

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | -50.4 | 13 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178095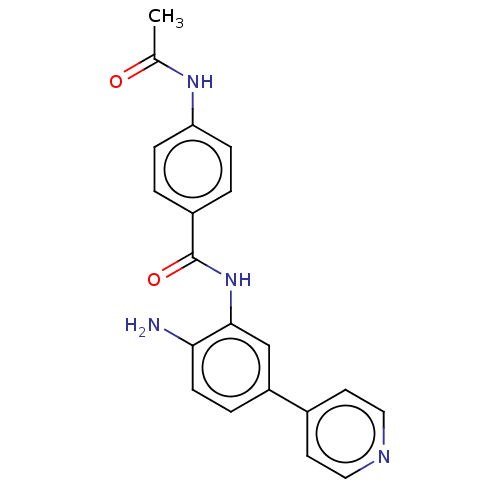 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM178095 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.8 | 19 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.4 | 46 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM178100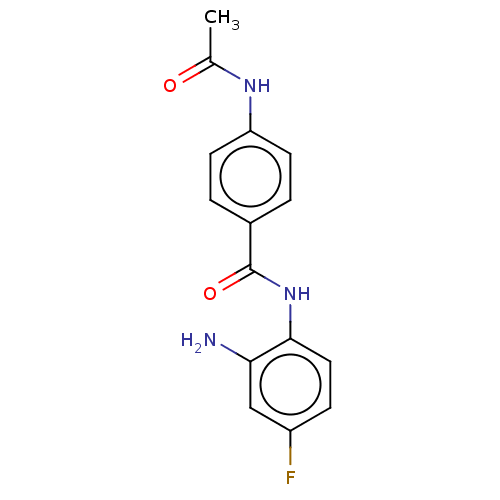 (BRD3308 | US11377423, Cmpd 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 29 | -43.0 | 64 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | -42.4 | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 223 | -38.0 | 147 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | -36.0 | 398 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572983 (CHEMBL4848846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178100 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | -30.2 | 1.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM178100 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | -29.7 | 1.15E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572968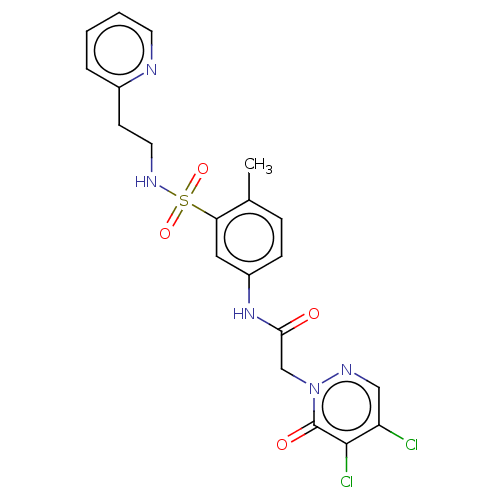 (CHEMBL4862851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM178095 (BRD2492) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | -27.7 | 2.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572964 (CHEMBL4867592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572989 (CHEMBL4846332) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572991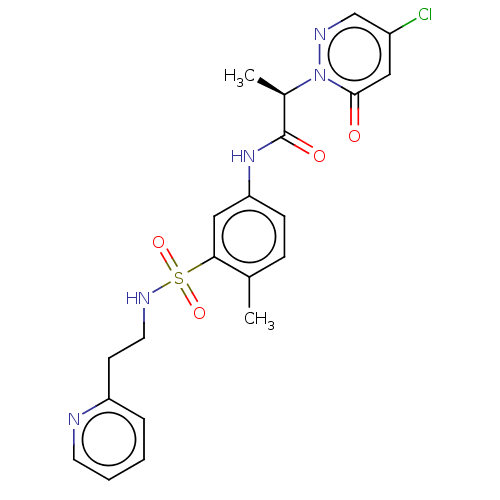 (CHEMBL4858967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572990 (CHEMBL4859105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572988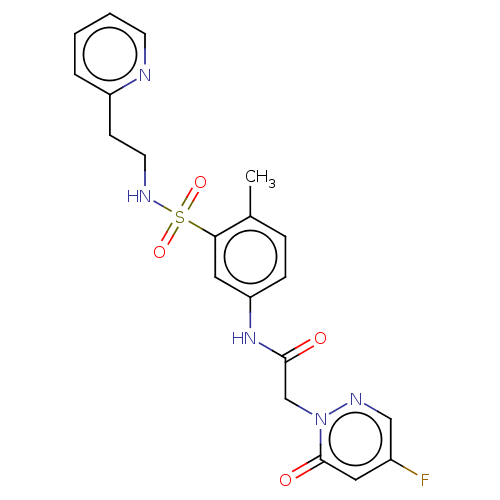 (CHEMBL4855695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572984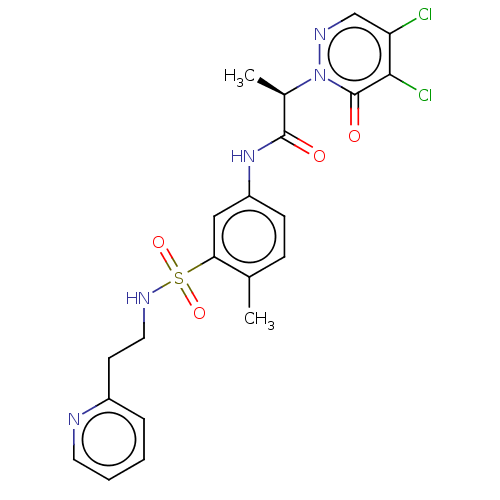 (CHEMBL4874198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571467 ((+)-4-chloro-15-(2-hydroxyethyl)-2,3-dimethyl-7-{3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43] (Homo sapiens (Human)) | BDBM562371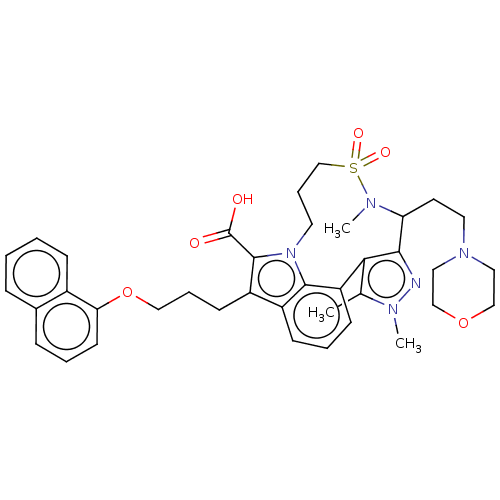 ((rac)-2,3,14-trimethyl-15-[2-(rac)-(morpholin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2930XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571478 ((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43] (Homo sapiens (Human)) | BDBM562401 (4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-yl)ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2930XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43] (Homo sapiens (Human)) | BDBM562369 ((−)-4-chloro-14-cyclopropyl-7-{3-[(6-fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2930XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571483 ((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571511 ((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571480 ((rac)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43] (Homo sapiens (Human)) | BDBM562377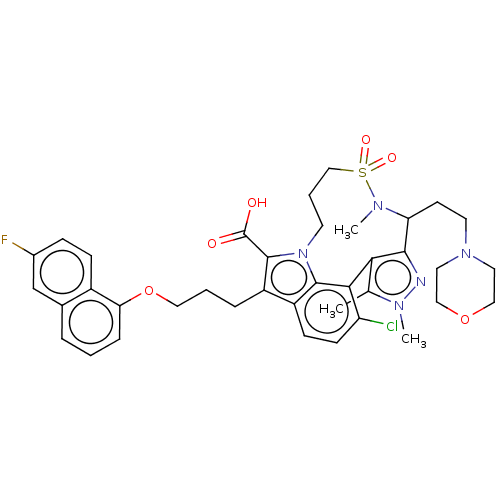 ((rac)-4-chloro-7-{3-[(6-fluoro-1-naphthyl)oxy]prop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2930XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571489 ((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571514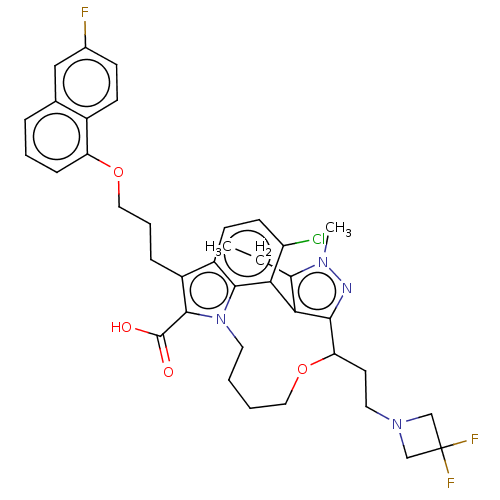 ((+)-4-chloro-15-[2-(3,3-difluoroazetidin-1-yl)ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM572169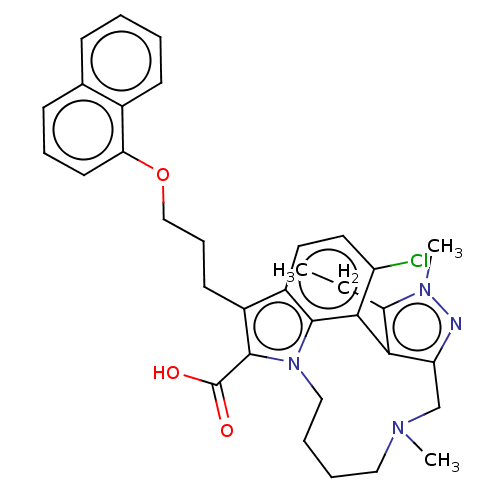 ((rac)-4-chloro-3-ethyl-2,14-dimethyl-7-[3-(naphtha...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2057K58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM544485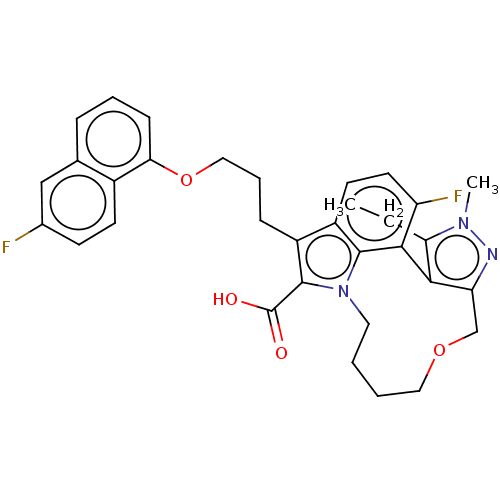 ((rac)-12-ethyl-13-fluoro-1-(3-((6-fluoronaphthalen...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1 Assay: In the assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WW7MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571497 (4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-yl)ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571525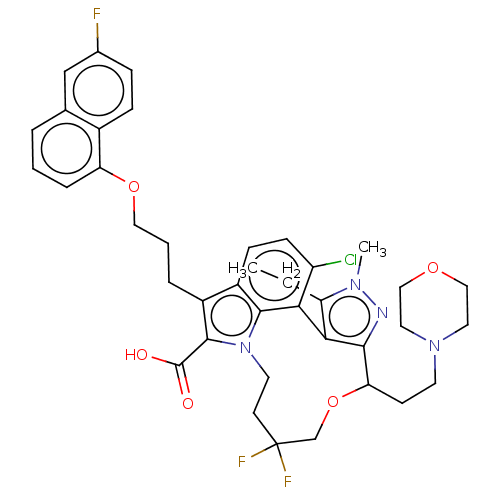 ((+)-4-chloro-3-ethyl-12,12-difluoro-7-{3-[(6-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM572155 ((rac)-4-chloro-3-ethyl-1-[2-(morpholin-4-yl)ethyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2057K58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43] (Homo sapiens (Human)) | BDBM562386 ((+)-4-chloro-3,14-diethyl-7-{3-[(6-fluoronaphthale...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2930XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571473 ((rac)-4-chloro-(15-rac)-(2-methoxyethyl)-2,3-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571486 ((+)-3-ethyl-4-fluoro-7-{3-[(6-fluoronaphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM572162 ((+)-4-chloro-7-{3-[(6-fluoronaphthalen-1-yl)oxy]pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2057K58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM572159 ((+)-4-chloro-2,3,14-trimethyl-7-[3-(naphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2057K58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-329]/Phorbol-Phorbol-12-myristate-13-acetate-induced protein 1 [26-43] (Homo sapiens (Human)) | BDBM562331 ((rac)-4-Chloro-2,3,14-trimethyl-7-{3-[(naphthalen-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1/Noxa BH3 Peptide (MCL-1 Assay): From there, 50 nl were transferred in a dark test plate (Greiner Bio-One, Frickenhausen, Germany). The assay wa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2930XCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571477 ((rac)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571522 ((+)-4-chloro-3-ethyl-12,12-difluoro-7-{3-[(6-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571449 ((rac)-4-chloro-2,3-dimethyl-15-[2-(morpholin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571446 ((rac)-3-ethyl-4-fluoro-2-methyl-15-[2-(morpholin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571502 ((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571481 ((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide (Homo sapiens (Human)) | BDBM571443 ((rac)-4-fluoro-2,3-dimethyl-15-[2-(morpholin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J38WT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM544485 ((rac)-12-ethyl-13-fluoro-1-(3-((6-fluoronaphthalen...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description MCL-1 Assay: In the assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WW7MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1198 total ) | Next | Last >> |