

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016323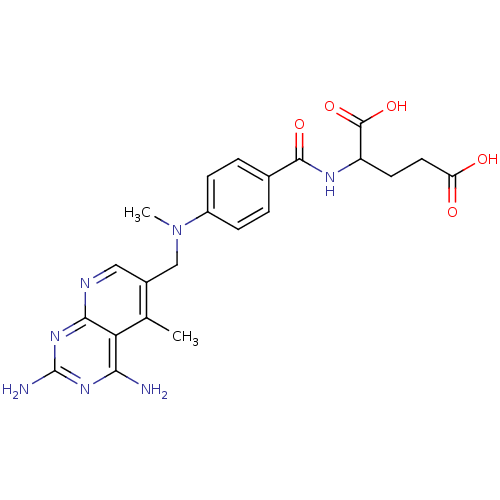 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016324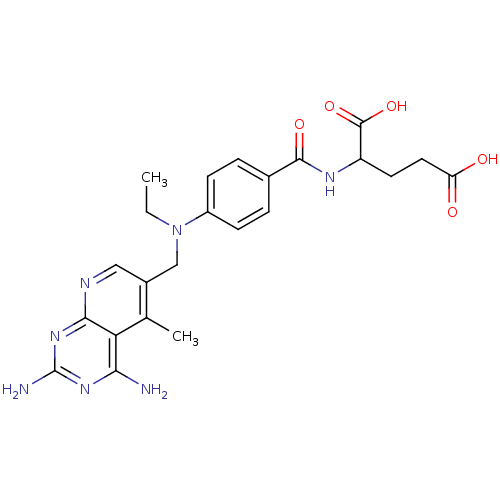 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016326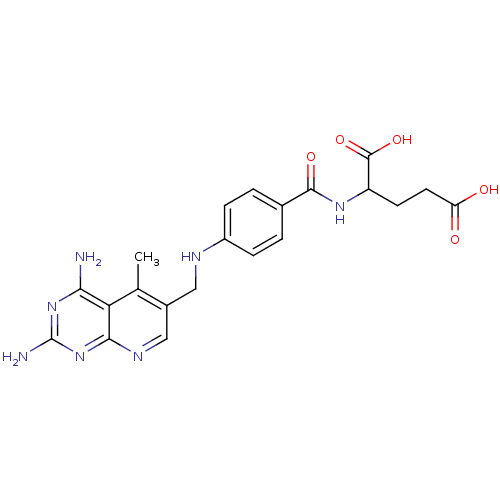 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00293 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028605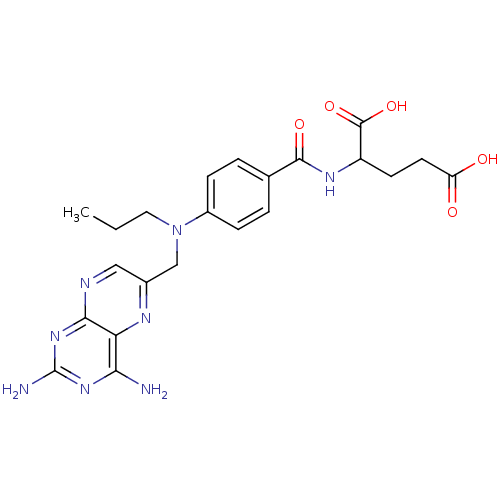 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-propyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.00355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016325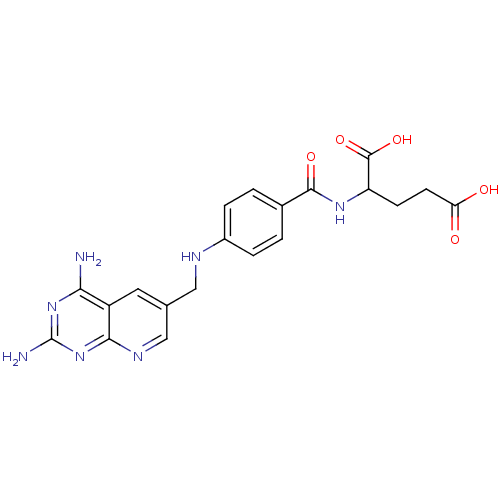 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026389 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026392 (2-[3-(2-Carboxy-2-{4-[(2,4-diamino-pteridin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00471 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026399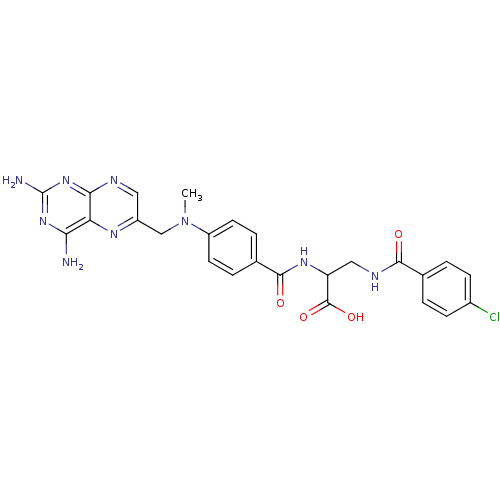 (3-(4-Chloro-benzoylamino)-2-{4-[(2,4-diamino-pteri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00498 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016322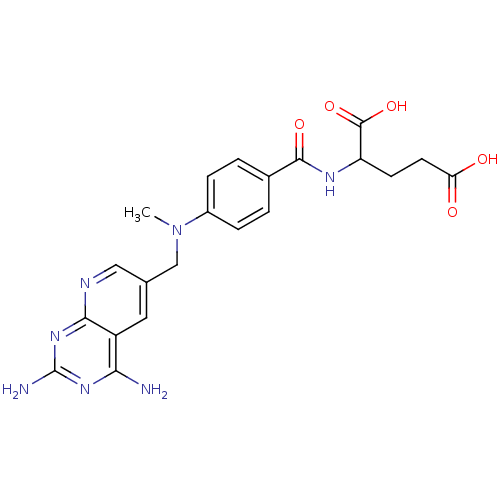 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026391 (5-(4-Chloro-benzoylamino)-2-{4-[(2,4-diamino-pteri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00531 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00581 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026402 (4-(4-Chloro-benzoylamino)-2-{4-[(2,4-diamino-pteri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00642 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022738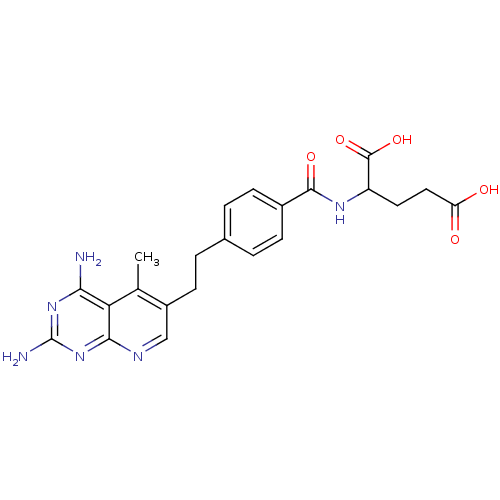 (2-{4-[2-(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0283 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028603 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-prop-2-yny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022739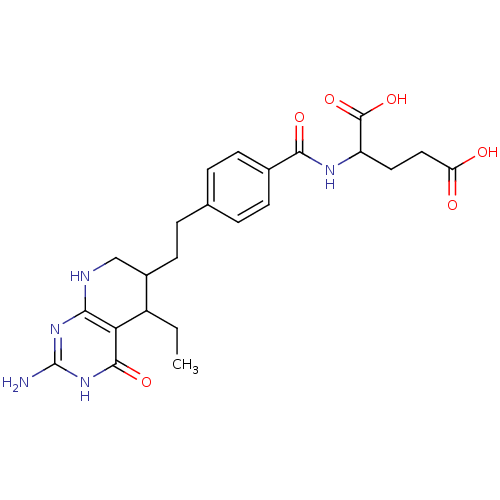 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022740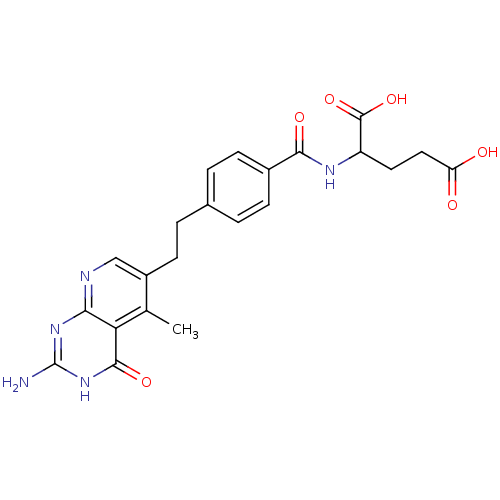 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50022741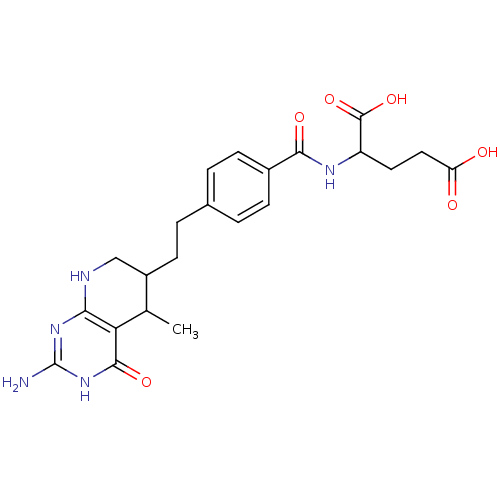 ((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026401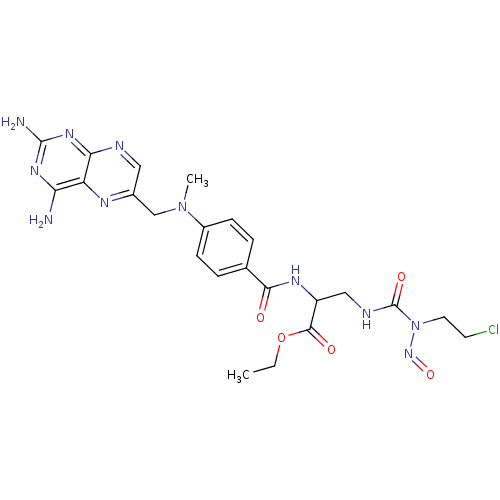 (2--[3-(2-Carboxy-2-{4-[(2,4-diamino-pteridin-6-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026390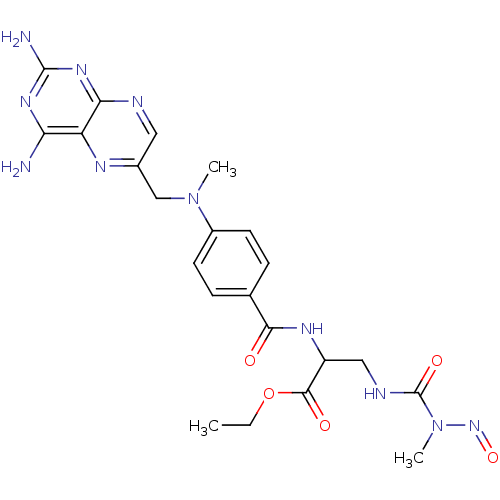 (2--[3-(2-Carboxy-2-{4-[(2,4-diamino-pteridin-6-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026398 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028604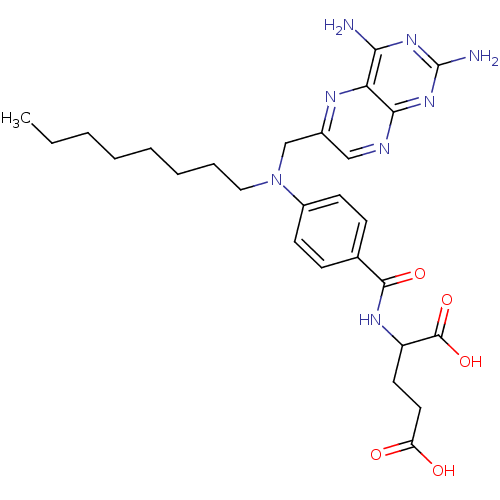 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-octyl-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50026394 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Dihydrofolate reductase in L1210 cell in mice | J Med Chem 28: 1016-25 (1985) BindingDB Entry DOI: 10.7270/Q2BP01TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50226275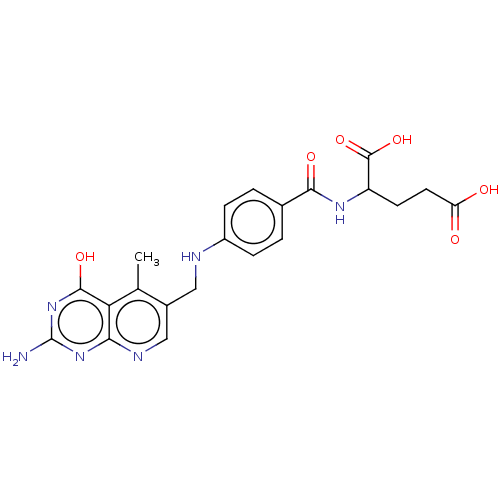 (CHEMBL267104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the thymidylate synthase (TS) of Lactobacillus casei | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50226275 (CHEMBL267104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the dihydrofolate reductase (DHFR) of Lactobacillus casei | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the thymidylate synthase (TS) of Lactobacillus casei | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50226277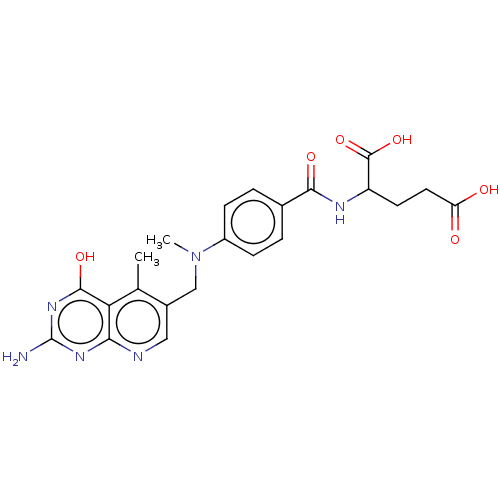 (CHEMBL273509) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the thymidylate synthase (TS) of Lactobacillus casei | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3.52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the dihydrofolate reductase (DHFR) of Lactobacillus casei. | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50226276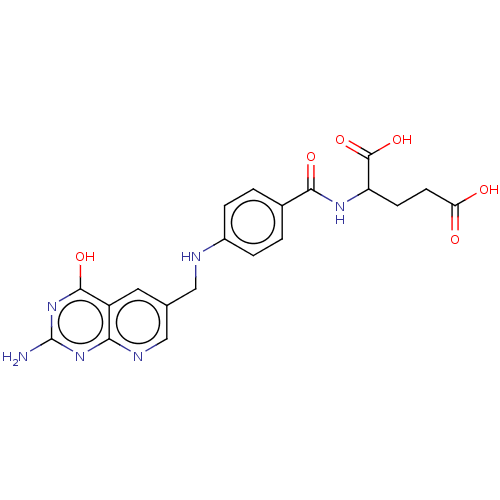 (CHEMBL11139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the thymidylate synthase (TS) of Lactobacillus casei | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50226277 (CHEMBL273509) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the dihydrofolate reductase (DHFR) of Lactobacillus casei | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50028603 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-prop-2-yny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Dihydrofolate reductase activity against Lactobacillus casei enzyme | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50028604 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-octyl-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Dihydrofolate reductase activity against Lactobacillus casei enzyme | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Dihydrofolate reductase activity against Lactobacillus casei enzyme | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50226276 (CHEMBL11139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on the dihydrofolate reductase (DHFR) of Lactobacillus casei | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022738 (2-{4-[2-(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian Manca | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022741 ((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian Manca | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50022738 (2-{4-[2-(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of thymidylate synthase (TS) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50022741 ((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022740 (2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50022739 ((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) from Lactobacillus casei | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of GAR transformylase from mammalian L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |