 Found 324 hits with Last Name = 'mcdonald' and Initial = 'nq'
Found 324 hits with Last Name = 'mcdonald' and Initial = 'nq' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM374727
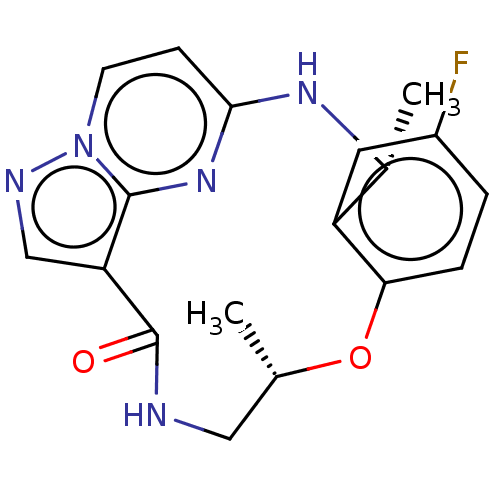
((7S,13R)-11-fluoro-7,13-dimethyl-6,7,13,14- tetrah...)Show SMILES C[C@H]1CNC(=O)c2cnn3ccc(N[C@H](C)c4cc(F)ccc4O1)nc23 |r| Show InChI InChI=1S/C18H18FN5O2/c1-10-8-20-18(25)14-9-21-24-6-5-16(23-17(14)24)22-11(2)13-7-12(19)3-4-15(13)26-10/h3-7,9-11H,8H2,1-2H3,(H,20,25)(H,22,23)/t10-,11+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA G595R mutant (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153906
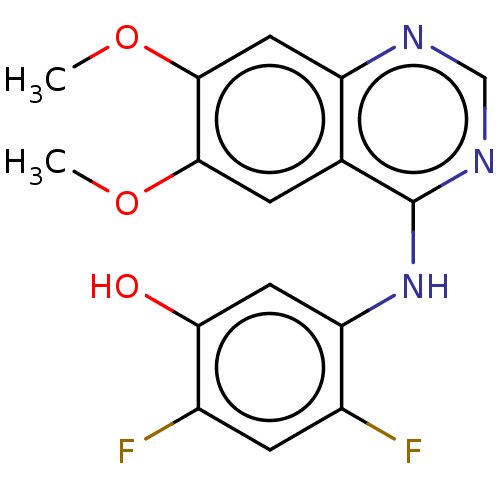
(CHEMBL3775169)Show InChI InChI=1S/C16H13F2N3O3/c1-23-14-3-8-11(6-15(14)24-2)19-7-20-16(8)21-12-5-13(22)10(18)4-9(12)17/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Coiled-coil domain-containing protein 6
(Homo sapiens (Human)) | BDBM50514844
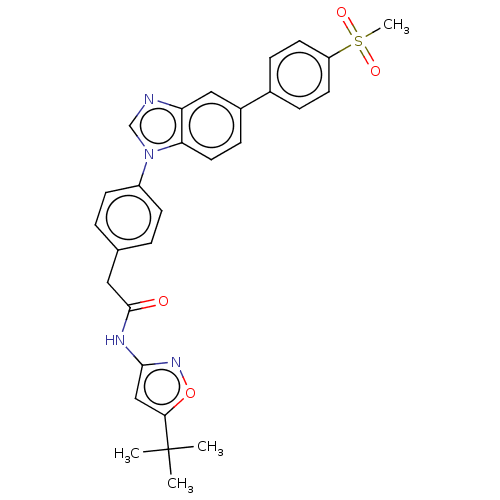
(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of CCDC6/RET in human TPC1 cells assessed as reduction in cell proliferation supplemented with fresh medium containing compound for every ... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of RET isoform 9 M918T mutant in human MZ-CRC-1 cells assessed as reduction in cell proliferation supplemented with fresh medium containin... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of RET isoform 9 C634W mutant in human TT cells assessed as reduction in cell proliferation supplemented with fresh medium containing comp... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Coiled-coil domain-containing protein 6
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of CCDC6/RET in human LC2/ad cells assessed as reduction in cell proliferation supplemented with fresh medium containing compound for ever... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM4627
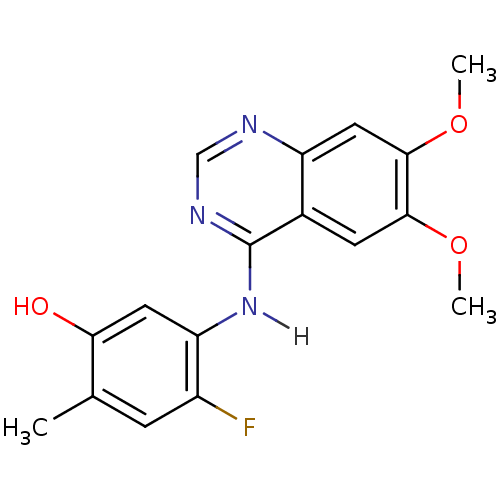
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...)Show InChI InChI=1S/C17H16FN3O3/c1-9-4-11(18)13(6-14(9)22)21-17-10-5-15(23-2)16(24-3)7-12(10)19-8-20-17/h4-8,22H,1-3H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50193395

(Altiratinib | DCC-2701 | DP-5164)Show SMILES Fc1ccc(NC(=O)C2(CC2)C(=O)Nc2cc(F)c(Oc3ccnc(NC(=O)C4CC4)c3)cc2F)cc1 Show InChI InChI=1S/C26H21F3N4O4/c27-15-3-5-16(6-4-15)31-24(35)26(8-9-26)25(36)32-20-12-19(29)21(13-18(20)28)37-17-7-10-30-22(11-17)33-23(34)14-1-2-14/h3-7,10-14H,1-2,8-9H2,(H,31,35)(H,32,36)(H,30,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50507492

(Loxo-195 | Selitrectinib | US10966985, Compound 33...)Show SMILES C[C@@H]1CCc2ncc(F)cc2[C@H]2CCCN2c2ccn3ncc(C(=O)N1)c3n2 Show InChI InChI=1S/C20H21FN6O/c1-12-4-5-16-14(9-13(21)10-22-16)17-3-2-7-26(17)18-6-8-27-19(25-18)15(11-23-27)20(28)24-12/h6,8-12,17H,2-5,7H2,1H3,(H,24,28)/t12-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA G667C mutant (unknown origin) using poly-EAY peptide as substrate in presence of gamma-33ATP by LanthaScreen assay |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus expression system using 5'FAM-EPLYW... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153979
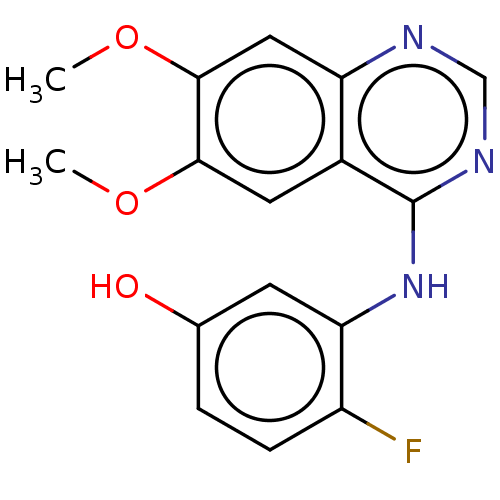
(CHEMBL3774904)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-13-5-9(21)3-4-11(13)17/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM296429

(US10112942, Example 163 | US10112942, Example 166 ...)Show SMILES COc1ccc(CN2C3CC2CN(C3)c2ccc(cn2)-c2cc(OCC(C)(C)O)cn3ncc(C#N)c23)cn1 Show InChI InChI=1S/C29H31N7O3/c1-29(2,37)18-39-24-9-25(28-21(10-30)13-33-36(28)17-24)20-5-6-26(31-12-20)34-15-22-8-23(16-34)35(22)14-19-4-7-27(38-3)32-11-19/h4-7,9,11-13,17,22-23,37H,8,14-16,18H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01280
BindingDB Entry DOI: 10.7270/Q24X5CPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50172078

(LY-2801653 | Merestinib)Show SMILES Cc1ccc(C(=O)Nc2ccc(Oc3cc4cnn(C)c4cc3-c3cn[nH]c3)c(F)c2)c(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C30H22F2N6O3/c1-17-3-9-23(30(40)38(17)22-7-4-20(31)5-8-22)29(39)36-21-6-10-27(25(32)12-21)41-28-11-18-16-35-37(2)26(18)13-24(28)19-14-33-34-15-19/h3-16H,1-2H3,(H,33,34)(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of RON (unknown origin) by FISH assay |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50467574

(MG-516 | MG-91516 | MGCD-516 | MGCD516 | Sitravati...)Show SMILES COCCNCc1ccc(nc1)-c1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2s1 Show InChI InChI=1S/C33H29F2N5O4S/c1-43-15-14-36-18-20-2-8-25(38-19-20)29-17-26-30(45-29)28(10-13-37-26)44-27-9-7-23(16-24(27)35)40-32(42)33(11-12-33)31(41)39-22-5-3-21(34)4-6-22/h2-10,13,16-17,19,36H,11-12,14-15,18H2,1H3,(H,39,41)(H,40,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AXL (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of RET isoform 15 M918T mutant (unknown origin) expressed in mouse BAF3 cells assessed as reduction in cell proliferation supplemented wit... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50299148
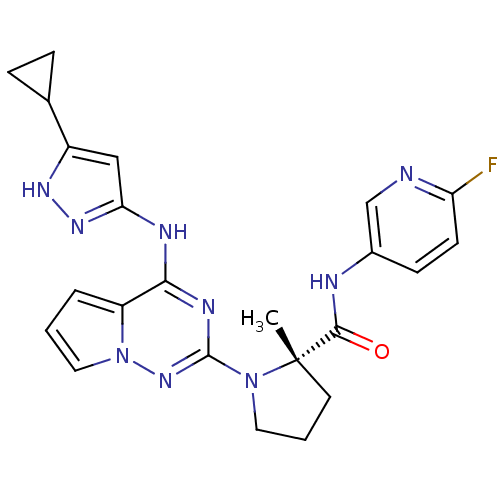
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of IR (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of RET isoform 15 C634R mutant (unknown origin) expressed in mouse BAF3 cells assessed as reduction in cell proliferation supplemented wit... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153902
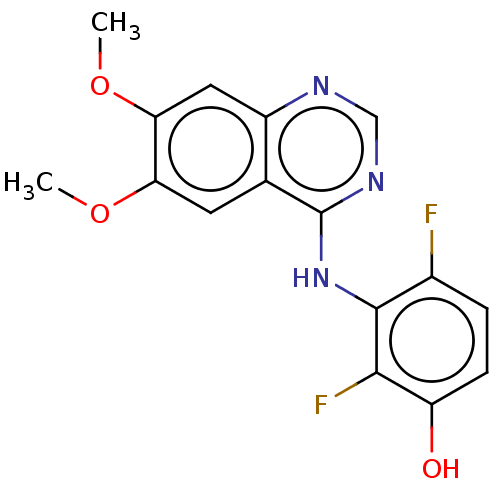
(CHEMBL3775557)Show InChI InChI=1S/C16H13F2N3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(17)3-4-11(22)14(15)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299148

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM136597
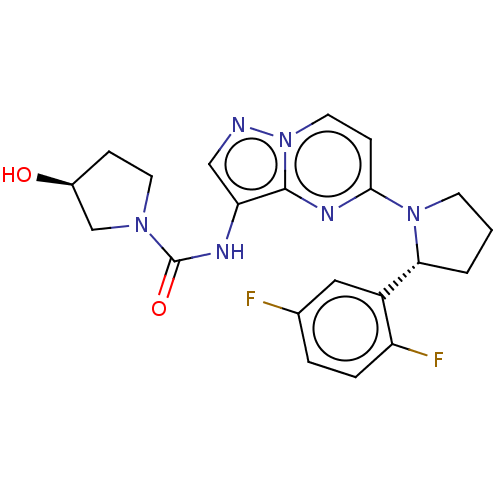
(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) by FISH assay |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153903
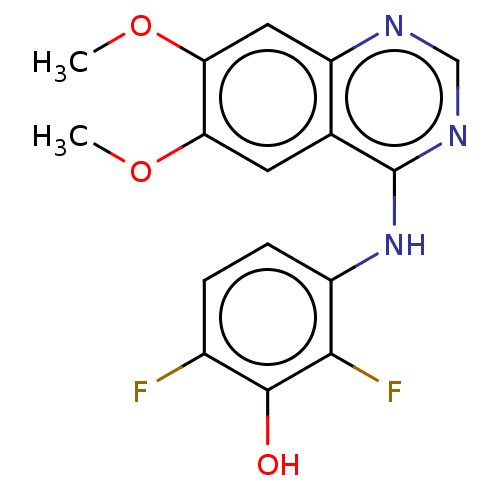
(CHEMBL3775336)Show InChI InChI=1S/C16H13F2N3O3/c1-23-12-5-8-11(6-13(12)24-2)19-7-20-16(8)21-10-4-3-9(17)15(22)14(10)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50507492

(Loxo-195 | Selitrectinib | US10966985, Compound 33...)Show SMILES C[C@@H]1CCc2ncc(F)cc2[C@H]2CCCN2c2ccn3ncc(C(=O)N1)c3n2 Show InChI InChI=1S/C20H21FN6O/c1-12-4-5-16-14(9-13(21)10-22-16)17-3-2-7-26(17)18-6-8-27-19(25-18)15(11-23-27)20(28)24-12/h6,8-12,17H,2-5,7H2,1H3,(H,24,28)/t12-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA G595R mutant (unknown origin) using poly-EAY peptide as substrate in presence of gamma-33ATP by LanthaScreen assay |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4627

(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...)Show InChI InChI=1S/C17H16FN3O3/c1-9-4-11(18)13(6-14(9)22)21-17-10-5-15(23-2)16(24-3)7-12(10)19-8-20-17/h4-8,22H,1-3H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153908
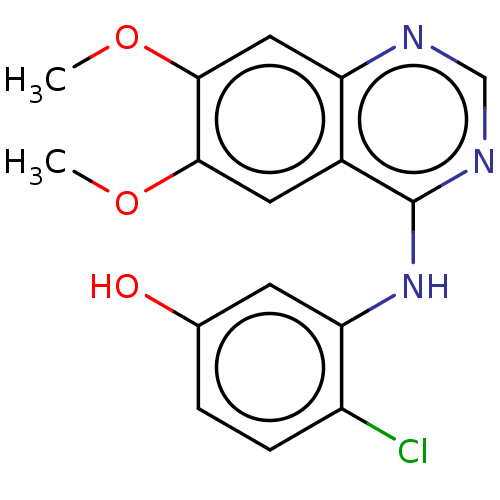
(CHEMBL3774489)Show InChI InChI=1S/C16H14ClN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-13-5-9(21)3-4-11(13)17/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154001
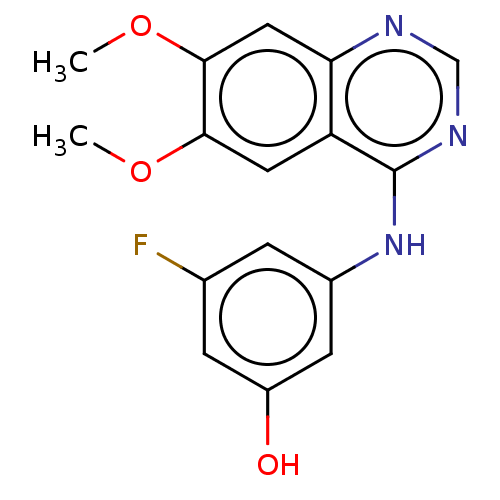
(CHEMBL3775934)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-12-13(7-15(14)23-2)18-8-19-16(12)20-10-3-9(17)4-11(21)5-10/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50193395

(Altiratinib | DCC-2701 | DP-5164)Show SMILES Fc1ccc(NC(=O)C2(CC2)C(=O)Nc2cc(F)c(Oc3ccnc(NC(=O)C4CC4)c3)cc2F)cc1 Show InChI InChI=1S/C26H21F3N4O4/c27-15-3-5-16(6-4-15)31-24(35)26(8-9-26)25(36)32-20-12-19(29)21(13-18(20)28)37-17-7-10-30-22(11-17)33-23(34)14-1-2-14/h3-7,10-14H,1-2,8-9H2,(H,31,35)(H,32,36)(H,30,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cMET (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged VEGFR2 cytoplasmic domain (789 to 1356 residues) expressed in baculovirus expression system using peptide ... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50396243
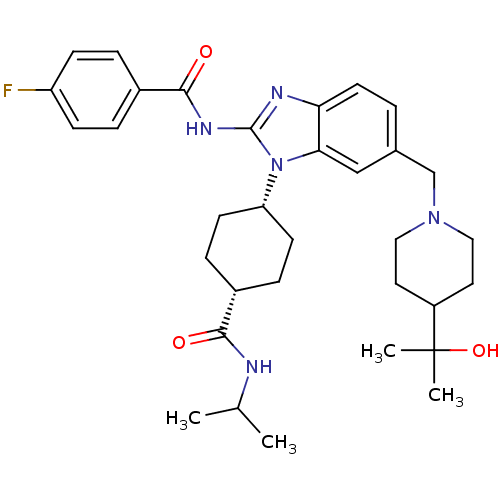
(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKC (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50396243

(CHEMBL2172308 | US10370379, Compound TSR-011)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2ccc(F)cc2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(21.34,-25.87,;19.83,-25.56,;19.35,-24.09,;18.81,-26.7,;17.3,-26.39,;16.27,-27.53,;16.82,-24.92,;15.31,-24.6,;14.83,-23.15,;15.87,-22,;17.37,-22.31,;17.85,-23.77,;15.38,-20.54,;16.29,-19.28,;17.83,-19.27,;18.59,-17.94,;17.81,-16.61,;20.13,-17.93,;20.9,-19.25,;22.44,-19.25,;23.2,-17.91,;24.74,-17.9,;22.42,-16.57,;20.88,-16.59,;15.37,-18.03,;13.9,-18.52,;12.56,-17.76,;11.23,-18.53,;11.23,-20.07,;9.9,-20.84,;8.56,-20.07,;8.56,-18.53,;7.24,-17.76,;5.9,-18.52,;5.9,-20.06,;7.23,-20.84,;4.56,-17.74,;3.79,-16.41,;5.33,-16.4,;3.23,-18.51,;12.56,-20.84,;13.9,-20.07,)| Show InChI InChI=1S/C33H44FN5O3/c1-21(2)35-30(40)24-8-12-27(13-9-24)39-29-19-22(20-38-17-15-25(16-18-38)33(3,4)42)5-14-28(29)36-32(39)37-31(41)23-6-10-26(34)11-7-23/h5-7,10-11,14,19,21,24-25,27,42H,8-9,12-13,15-18,20H2,1-4H3,(H,35,40)(H,36,37,41)/t24-,27+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKB (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154249
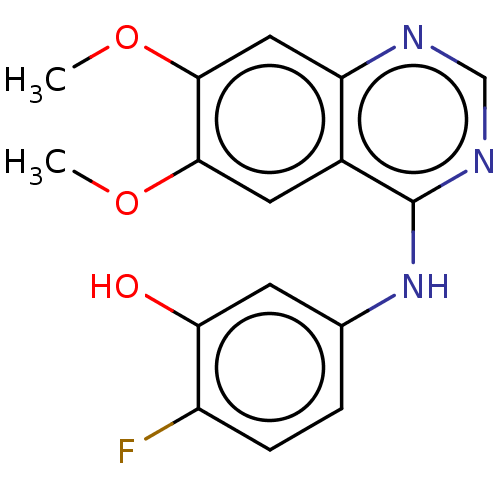
(CHEMBL3775879)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-9-3-4-11(17)13(21)5-9/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154246
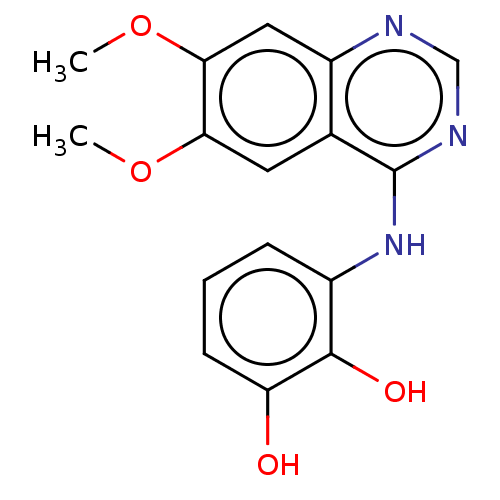
(CHEMBL3774580)Show InChI InChI=1S/C16H15N3O4/c1-22-13-6-9-11(7-14(13)23-2)17-8-18-16(9)19-10-4-3-5-12(20)15(10)21/h3-8,20-21H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
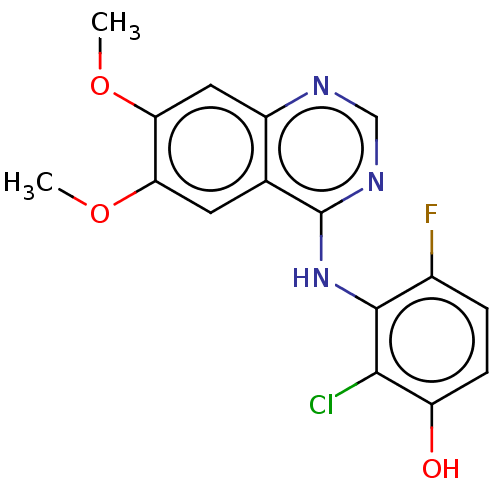
(Homo sapiens (Human)) | BDBM50153895
(CHEMBL3774953)Show InChI InChI=1S/C16H13ClFN3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(18)3-4-11(22)14(15)17/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50299148

((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKB (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50514844

(CHEMBL4473213)Show SMILES CC(C)(C)c1cc(NC(=O)Cc2ccc(cc2)-n2cnc3cc(ccc23)-c2ccc(cc2)S(C)(=O)=O)no1 Show InChI InChI=1S/C29H28N4O4S/c1-29(2,3)26-17-27(32-37-26)31-28(34)15-19-5-10-22(11-6-19)33-18-30-24-16-21(9-14-25(24)33)20-7-12-23(13-8-20)38(4,35)36/h5-14,16-18H,15H2,1-4H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli "Federico II"
Curated by ChEMBL
| Assay Description
Inhibition of RET isoform 9 C634R mutant (unknown origin) expressed in mouse NIH/3T3 cells assessed as reduction in cell proliferation supplemented w... |
J Med Chem 63: 4506-4516 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01336
BindingDB Entry DOI: 10.7270/Q2154MD0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM4622
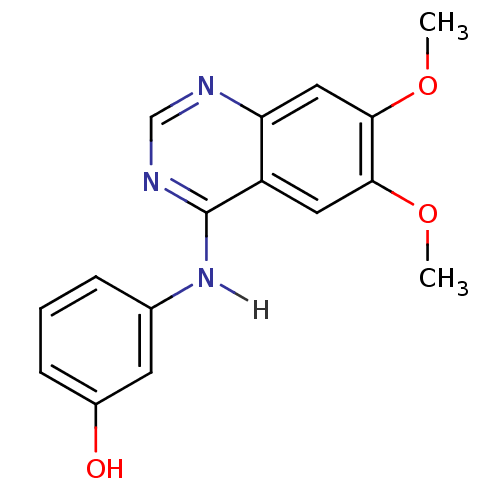
(3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol | An...)Show InChI InChI=1S/C16H15N3O3/c1-21-14-7-12-13(8-15(14)22-2)17-9-18-16(12)19-10-4-3-5-11(20)6-10/h3-9,20H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50193395

(Altiratinib | DCC-2701 | DP-5164)Show SMILES Fc1ccc(NC(=O)C2(CC2)C(=O)Nc2cc(F)c(Oc3ccnc(NC(=O)C4CC4)c3)cc2F)cc1 Show InChI InChI=1S/C26H21F3N4O4/c27-15-3-5-16(6-4-15)31-24(35)26(8-9-26)25(36)32-20-12-19(29)21(13-18(20)28)37-17-7-10-30-22(11-17)33-23(34)14-1-2-14/h3-7,10-14H,1-2,8-9H2,(H,31,35)(H,32,36)(H,30,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKB (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM26477
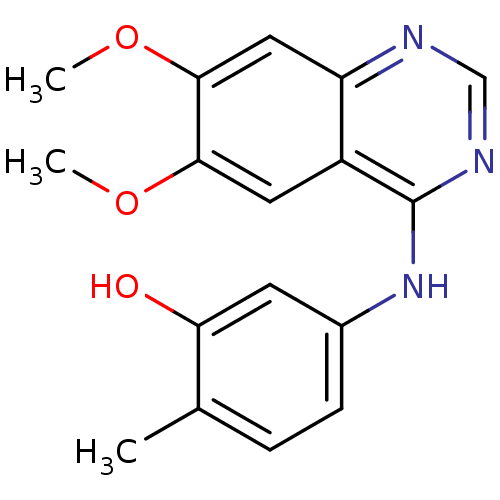
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-2-methylph...)Show InChI InChI=1S/C17H17N3O3/c1-10-4-5-11(6-14(10)21)20-17-12-7-15(22-2)16(23-3)8-13(12)18-9-19-17/h4-9,21H,1-3H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50172078

(LY-2801653 | Merestinib)Show SMILES Cc1ccc(C(=O)Nc2ccc(Oc3cc4cnn(C)c4cc3-c3cn[nH]c3)c(F)c2)c(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C30H22F2N6O3/c1-17-3-9-23(30(40)38(17)22-7-4-20(31)5-8-22)29(39)36-21-6-10-27(25(32)12-21)41-28-11-18-16-35-37(2)26(18)13-24(28)19-14-33-34-15-19/h3-16H,1-2H3,(H,33,34)(H,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cMET (unknown origin) by FISH assay |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153901
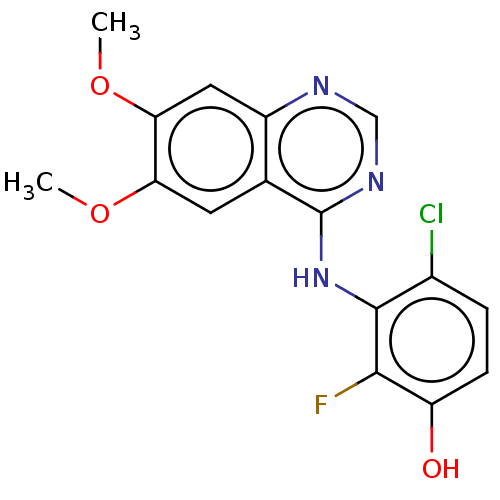
(CHEMBL3775511)Show InChI InChI=1S/C16H13ClFN3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(17)3-4-11(22)14(15)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153905
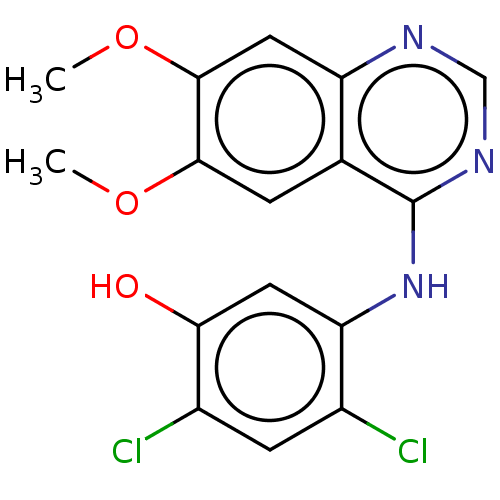
(CHEMBL3775190)Show InChI InChI=1S/C16H13Cl2N3O3/c1-23-14-3-8-11(6-15(14)24-2)19-7-20-16(8)21-12-5-13(22)10(18)4-9(12)17/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50467574

(MG-516 | MG-91516 | MGCD-516 | MGCD516 | Sitravati...)Show SMILES COCCNCc1ccc(nc1)-c1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2s1 Show InChI InChI=1S/C33H29F2N5O4S/c1-43-15-14-36-18-20-2-8-25(38-19-20)29-17-26-30(45-29)28(10-13-37-26)44-27-9-7-23(16-24(27)35)40-32(42)33(11-12-33)31(41)39-22-5-3-21(34)4-6-22/h2-10,13,16-17,19,36H,11-12,14-15,18H2,1H3,(H,39,41)(H,40,42) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50467574

(MG-516 | MG-91516 | MGCD-516 | MGCD516 | Sitravati...)Show SMILES COCCNCc1ccc(nc1)-c1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2s1 Show InChI InChI=1S/C33H29F2N5O4S/c1-43-15-14-36-18-20-2-8-25(38-19-20)29-17-26-30(45-29)28(10-13-37-26)44-27-9-7-23(16-24(27)35)40-32(42)33(11-12-33)31(41)39-22-5-3-21(34)4-6-22/h2-10,13,16-17,19,36H,11-12,14-15,18H2,1H3,(H,39,41)(H,40,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50585629

(CHEMBL5087283)Show SMILES CC(C)c1ccc(NC(=O)Cc2ccc(Nc3nc(Nc4cc(C)n[nH]4)cc(n3)N3CCN(C)CC3)cc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01280
BindingDB Entry DOI: 10.7270/Q24X5CPK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50585635

(CHEMBL5074882)Show SMILES C[C@H](NC(=O)Cc1ccc(Nc2nc(Nc3cc(C)n[nH]3)cc(n2)N2CCN(C)CC2)cc1)c1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01280
BindingDB Entry DOI: 10.7270/Q24X5CPK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50585640
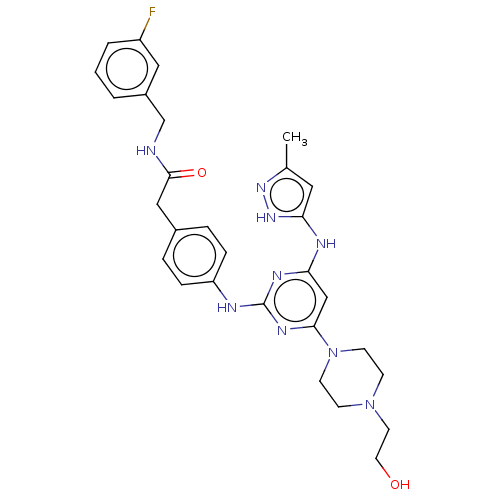
(CHEMBL5080296)Show SMILES Cc1cc(Nc2cc(nc(Nc3ccc(CC(=O)NCc4cccc(F)c4)cc3)n2)N2CCN(CCO)CC2)[nH]n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01280
BindingDB Entry DOI: 10.7270/Q24X5CPK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50585633
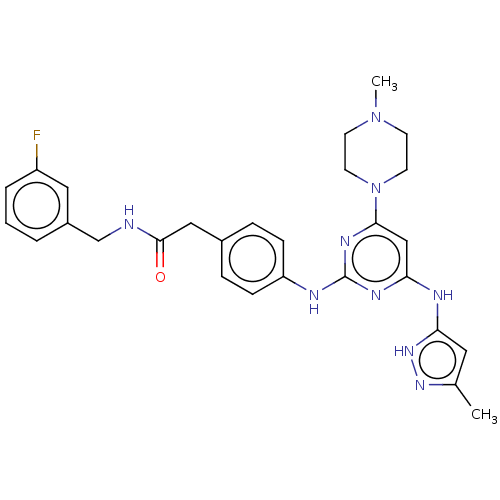
(CHEMBL5073804)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Nc2ccc(CC(=O)NCc3cccc(F)c3)cc2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01280
BindingDB Entry DOI: 10.7270/Q24X5CPK |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50467574

(MG-516 | MG-91516 | MGCD-516 | MGCD516 | Sitravati...)Show SMILES COCCNCc1ccc(nc1)-c1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)c2s1 Show InChI InChI=1S/C33H29F2N5O4S/c1-43-15-14-36-18-20-2-8-25(38-19-20)29-17-26-30(45-29)28(10-13-37-26)44-27-9-7-23(16-24(27)35)40-32(42)33(11-12-33)31(41)39-22-5-3-21(34)4-6-22/h2-10,13,16-17,19,36H,11-12,14-15,18H2,1H3,(H,39,41)(H,40,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cKIT (unknown origin) |
J Med Chem 62: 1731-1760 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01092
BindingDB Entry DOI: 10.7270/Q29Z986G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50585632
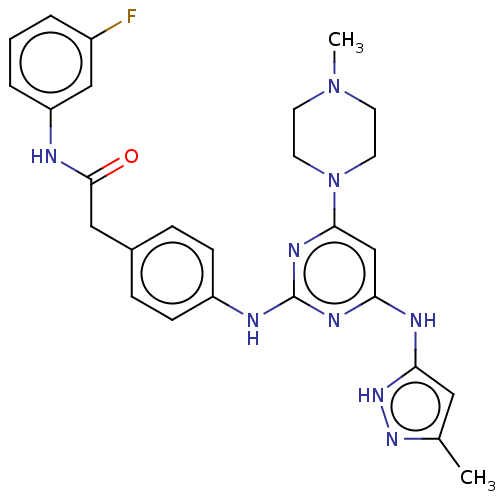
(CHEMBL5070593)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Nc2ccc(CC(=O)Nc3cccc(F)c3)cc2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01280
BindingDB Entry DOI: 10.7270/Q24X5CPK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50585631

(CHEMBL5078090)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Nc2ccc(CC(=O)Nc3ccc(F)cc3)cc2)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-3C fused human RET (705 to 1013 residues) expressed in sf9 baculovirus expression system using 5-FAM- peptide as substrate preincub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01280
BindingDB Entry DOI: 10.7270/Q24X5CPK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data