

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50113787 (CHEMBL81056 | S-(2-{2-[2-(4-Carbamimidoyl-phenoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50201438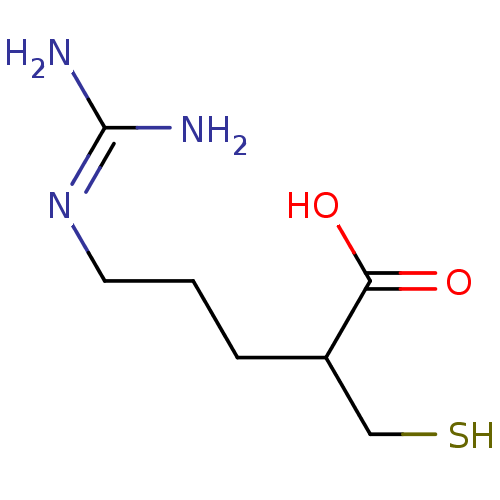 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic carboxypeptidase B | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113790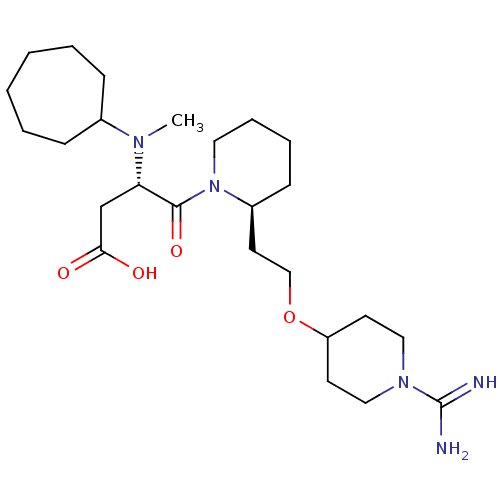 (CHEMBL84389 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113791 (CHEMBL84229 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113785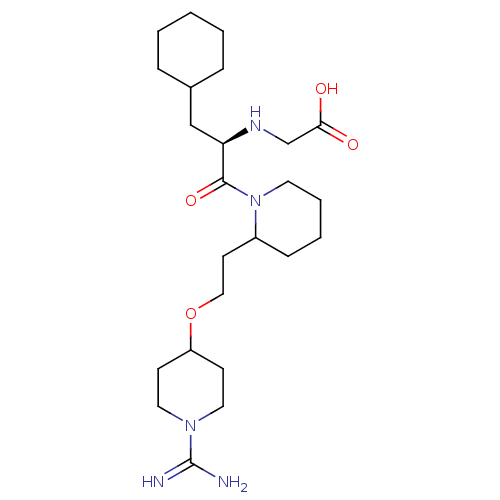 (CHEMBL309670 | RS-(2-{2-[2-(1-Carbamimidoyl-piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470598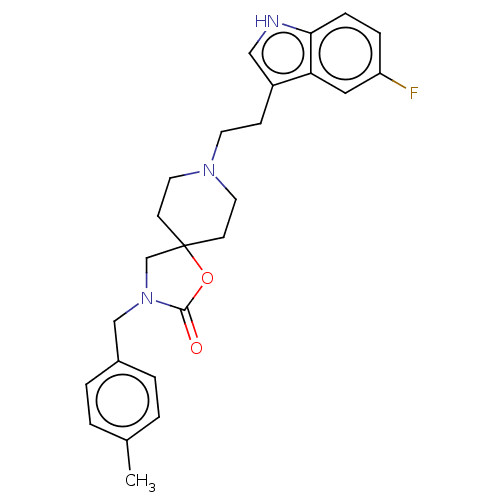 (CHEMBL126050) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113799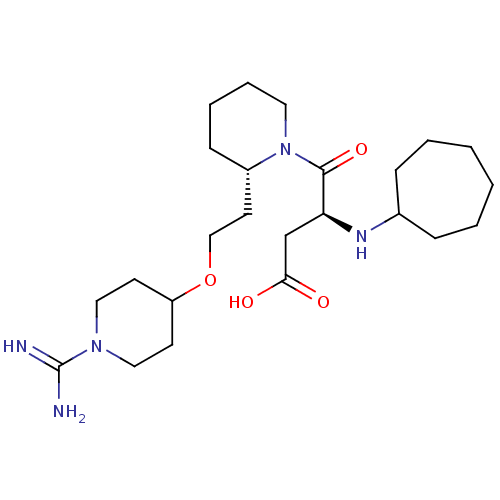 (CHEMBL309403 | S-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470604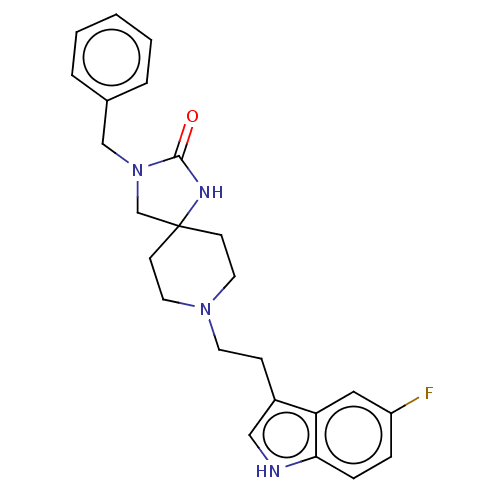 (CHEMBL338825) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470590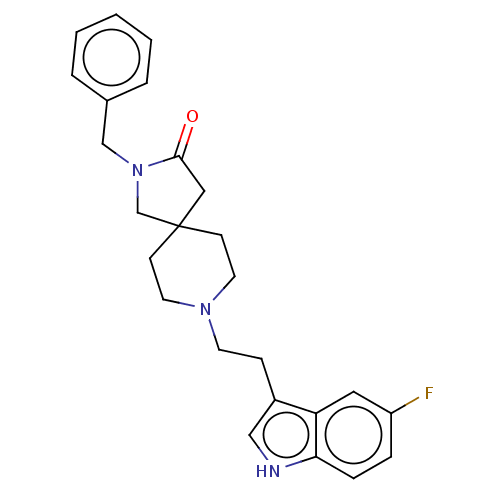 (CHEMBL341357) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113794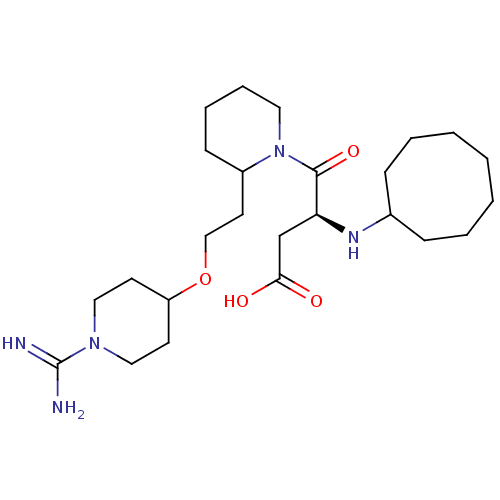 (CHEMBL82658 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470591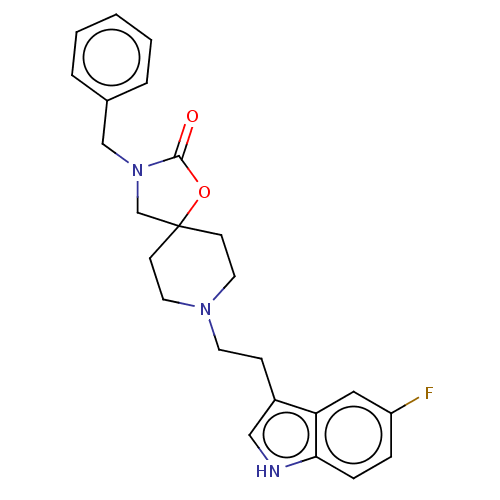 (CHEMBL124208) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470583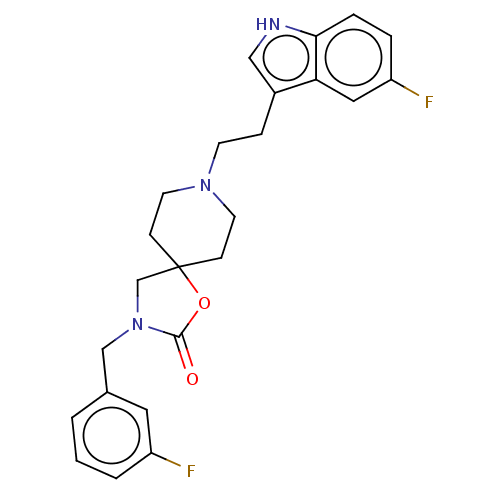 (CHEMBL125696) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470596 (CHEMBL125310) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113802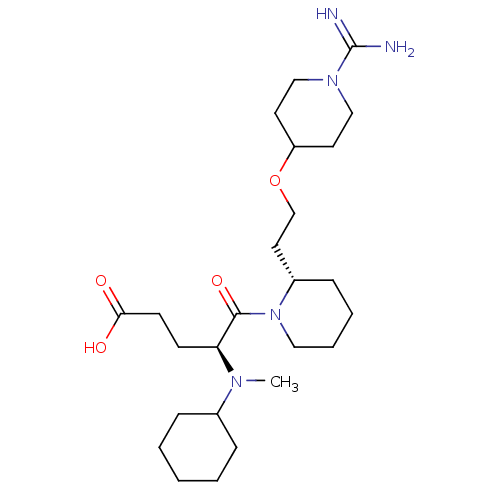 (5-{2-[2-(1-Carbamimidoyl-piperidin-4-yloxy)-ethyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase N catalytic chain (Homo sapiens (Human)) | BDBM50201438 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human plasma carboxypeptidase N | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113792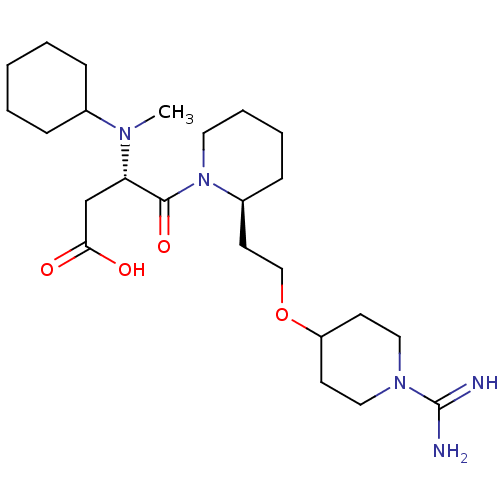 (CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470587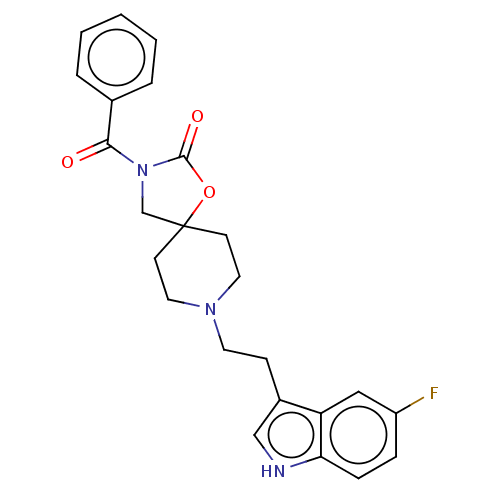 (CHEMBL124648) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50470591 (CHEMBL124208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for binding affinity against Tachykinin receptor 2 from guinea pig trachea | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Bos taurus) | BDBM50113792 (CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory effect on plasmin in bovine plasma | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470603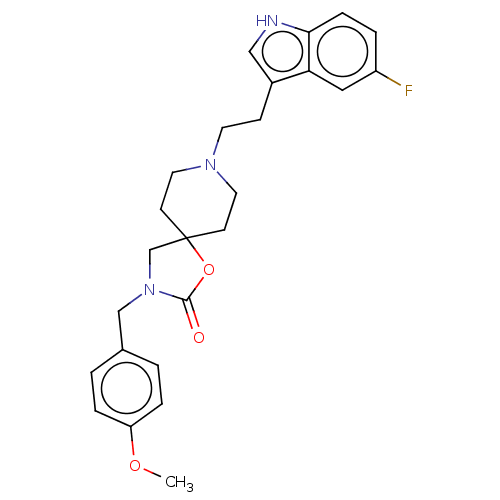 (CHEMBL124013) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50201438 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human TAFIa | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470605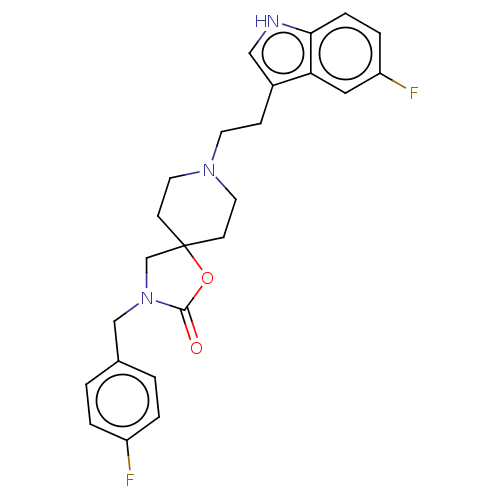 (CHEMBL122169) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM50113792 (CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory effect on Coagulation factor Xa (FXa) from bovine plasma | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470582 (CHEMBL126043) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470599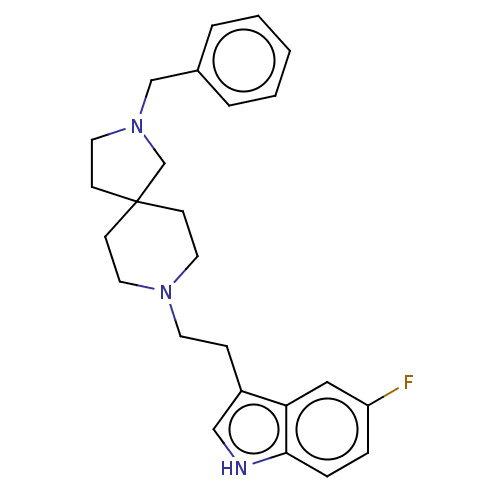 (CHEMBL330766) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470595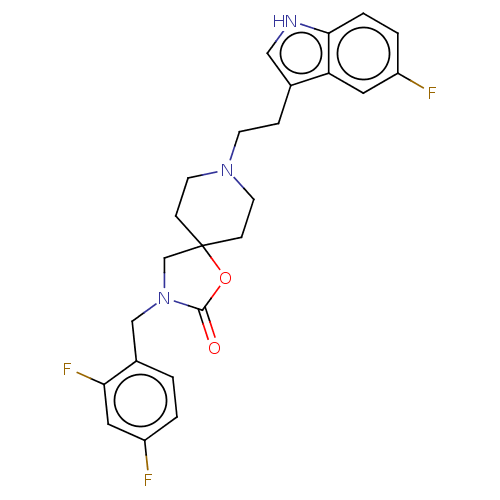 (CHEMBL122019) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113798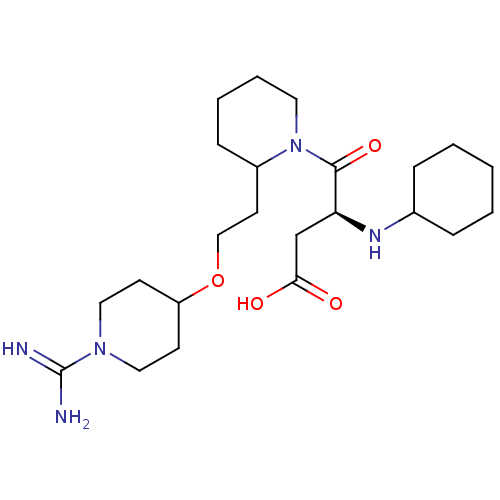 (CHEMBL82535 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470592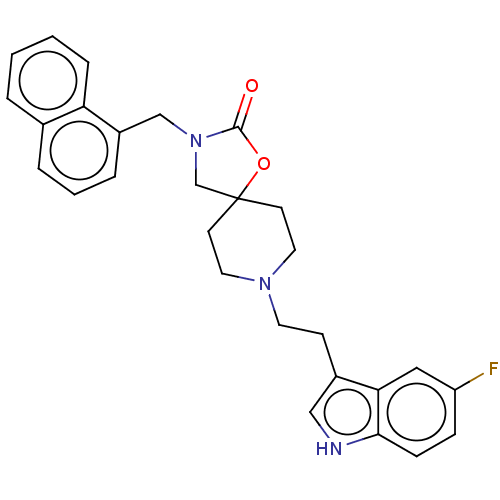 (CHEMBL339311) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50470591 (CHEMBL124208) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for binding affinity against Tachykinin receptor 2 from human expressed in CHO cells | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113796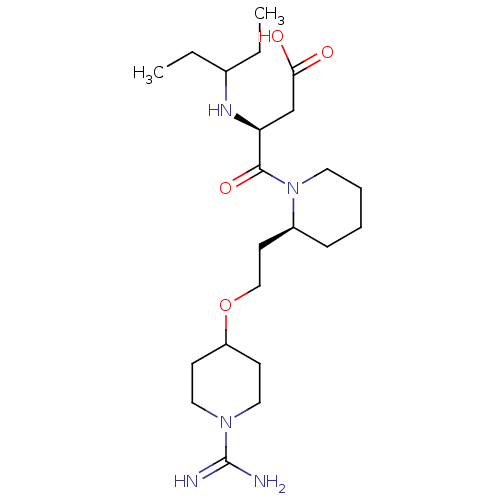 (CHEMBL418923 | S-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470597 (CHEMBL330826) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470606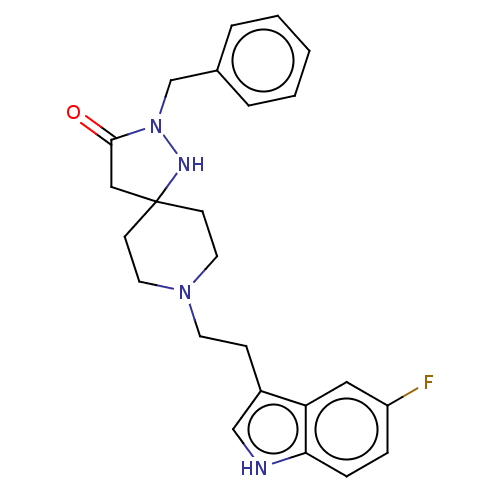 (CHEMBL446099) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470581 (CHEMBL341008) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50226610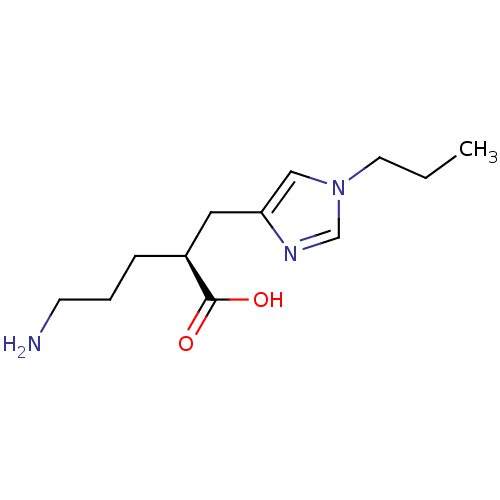 ((2S)-5-AMINO-2-[(1-PROPYL-1H-IMIDAZOL-4-YL)METHYL]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human TAFIa | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50109593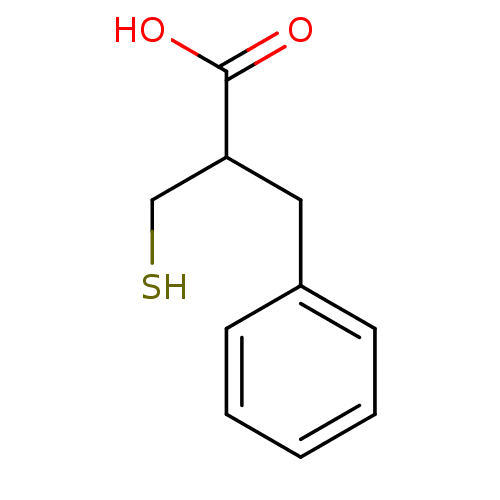 (2-Benzyl-3-mercapto-propionic acid | 2-Mercaptomet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of bovine pancreatic carboxypeptidase A | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470584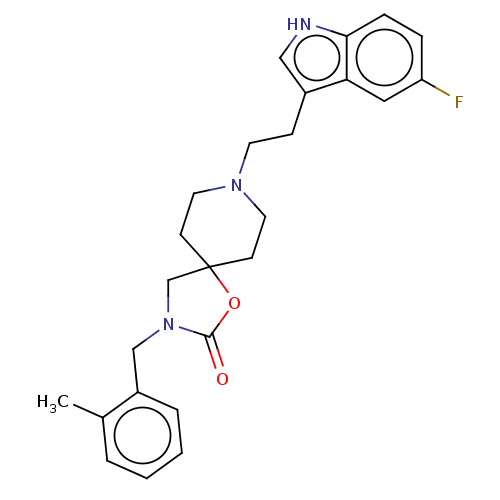 (CHEMBL125001) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113793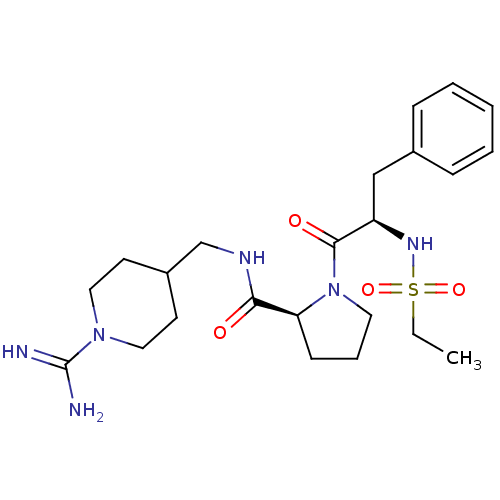 (1-(2-Ethanesulfonylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470585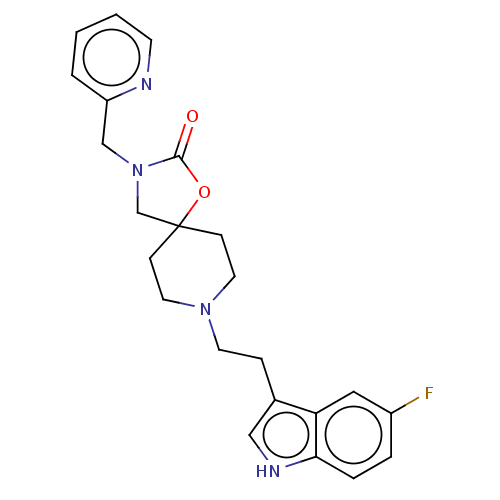 (CHEMBL415518) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113788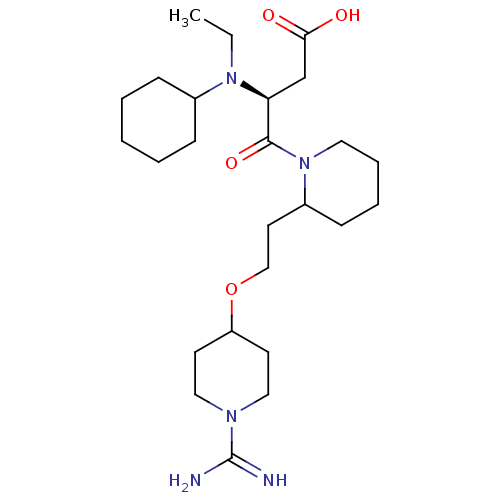 (4-{2-[2-(1-Carbamimidoyl-piperidin-4-yloxy)-ethyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113801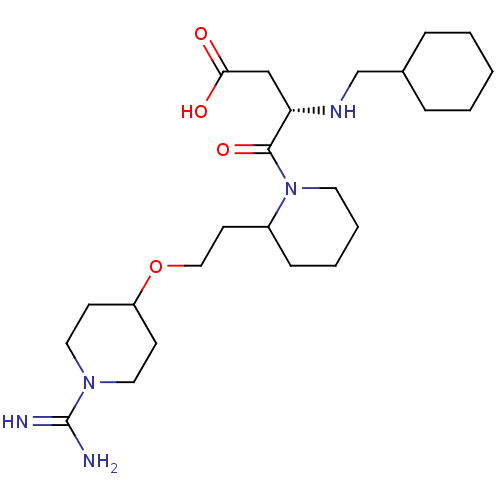 (CHEMBL81436 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470608 (CHEMBL338030) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470601 (CHEMBL123731) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470607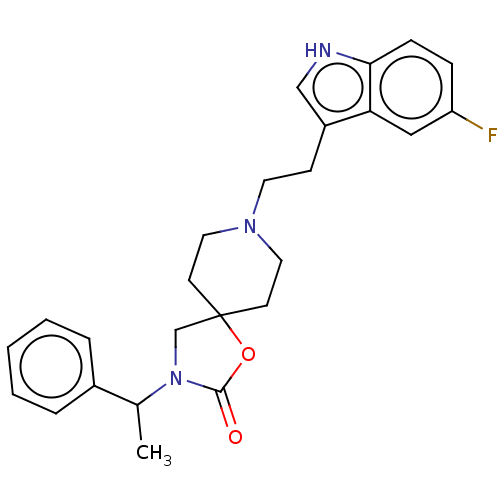 (CHEMBL124624) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113795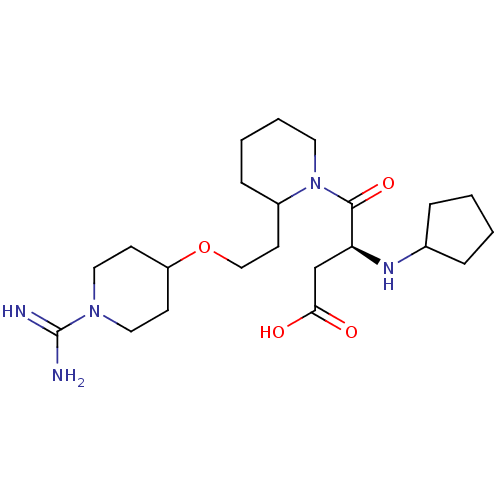 (CHEMBL81251 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50226605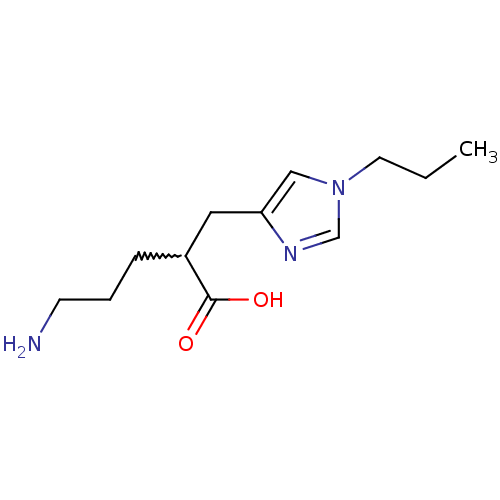 ((+/-)-5-amino-2-((1-propyl-1H-imidazol-4-yl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human TAFIa | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113786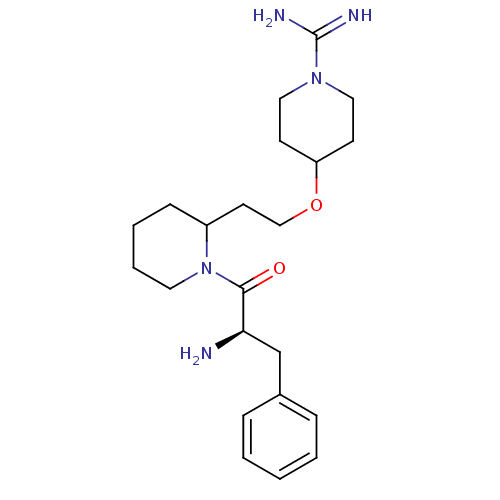 (4-{2-[1-(2-Amino-3-phenyl-propionyl)-piperidin-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50226598 ((S)-2-(2-aminoethylamino)-3-(1-butyl-1H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human TAFIa | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50470589 (CHEMBL331011) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity against tachykinin receptor 2 from rat colon. | J Med Chem 38: 3772-9 (1995) Article DOI: 10.1021/jm00019a006 BindingDB Entry DOI: 10.7270/Q2JM2DCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50226606 ((S)-2-(2-aminoethylamino)-3-(1-propyl-1H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human TAFIa | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113787 (CHEMBL81056 | S-(2-{2-[2-(4-Carbamimidoyl-phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human trypsin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 311 total ) | Next | Last >> |