

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-A2/Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.90 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM17054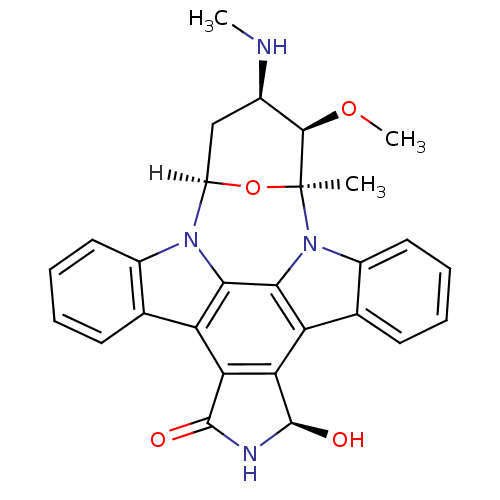 ((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline | Assay Description In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM17140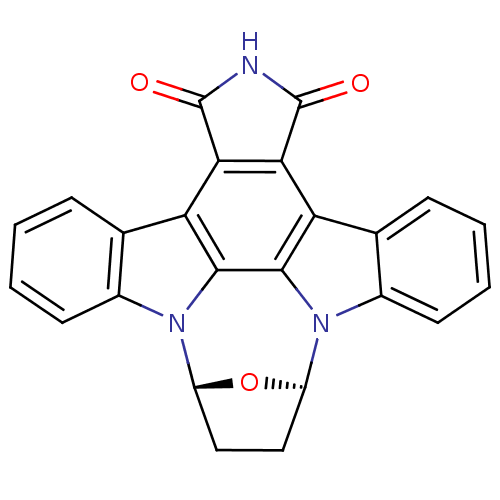 ((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 5.60 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.80 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline | Assay Description In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM17140 ((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline | Assay Description In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM17140 ((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 16 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM17140 ((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 23 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM17054 ((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 41 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM17054 ((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 95 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169013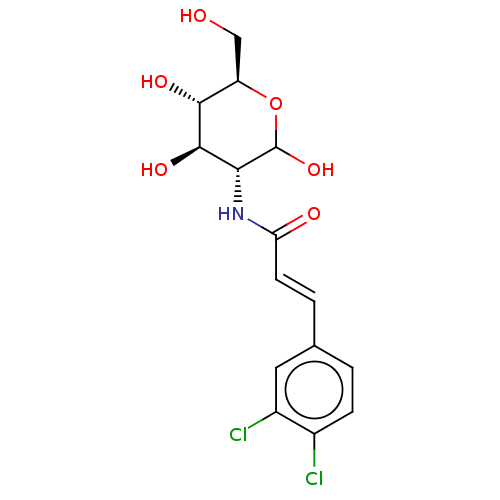 (CHEMBL3805703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) assessed as formation of G6P by continuou... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM17054 ((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.60E+3 | -31.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline | Assay Description CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... | J Biol Chem 277: 46609-15 (2002) Article DOI: 10.1074/jbc.M201233200 BindingDB Entry DOI: 10.7270/Q2D798PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169038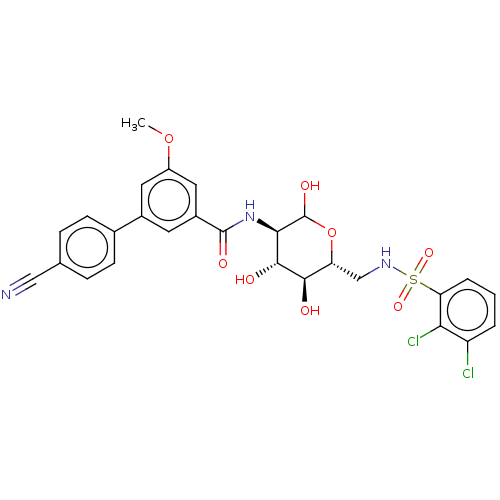 (CHEMBL3804841) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169042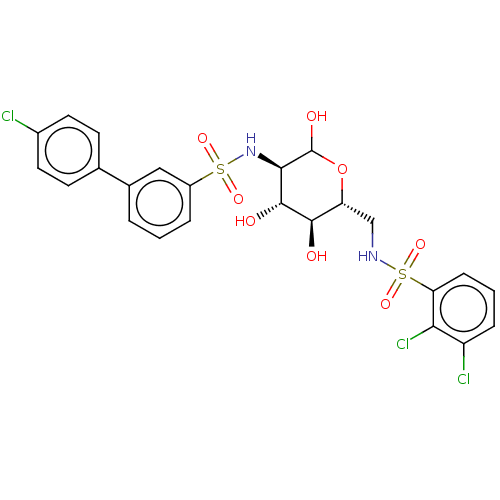 (CHEMBL3806103) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169033 (CHEMBL3806069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169037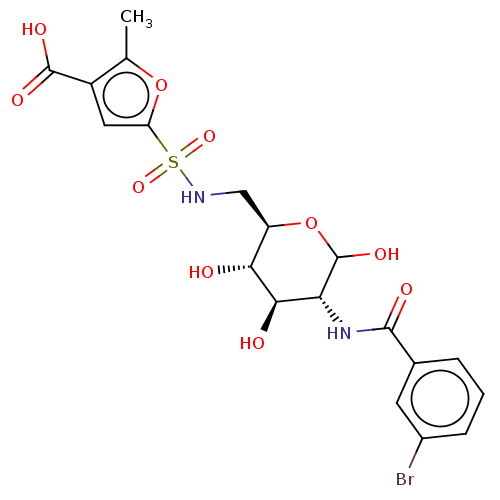 (CHEMBL3805205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169026 (CHEMBL3805148) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169037 (CHEMBL3805205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169032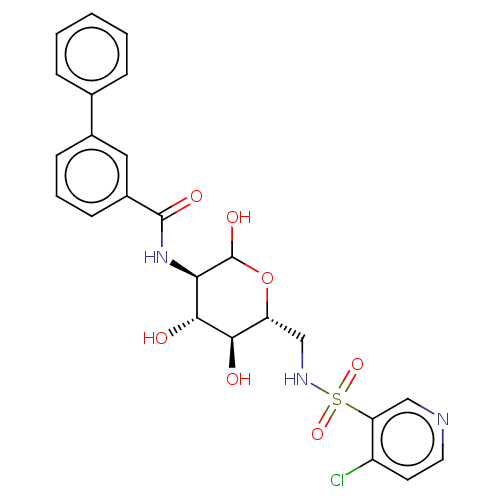 (CHEMBL3805398) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169041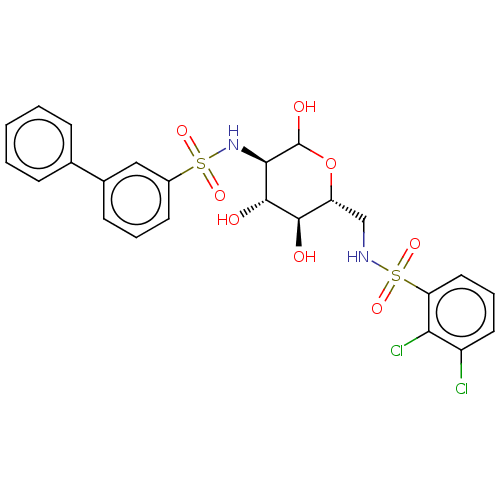 (CHEMBL3805653) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169038 (CHEMBL3804841) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169031 (CHEMBL3805905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169034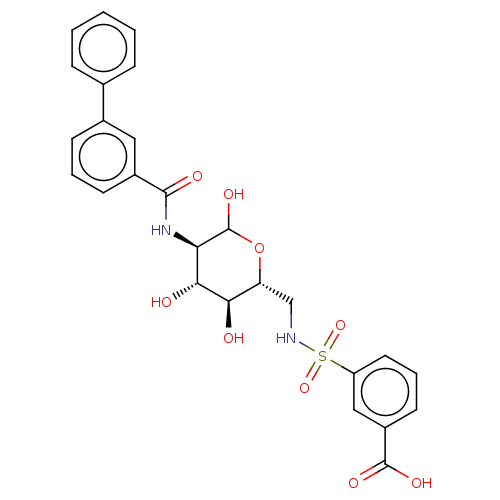 (CHEMBL3805734) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169031 (CHEMBL3805905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169017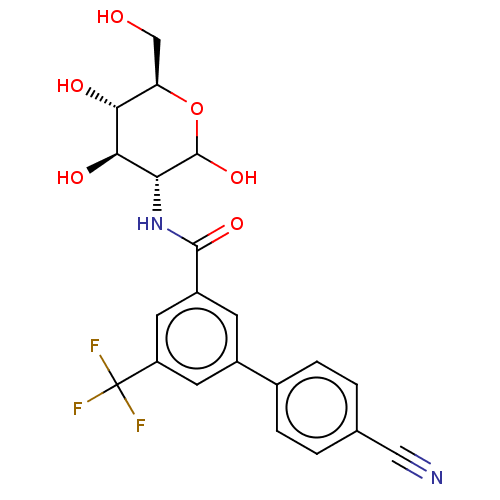 (CHEMBL3804930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169039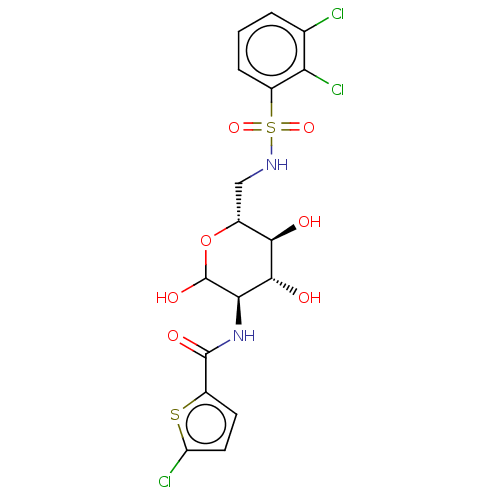 (CHEMBL3806132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169034 (CHEMBL3805734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169043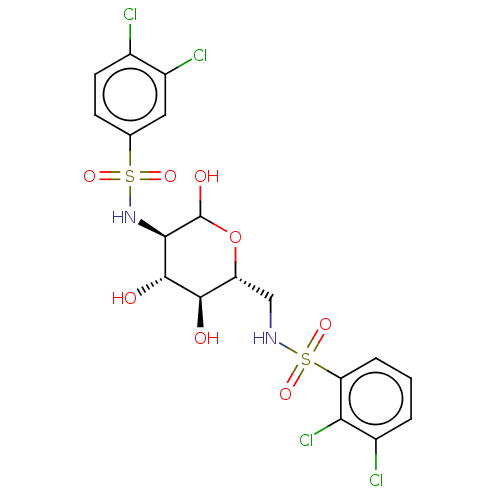 (CHEMBL3806095) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169026 (CHEMBL3805148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169032 (CHEMBL3805398) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169040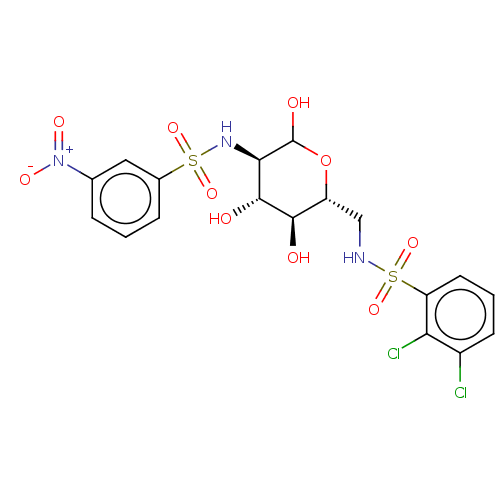 (CHEMBL3804874) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169046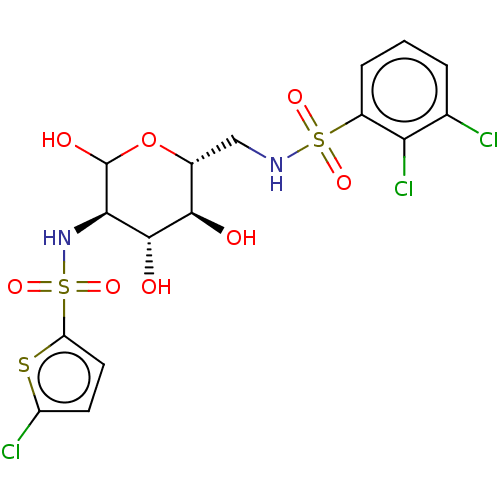 (CHEMBL3805459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169033 (CHEMBL3806069) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169023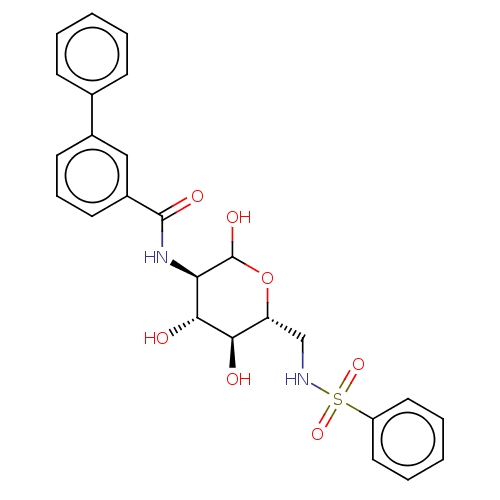 (CHEMBL3805753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169028 (CHEMBL3805598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169031 (CHEMBL3805905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HK2 in human UM-UC-3 cells using 2FDG as substrate assessed as suppression of G6P production preincubated for 30 mins followed by subst... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169028 (CHEMBL3805598) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169041 (CHEMBL3805653) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HK2 in human UM-UC-3 cells using 2FDG as substrate assessed as suppression of G6P production preincubated for 30 mins followed by subst... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169046 (CHEMBL3805459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HK2 in human UM-UC-3 cells using 2FDG as substrate assessed as suppression of G6P production preincubated for 30 mins followed by subst... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169036 (CHEMBL3806028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169015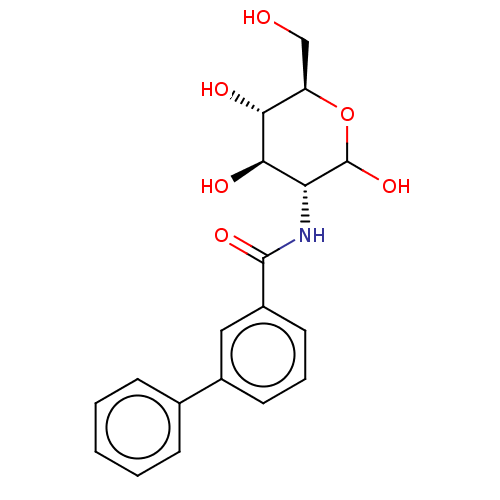 (CHEMBL3805460) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169023 (CHEMBL3805753) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169017 (CHEMBL3804930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169042 (CHEMBL3806103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169036 (CHEMBL3806028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169039 (CHEMBL3806132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169025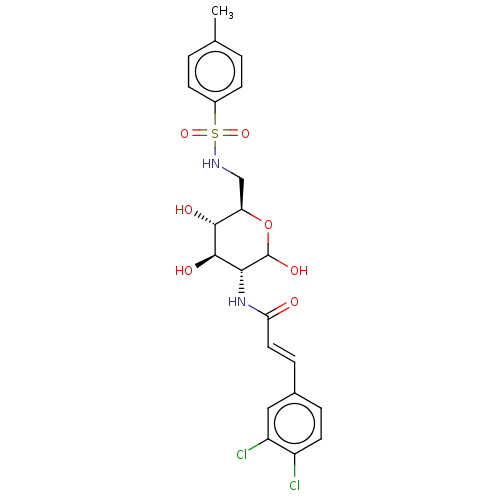 (CHEMBL3806250) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169025 (CHEMBL3806250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-2 (Homo sapiens (Human)) | BDBM50169019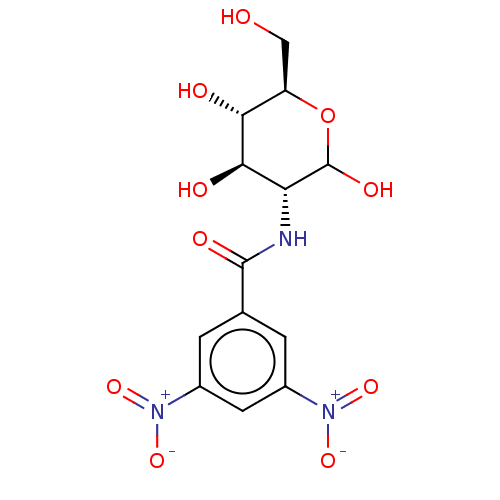 (CHEMBL3805765) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |