

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341578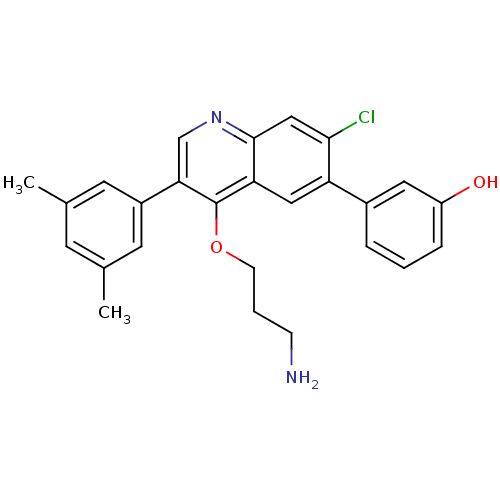 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341575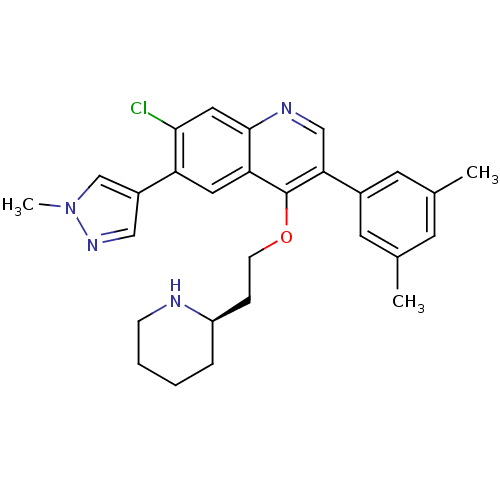 (7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341572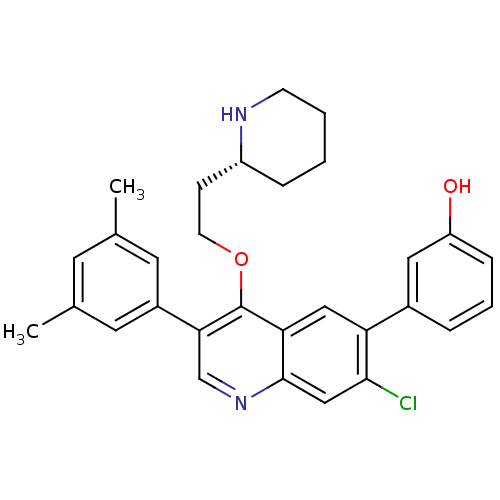 (3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341574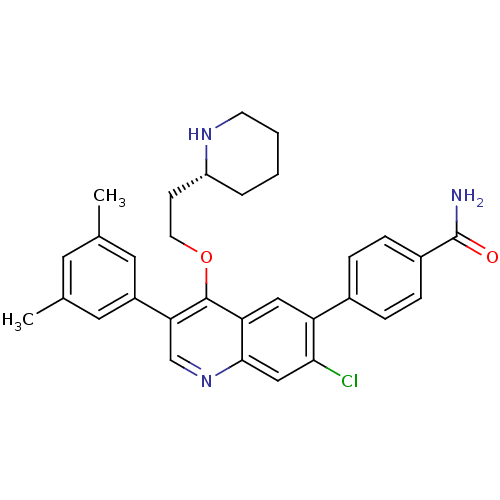 (3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341562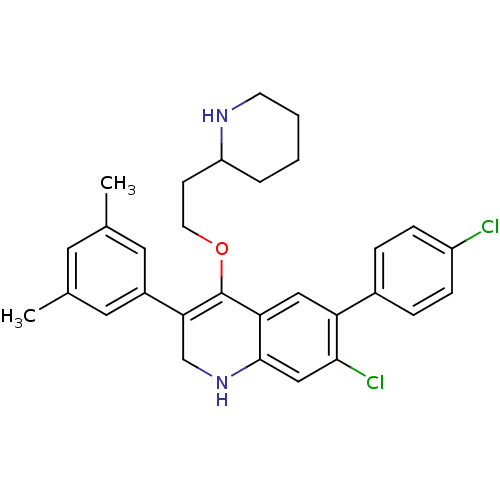 (7-chloro-6-(4-chlorophenyl)-3-(3,5-dimethylphenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341571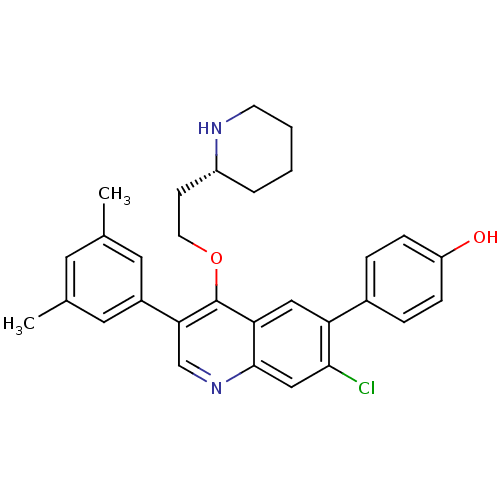 (4-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341577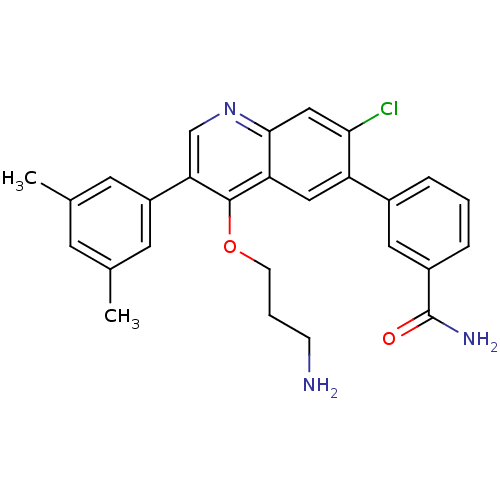 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341573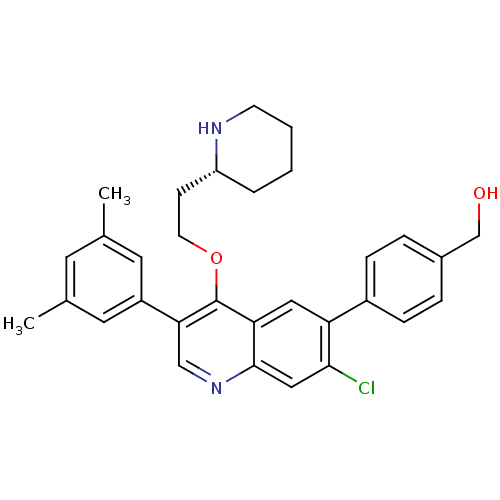 (CHEMBL1766098 | {4-[7-Chloro-3-(3,5-dimethylphenyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341570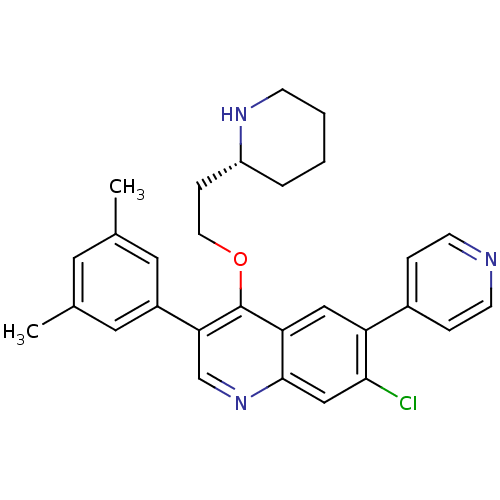 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341569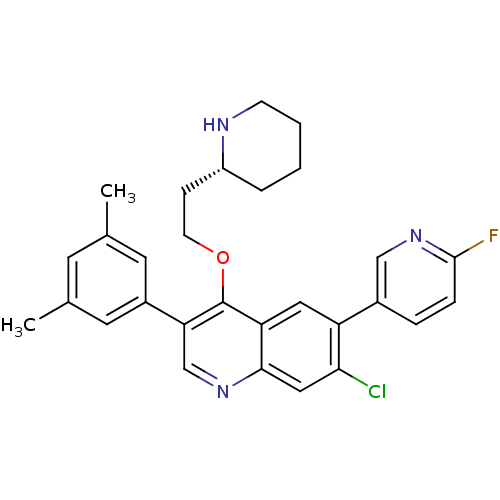 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-fluoropyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341568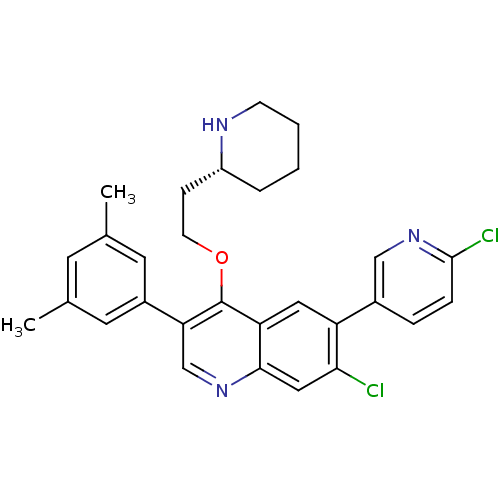 (7-Chloro-6-(6-chloropyridin-3-yl)-3-(3,5-dimethylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341580 (CHEMBL1766105 | [(4-{2-[(2R)-Piperidin-2-yl]ethoxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341564 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341579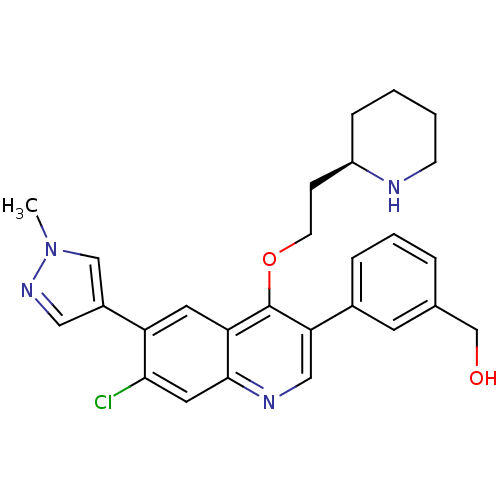 (CHEMBL1766104 | {3-[7-Chloro-6-(1-methyl-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341563 (7-chloro-3-(3,5-dimethylphenyl)-6-phenyl-4-(2-(pip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM85790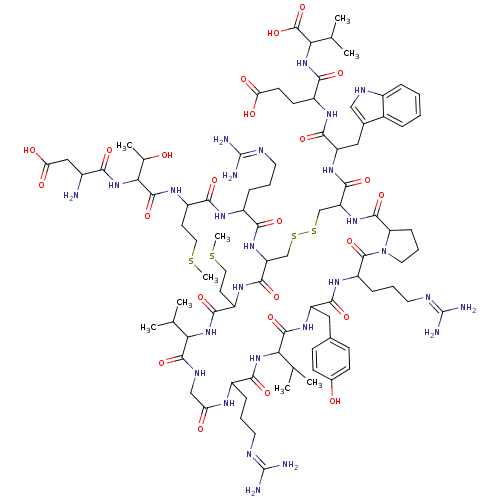 (Salmon MCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341565 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM85789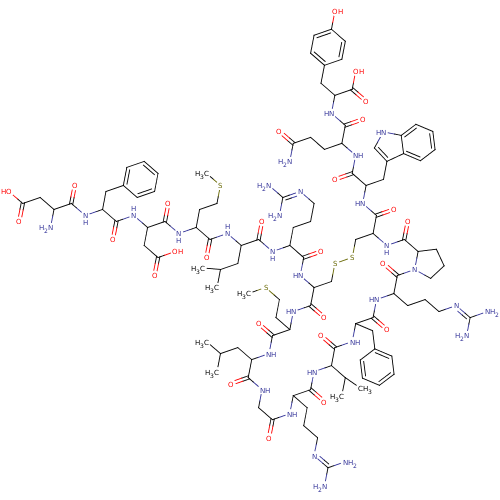 ([Phe13,Tyr19]MCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341566 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341567 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-methoxypyridi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 2 (Homo sapiens (Human)) | BDBM85789 ([Phe13,Tyr19]MCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 2 (Homo sapiens (Human)) | BDBM85788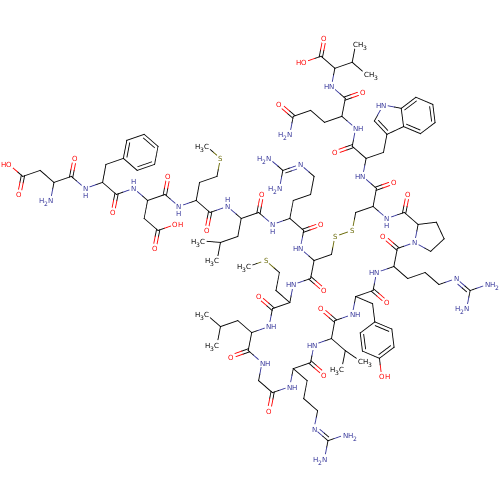 (MCH | hMCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341576 ((S)-7-chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341560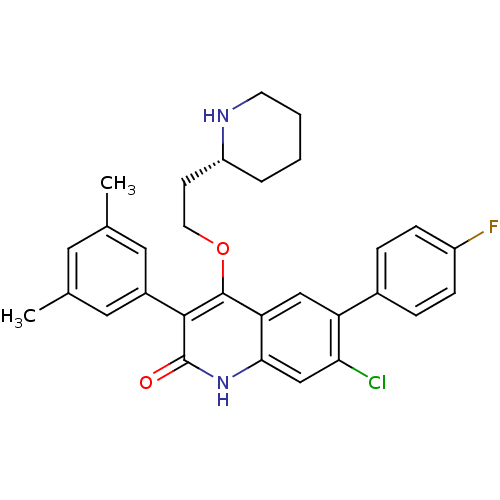 ((R)-7-chloro-3-(3,5-dimethylphenyl)-6-(4-fluorophe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203736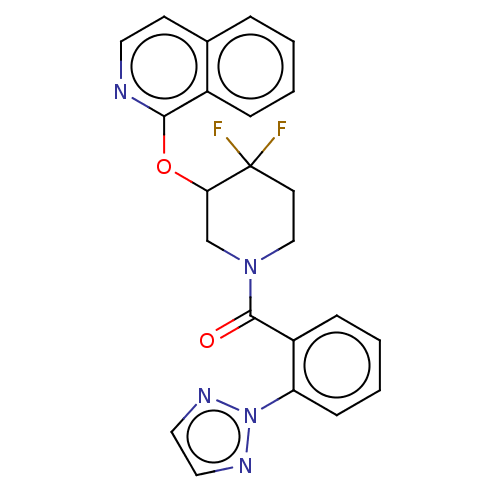 (CHEMBL3915136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203756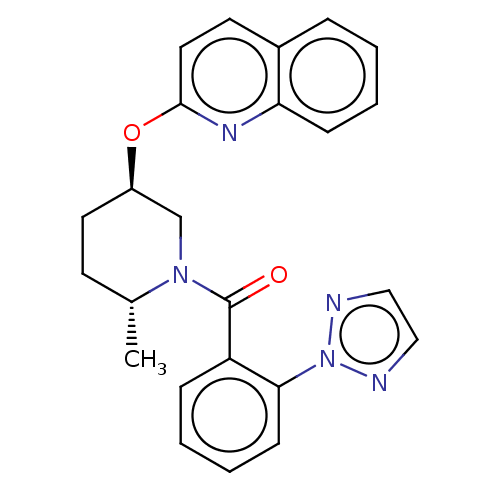 (CHEMBL3951917) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203741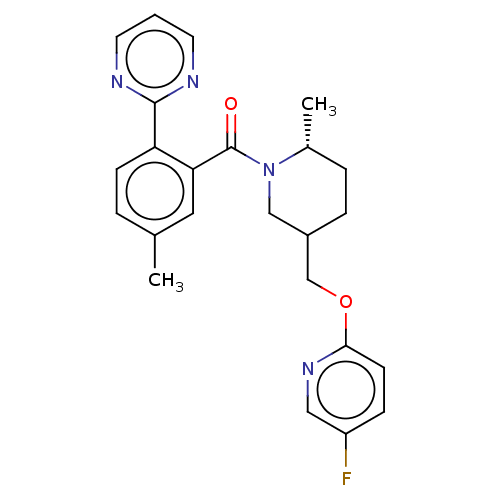 (CHEMBL3928138) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203768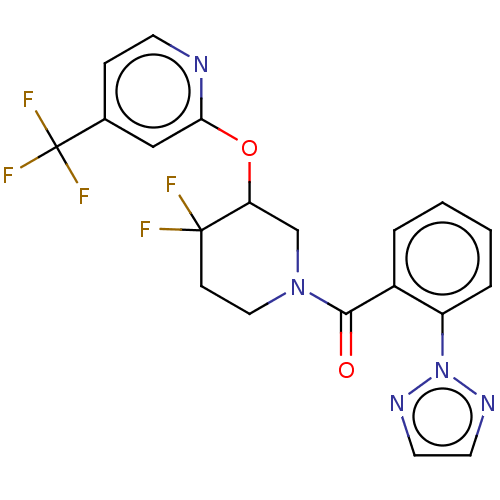 (CHEMBL3959896) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203761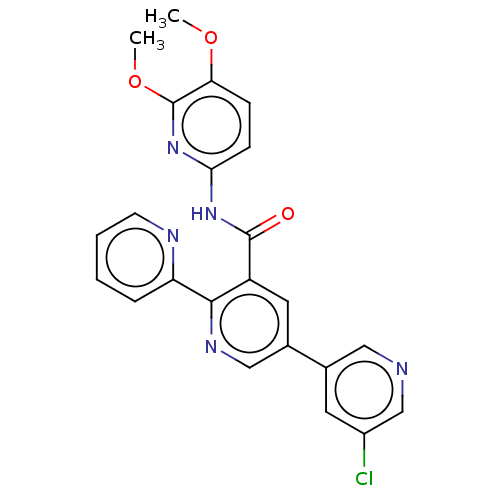 (CHEMBL3891586) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50384416 (CHEMBL2111553 | CHEMBL291536 | SB-334867) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of N6,10-rhodamine green-tagged orexin-A from human OX1 receptor expressed in CHO cells measured after 30 mins by syto62 staining based ... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341561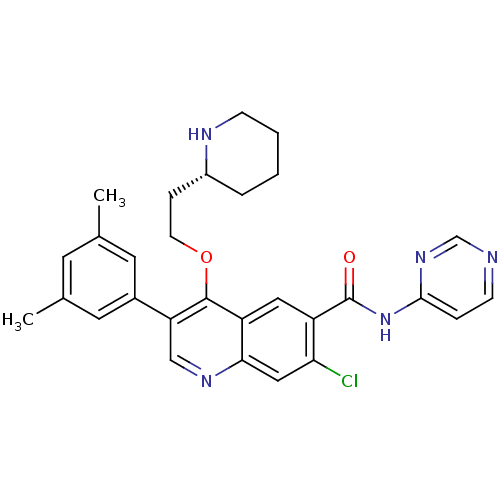 ((R)-7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203725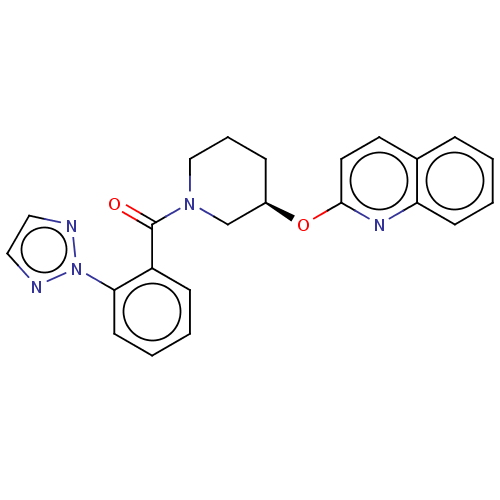 (CHEMBL3972735) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203950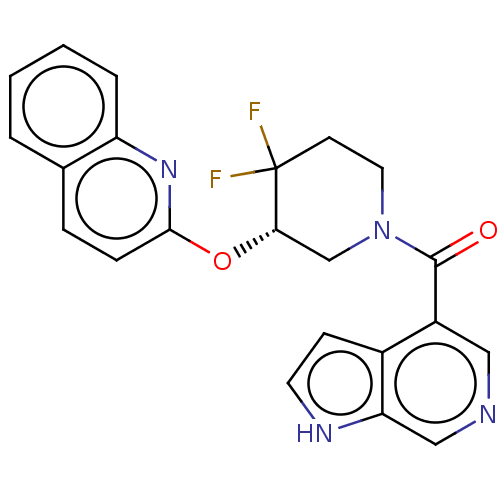 (CHEMBL3900983) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203751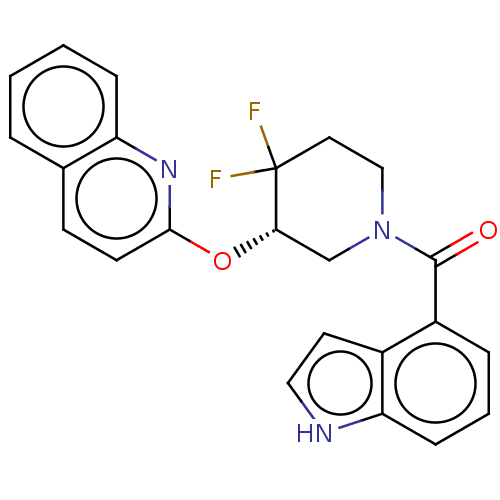 (CHEMBL3971815) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203949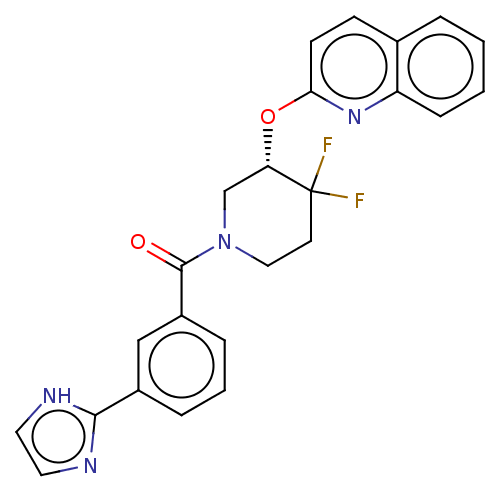 (CHEMBL3930252) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203743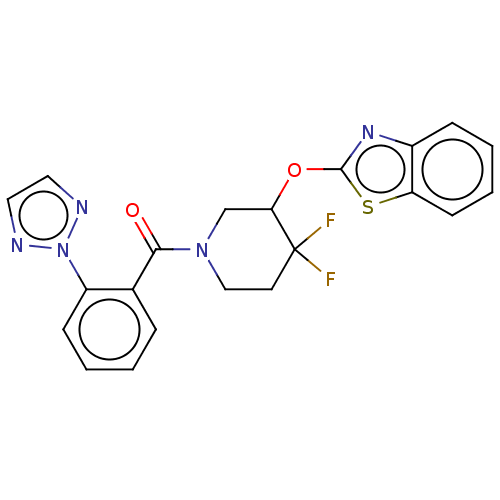 (CHEMBL3923070) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203758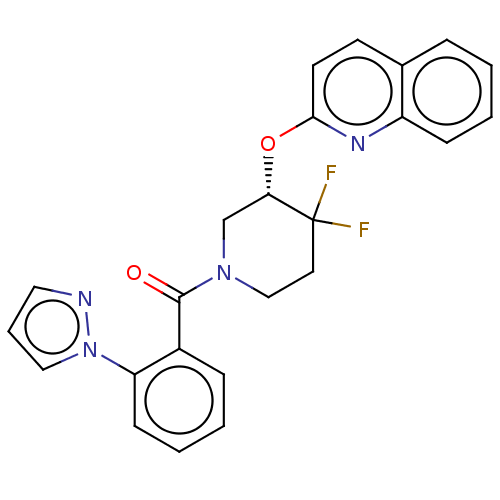 (CHEMBL3892723) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203760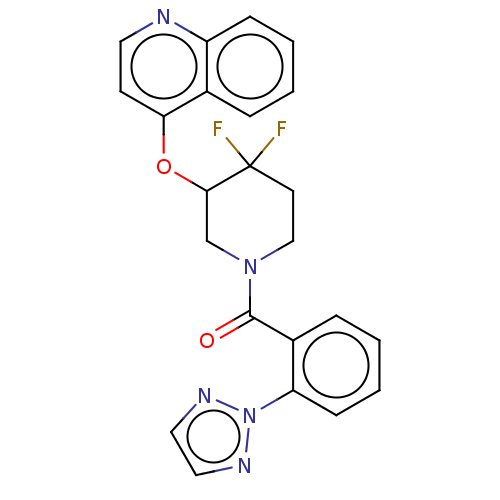 (CHEMBL3924149) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 277 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203748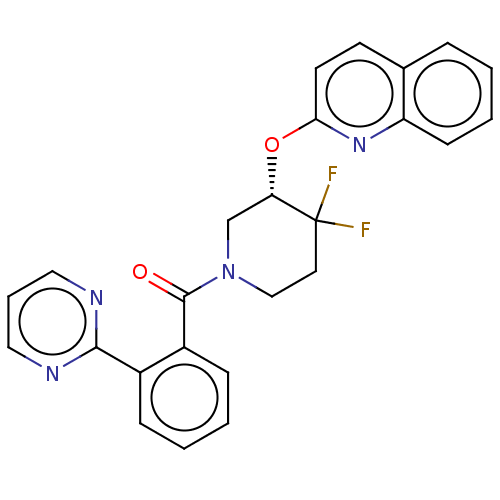 (CHEMBL3919876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203737 (CHEMBL3929466) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50438822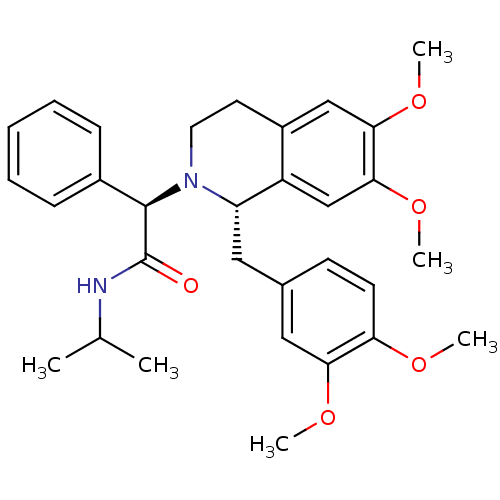 (CHEMBL2413367) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 2 (Homo sapiens (Human)) | BDBM85790 (Salmon MCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203929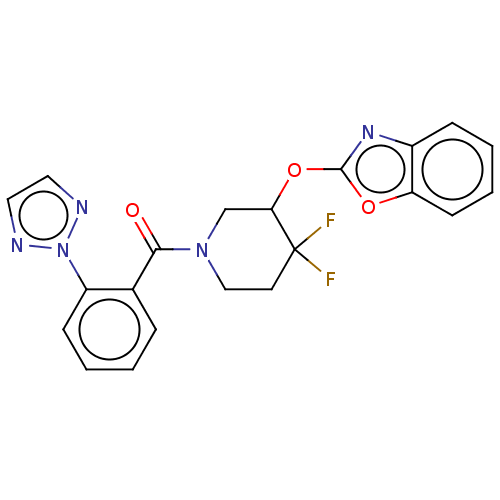 (CHEMBL3906164) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203724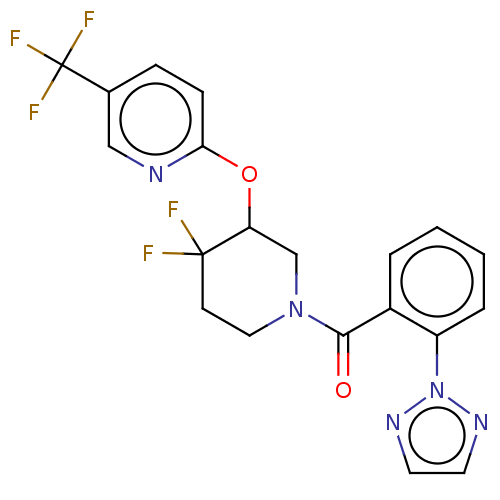 (CHEMBL3941376) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50060937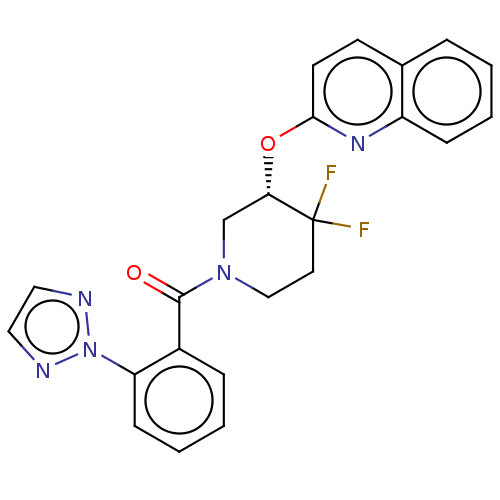 (CHEMBL3394848) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 521 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203729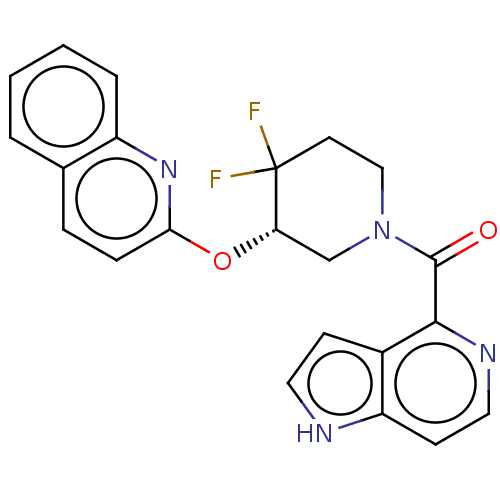 (CHEMBL3928942) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 521 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203750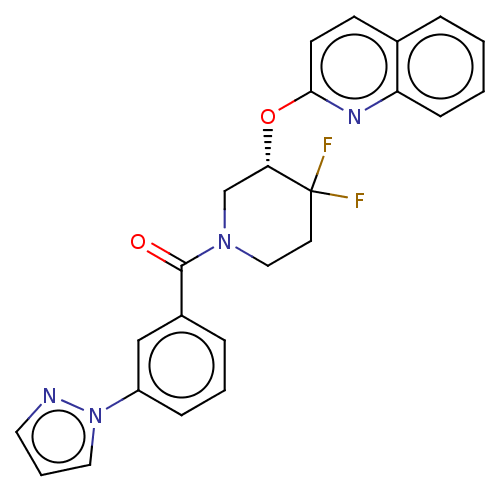 (CHEMBL3958483) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50384416 (CHEMBL2111553 | CHEMBL291536 | SB-334867) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 835 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human OX2 receptor expressed in CHO cells by fluorescence assay | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203755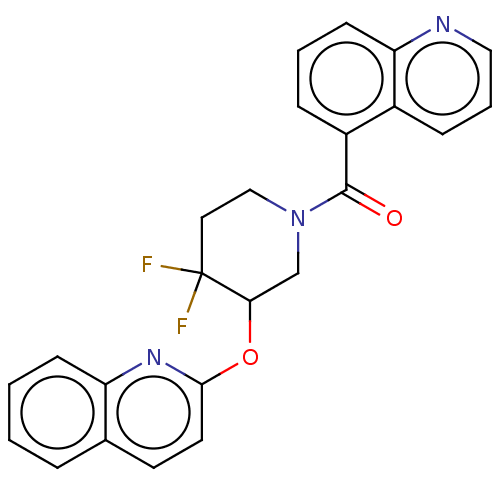 (CHEMBL3900658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50203728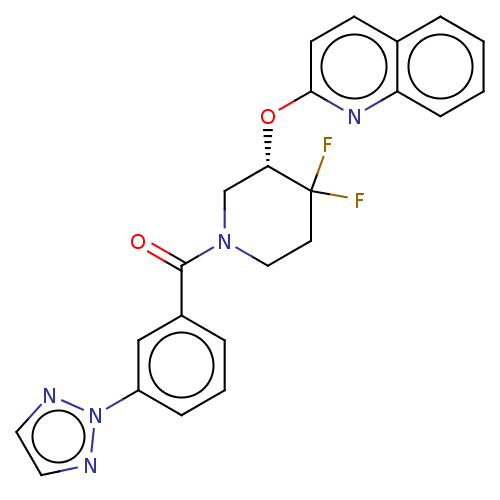 (CHEMBL3944339) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... | Bioorg Med Chem Lett 26: 5809-5814 (2016) Article DOI: 10.1016/j.bmcl.2016.10.019 BindingDB Entry DOI: 10.7270/Q2MS3VRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |