11-17)15-5-3-2-4-6-15/h2-11,14,18,32H,12-13H2,1H3,(H2,26,27,28)/t18-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437363

(CHEMBL3037919)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3c(Oc4ccccc4)cc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:9.12,7.7,(67.57,-36.98,;66.66,-35.74,;65.13,-35.9,;64.22,-34.66,;64.85,-33.25,;66.38,-33.07,;67.29,-34.33,;63.94,-32.01,;62.42,-31.78,;62.65,-30.26,;64.18,-30.49,;61.74,-29.01,;62.21,-27.55,;60.96,-26.65,;60.95,-25.1,;59.6,-24.34,;59.6,-22.79,;60.93,-22.02,;60.94,-20.48,;59.6,-19.71,;59.6,-18.16,;60.94,-17.4,;60.94,-15.86,;59.6,-15.09,;58.27,-15.86,;58.27,-17.4,;62.27,-19.71,;63.6,-20.47,;63.61,-22.02,;62.27,-22.79,;62.27,-24.33,;64.93,-19.7,;66.27,-20.47,;67.6,-19.69,;67.59,-18.15,;66.25,-17.38,;64.92,-18.16,;59.72,-27.56,;58.23,-27.24,;57.75,-25.78,;57.2,-28.38,;57.67,-29.84,;59.18,-30.17,;60.2,-29.02,)| Show InChI InChI=1S/C36H35N7O/c1-41-16-18-42(19-17-41)27-20-26(21-27)36-40-33(34-35(37)38-14-15-43(34)36)25-12-13-29-31(22-25)39-30(24-8-4-2-5-9-24)23-32(29)44-28-10-6-3-7-11-28/h2-15,22-23,26-27H,16-21H2,1H3,(H2,37,38)/t26-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339061

(4-(3-(4-amino-5-(2-phenylquinolin-7-yl)imidazo[1,5...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:26.30,28.35,(-7.24,-13.78,;-7.23,-15.32,;-8.56,-16.09,;-8.56,-17.63,;-7.23,-18.41,;-5.9,-17.63,;-4.43,-18.1,;-3.52,-16.85,;-4.43,-15.61,;-3.68,-14.27,;-4.46,-12.95,;-3.7,-11.61,;-2.16,-11.59,;-1.4,-10.26,;.14,-10.24,;.92,-11.58,;.15,-12.92,;-1.39,-12.92,;-2.13,-14.25,;2.45,-11.57,;3.23,-12.9,;4.77,-12.9,;5.53,-11.56,;4.75,-10.22,;3.21,-10.24,;-5.9,-16.08,;-3.96,-19.56,;-4.66,-20.93,;-3.28,-21.63,;-2.58,-20.26,;-2.8,-23.1,;-3.84,-24.24,;-3.37,-25.7,;-1.86,-26.03,;-.83,-24.88,;-1.3,-23.41,;-1.39,-27.49,;-2.43,-28.63,)| Show InChI InChI=1S/C29H28N8O/c30-28-27-26(21-7-6-20-8-9-24(33-25(20)16-21)19-4-2-1-3-5-19)34-29(37(27)32-17-31-28)22-14-23(15-22)36-12-10-35(18-38)11-13-36/h1-9,16-18,22-23H,10-15H2,(H2,30,31,32)/t22-,23+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339058
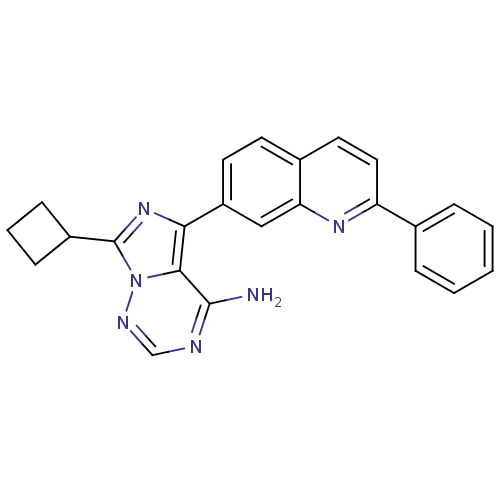
(7-cyclobutyl-5-(2-phenylquinolin-7-yl)imidazo[1,5-...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C24H20N6/c25-23-22-21(29-24(17-7-4-8-17)30(22)27-14-26-23)18-10-9-16-11-12-19(28-20(16)13-18)15-5-2-1-3-6-15/h1-3,5-6,9-14,17H,4,7-8H2,(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R in presence of 100 uM ATP |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437358

(CHEMBL3037908)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.20,15.16,wD:17.19,(2.3,-.85,;2.3,-2.39,;3.63,-3.16,;4.96,-2.39,;6.3,-3.15,;6.31,-4.71,;4.97,-5.48,;4.97,-7.01,;3.65,-7.79,;2.3,-7.02,;2.3,-5.48,;3.63,-4.71,;3.66,-9.33,;4.91,-10.23,;4.44,-11.7,;5.35,-12.94,;6.87,-13.17,;6.63,-14.69,;8.12,-15.09,;7.03,-16.18,;5.12,-14.46,;2.9,-11.71,;1.88,-12.85,;.37,-12.53,;-.11,-11.07,;.93,-9.93,;.45,-8.46,;2.42,-10.25,;7.63,-2.38,;8.97,-3.15,;10.3,-2.38,;10.29,-.83,;8.94,-.07,;7.62,-.85,)| Show InChI InChI=1S/C27H25N5O2/c1-27(33)14-18(15-27)26-31-23(24-25(28)29-10-11-32(24)26)17-8-9-19-21(12-17)30-20(13-22(19)34-2)16-6-4-3-5-7-16/h3-13,18,33H,14-15H2,1-2H3,(H2,28,29)/t18-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339063

(7-(3-(4-methylpiperazin-1-yl)cyclobutyl)-5-(2-phen...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(25.86,-27.54,;25.39,-26.08,;23.89,-25.75,;23.42,-24.29,;24.45,-23.15,;25.96,-23.46,;26.43,-24.93,;23.97,-21.68,;22.6,-20.98,;23.3,-19.61,;24.67,-20.31,;22.82,-18.15,;23.73,-16.9,;22.82,-15.66,;23.58,-14.32,;22.79,-13,;23.55,-11.66,;25.09,-11.64,;25.85,-10.31,;27.39,-10.3,;28.17,-11.63,;27.41,-12.97,;25.87,-12.98,;25.12,-14.3,;29.71,-11.62,;30.48,-12.95,;32.02,-12.95,;32.79,-11.61,;32,-10.27,;30.47,-10.29,;21.36,-16.13,;20.02,-15.37,;20.02,-13.83,;18.69,-16.14,;18.69,-17.68,;20.03,-18.45,;21.36,-17.68,)| Show InChI InChI=1S/C29H30N8/c1-35-11-13-36(14-12-35)23-15-22(16-23)29-34-26(27-28(30)31-18-32-37(27)29)21-8-7-20-9-10-24(33-25(20)17-21)19-5-3-2-4-6-19/h2-10,17-18,22-23H,11-16H2,1H3,(H2,30,31,32)/t22-,23+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339058

(7-cyclobutyl-5-(2-phenylquinolin-7-yl)imidazo[1,5-...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C24H20N6/c25-23-22-21(29-24(17-7-4-8-17)30(22)27-14-26-23)18-10-9-16-11-12-19(28-20(16)13-18)15-5-2-1-3-6-15/h1-3,5-6,9-14,17H,4,7-8H2,(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437357

(CHEMBL3037909)Show SMILES CCOc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.21,16.17,wD:18.20,(12.1,-.5,;13.44,-1.27,;13.44,-2.81,;14.77,-3.58,;16.1,-2.81,;17.44,-3.57,;17.45,-5.13,;16.11,-5.9,;16.11,-7.43,;14.79,-8.21,;13.44,-7.44,;13.44,-5.9,;14.77,-5.13,;14.8,-9.75,;16.05,-10.65,;15.58,-12.12,;16.49,-13.36,;18.01,-13.59,;17.77,-15.11,;19.26,-15.51,;18.17,-16.6,;16.26,-14.88,;14.04,-12.13,;13.02,-13.27,;11.51,-12.95,;11.04,-11.49,;12.07,-10.35,;11.59,-8.88,;13.56,-10.67,;18.77,-2.8,;20.11,-3.57,;21.44,-2.8,;21.43,-1.25,;20.09,-.49,;18.76,-1.27,)| Show InChI InChI=1S/C28H27N5O2/c1-3-35-23-14-21(17-7-5-4-6-8-17)31-22-13-18(9-10-20(22)23)24-25-26(29)30-11-12-33(25)27(32-24)19-15-28(2,34)16-19/h4-14,19,34H,3,15-16H2,1-2H3,(H2,29,30)/t19-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339060

(CHEMBL1688359 | cis-3-(4-amino-5-(2-phenylquinolin...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(26.46,-7.08,;25.39,-6.01,;24.98,-7.48,;26.09,-4.63,;24.71,-3.93,;24.01,-5.31,;24.24,-2.48,;25.15,-1.23,;24.24,.02,;24.99,1.36,;24.21,2.68,;24.97,4.02,;26.51,4.03,;27.27,5.37,;28.81,5.38,;29.59,4.04,;28.82,2.71,;27.29,2.7,;26.54,1.37,;31.12,4.05,;31.9,2.72,;33.44,2.73,;34.2,4.07,;33.42,5.4,;31.88,5.39,;22.77,-.46,;21.44,.31,;21.44,1.85,;20.11,-.46,;20.11,-2.01,;21.44,-2.78,;22.77,-2,)| Show InChI InChI=1S/C25H22N6O/c1-25(32)12-18(13-25)24-30-21(22-23(26)27-14-28-31(22)24)17-8-7-16-9-10-19(29-20(16)11-17)15-5-3-2-4-6-15/h2-11,14,18,32H,12-13H2,1H3,(H2,26,27,28)/t18-,25+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50336317

(CHEMBL1667935 | cis-3-[4-Amino-5-(8-fluoro-2-pheny...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(-2.6,-22.44,;-3.93,-21.67,;-4.34,-23.15,;-3.24,-20.29,;-4.61,-19.61,;-5.31,-20.98,;-5.1,-18.14,;-4.2,-16.89,;-5.11,-15.65,;-4.34,-14.32,;-5.11,-12.99,;-4.34,-11.66,;-2.79,-11.65,;-2.03,-10.33,;-.5,-10.33,;.27,-11.66,;-.5,-12.99,;-2.03,-12.99,;-2.8,-14.32,;-2.03,-15.66,;1.81,-11.66,;2.58,-13,;4.12,-13,;4.89,-11.66,;4.11,-10.32,;2.57,-10.33,;-6.58,-16.13,;-7.91,-15.37,;-7.92,-13.83,;-9.24,-16.14,;-9.25,-17.68,;-7.91,-18.46,;-6.57,-17.68,)| Show InChI InChI=1S/C25H21FN6O/c1-25(33)11-16(12-25)24-31-21(22-23(27)28-13-29-32(22)24)17-9-7-15-8-10-18(30-20(15)19(17)26)14-5-3-2-4-6-14/h2-10,13,16,33H,11-12H2,1H3,(H2,27,28,29)/t16-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50336317

(CHEMBL1667935 | cis-3-[4-Amino-5-(8-fluoro-2-pheny...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(-2.6,-22.44,;-3.93,-21.67,;-4.34,-23.15,;-3.24,-20.29,;-4.61,-19.61,;-5.31,-20.98,;-5.1,-18.14,;-4.2,-16.89,;-5.11,-15.65,;-4.34,-14.32,;-5.11,-12.99,;-4.34,-11.66,;-2.79,-11.65,;-2.03,-10.33,;-.5,-10.33,;.27,-11.66,;-.5,-12.99,;-2.03,-12.99,;-2.8,-14.32,;-2.03,-15.66,;1.81,-11.66,;2.58,-13,;4.12,-13,;4.89,-11.66,;4.11,-10.32,;2.57,-10.33,;-6.58,-16.13,;-7.91,-15.37,;-7.92,-13.83,;-9.24,-16.14,;-9.25,-17.68,;-7.91,-18.46,;-6.57,-17.68,)| Show InChI InChI=1S/C25H21FN6O/c1-25(33)11-16(12-25)24-31-21(22-23(27)28-13-29-32(22)24)17-9-7-15-8-10-18(30-20(15)19(17)26)14-5-3-2-4-6-14/h2-10,13,16,33H,11-12H2,1H3,(H2,27,28,29)/t16-,25+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data