 Found 252 hits with Last Name = 'mckay' and Initial = 'f'
Found 252 hits with Last Name = 'mckay' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50477760
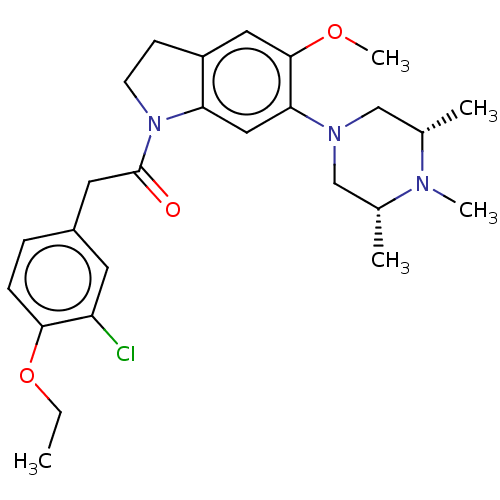
(CHEMBL250973)Show SMILES CCOc1ccc(CC(=O)N2CCc3cc(OC)c(cc23)N2C[C@H](C)N(C)[C@H](C)C2)cc1Cl Show InChI InChI=1S/C26H34ClN3O3/c1-6-33-24-8-7-19(11-21(24)27)12-26(31)30-10-9-20-13-25(32-5)23(14-22(20)30)29-15-17(2)28(4)18(3)16-29/h7-8,11,13-14,17-18H,6,9-10,12,15-16H2,1-5H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at 5HT1B receptor |
Bioorg Med Chem Lett 17: 6584-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.067
BindingDB Entry DOI: 10.7270/Q2MW2KX2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50477753
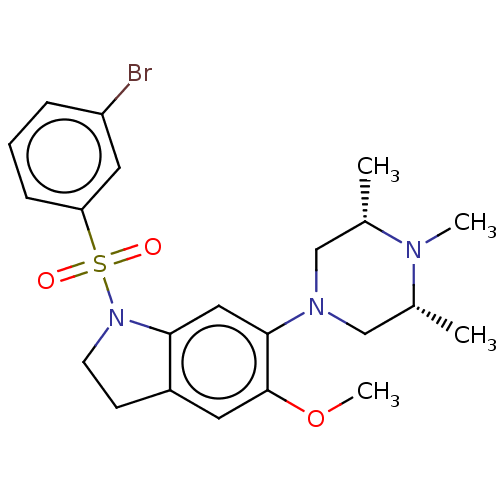
(CHEMBL399704)Show SMILES COc1cc2CCN(c2cc1N1C[C@H](C)N(C)[C@H](C)C1)S(=O)(=O)c1cccc(Br)c1 Show InChI InChI=1S/C22H28BrN3O3S/c1-15-13-25(14-16(2)24(15)3)21-12-20-17(10-22(21)29-4)8-9-26(20)30(27,28)19-7-5-6-18(23)11-19/h5-7,10-12,15-16H,8-9,13-14H2,1-4H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at 5HT1B receptor |
Bioorg Med Chem Lett 17: 6584-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.067
BindingDB Entry DOI: 10.7270/Q2MW2KX2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50412955

(CHEMBL407706)Show SMILES COc1ccc(cc1NS(=O)(=O)c1ccc(cc1)-c1ccc(C)o1)N1C[C@H](C)N[C@H](C)C1 |r| Show InChI InChI=1S/C24H29N3O4S/c1-16-14-27(15-17(2)25-16)20-8-12-24(30-4)22(13-20)26-32(28,29)21-9-6-19(7-10-21)23-11-5-18(3)31-23/h5-13,16-17,25-26H,14-15H2,1-4H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1B receptor |
Bioorg Med Chem Lett 18: 2203-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.021
BindingDB Entry DOI: 10.7270/Q2KS6VBX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50478241

(CHEMBL256263)Show SMILES COc1ccc(cc1NS(=O)(=O)c1ccc(cc1)-c1cccs1)N1C[C@H](C)N[C@H](C)C1 Show InChI InChI=1S/C23H27N3O3S2/c1-16-14-26(15-17(2)24-16)19-8-11-22(29-3)21(13-19)25-31(27,28)20-9-6-18(7-10-20)23-5-4-12-30-23/h4-13,16-17,24-25H,14-15H2,1-3H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1B receptor |
Bioorg Med Chem Lett 18: 2203-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.021
BindingDB Entry DOI: 10.7270/Q2KS6VBX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311424
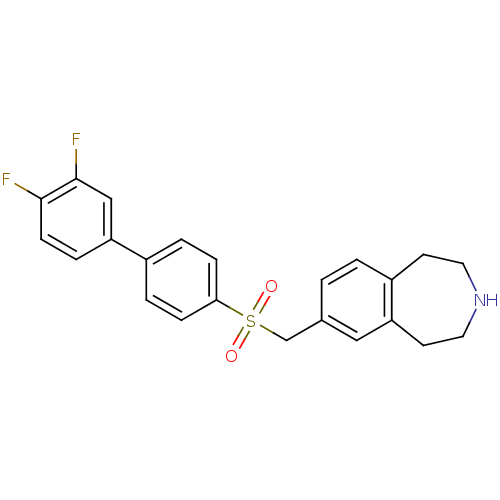
(7-((3',4'-difluorobiphenyl-4-ylsulfonyl)methyl)-2,...)Show SMILES Fc1ccc(cc1F)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H21F2NO2S/c24-22-8-5-19(14-23(22)25)17-3-6-21(7-4-17)29(27,28)15-16-1-2-18-9-11-26-12-10-20(18)13-16/h1-8,13-14,26H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50344942
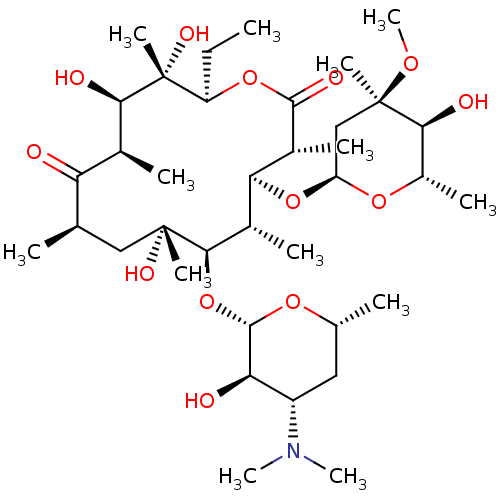
(CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O |r| Show InChI InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311425
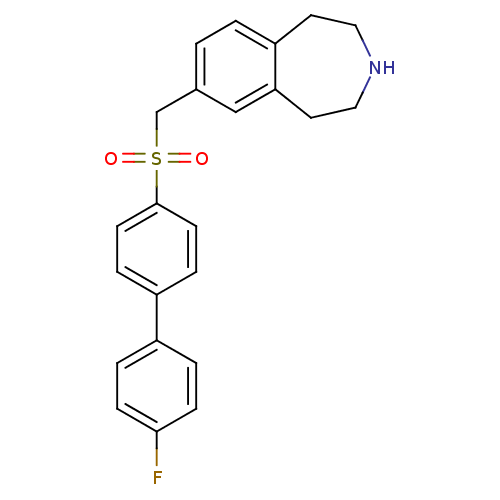
(7-((4'-fluorobiphenyl-4-ylsulfonyl)methyl)-2,3,4,5...)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H22FNO2S/c24-22-7-3-18(4-8-22)19-5-9-23(10-6-19)28(26,27)16-17-1-2-20-11-13-25-14-12-21(20)15-17/h1-10,15,25H,11-14,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311413
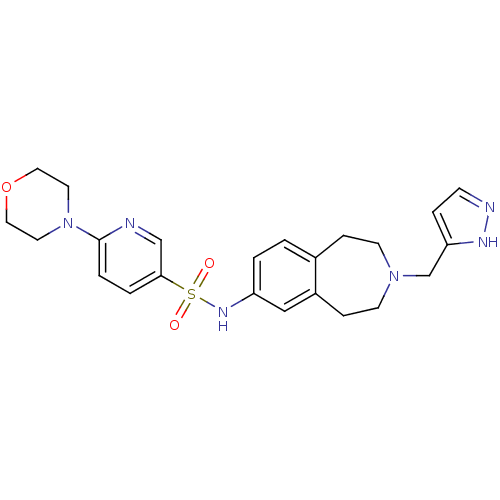
(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311414
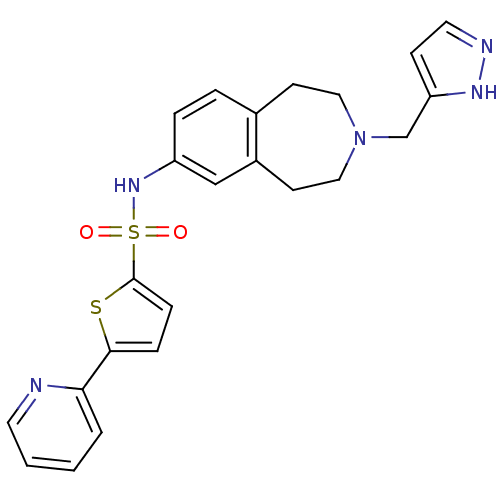
(CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C23H23N5O2S2/c29-32(30,23-7-6-22(31-23)21-3-1-2-11-24-21)27-19-5-4-17-9-13-28(14-10-18(17)15-19)16-20-8-12-25-26-20/h1-8,11-12,15,27H,9-10,13-14,16H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311419

(CHEMBL1078464 | N-(4-fluorophenyl)-5-((2,3,4,5-tet...)Show SMILES Fc1ccc(Nc2ccc(cn2)S(=O)(=O)Cc2ccc3CCNCCc3c2)cc1 Show InChI InChI=1S/C22H22FN3O2S/c23-19-3-5-20(6-4-19)26-22-8-7-21(14-25-22)29(27,28)15-16-1-2-17-9-11-24-12-10-18(17)13-16/h1-8,13-14,24H,9-12,15H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311414

(CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C23H23N5O2S2/c29-32(30,23-7-6-22(31-23)21-3-1-2-11-24-21)27-19-5-4-17-9-13-28(14-10-18(17)15-19)16-20-8-12-25-26-20/h1-8,11-12,15,27H,9-10,13-14,16H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311415
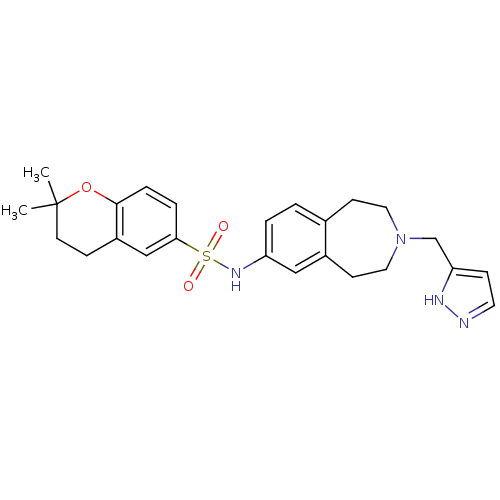
(CHEMBL1078490 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C25H30N4O3S/c1-25(2)11-7-20-16-23(5-6-24(20)32-25)33(30,31)28-21-4-3-18-9-13-29(14-10-19(18)15-21)17-22-8-12-26-27-22/h3-6,8,12,15-16,28H,7,9-11,13-14,17H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311423
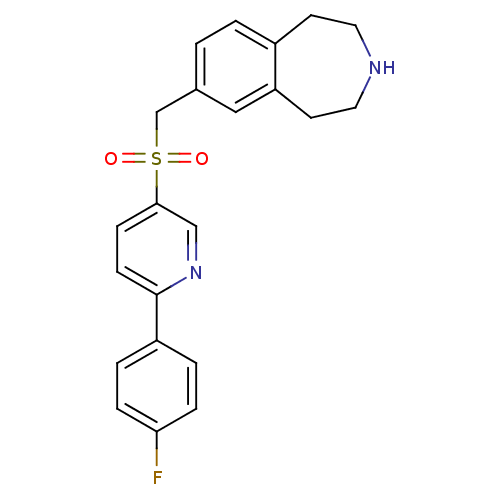
(7-((6-(4-fluorophenyl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES Fc1ccc(cc1)-c1ccc(cn1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C22H21FN2O2S/c23-20-5-3-18(4-6-20)22-8-7-21(14-25-22)28(26,27)15-16-1-2-17-9-11-24-12-10-19(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311412
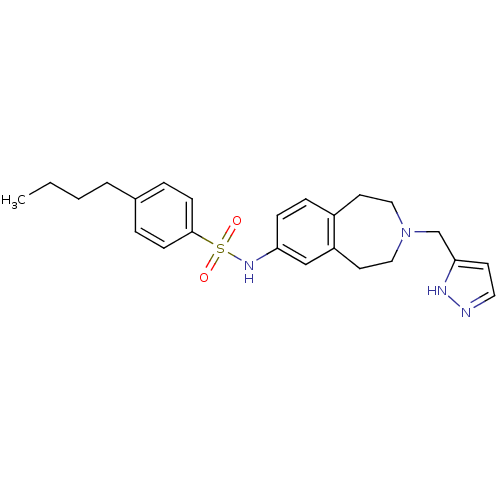
(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311420
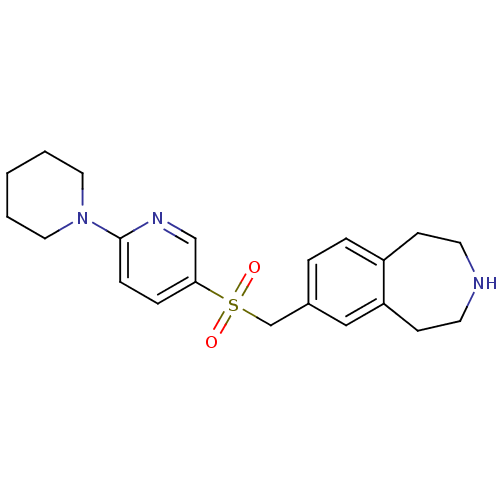
(7-((6-(piperidin-1-yl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)N1CCCCC1 Show InChI InChI=1S/C21H27N3O2S/c25-27(26,16-17-4-5-18-8-10-22-11-9-19(18)14-17)20-6-7-21(23-15-20)24-12-2-1-3-13-24/h4-7,14-15,22H,1-3,8-13,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311416
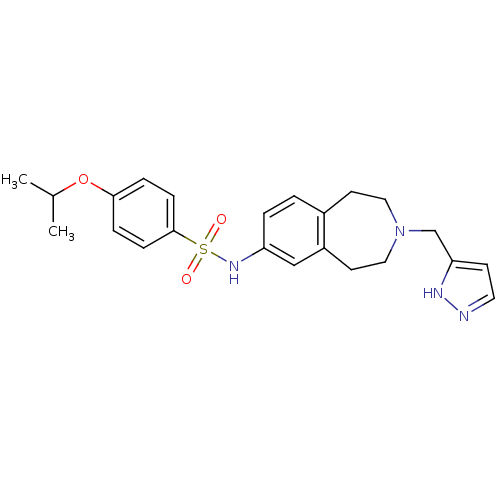
(CHEMBL1078208 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC(C)Oc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C23H28N4O3S/c1-17(2)30-22-5-7-23(8-6-22)31(28,29)26-20-4-3-18-10-13-27(14-11-19(18)15-20)16-21-9-12-24-25-21/h3-9,12,15,17,26H,10-11,13-14,16H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50247157
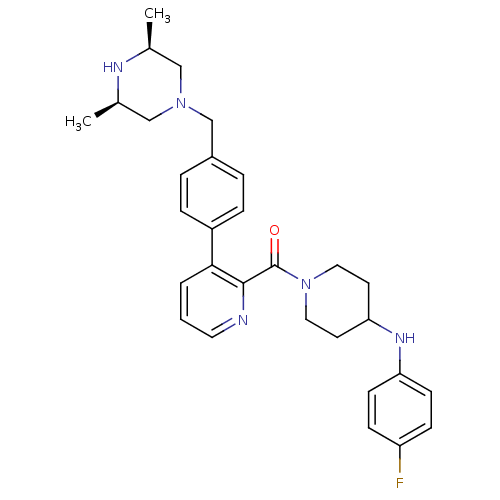
((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 25 to 30 mins by time dependent inhibition a... |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311421
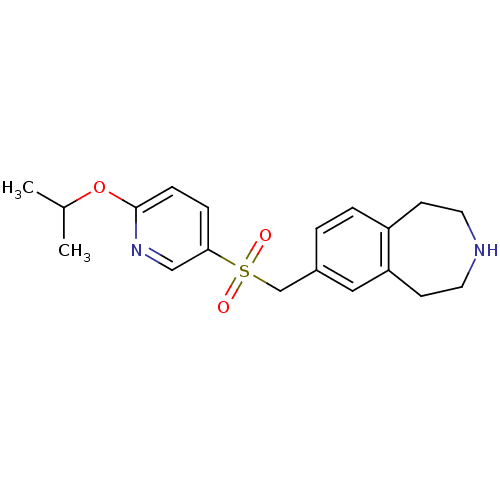
(7-((6-isopropoxypyridin-3-ylsulfonyl)methyl)-2,3,4...)Show InChI InChI=1S/C19H24N2O3S/c1-14(2)24-19-6-5-18(12-21-19)25(22,23)13-15-3-4-16-7-9-20-10-8-17(16)11-15/h3-6,11-12,14,20H,7-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311422
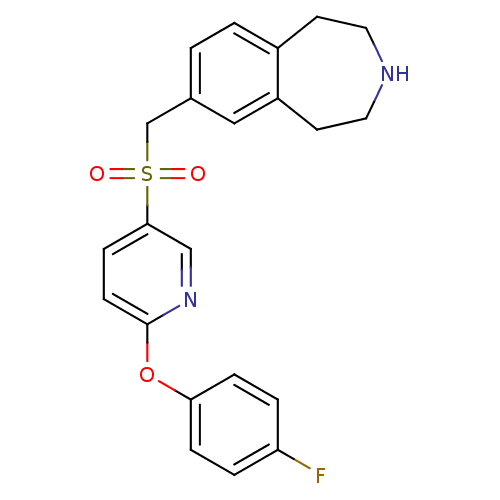
(7-((6-(4-fluorophenoxy)pyridin-3-ylsulfonyl)methyl...)Show SMILES Fc1ccc(Oc2ccc(cn2)S(=O)(=O)Cc2ccc3CCNCCc3c2)cc1 Show InChI InChI=1S/C22H21FN2O3S/c23-19-3-5-20(6-4-19)28-22-8-7-21(14-25-22)29(26,27)15-16-1-2-17-9-11-24-12-10-18(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using 7-{3-(4-phenylpiperazin-1-ylmethyl)benzyl}resorufin as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50344942

(CHEMBL532 | E-MYCIN E | ERYTHROMYCIN | ERYTHROMYCI...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O |r| Show InChI InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 0 to 5 mins by time dependent inhibition ass... |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311417
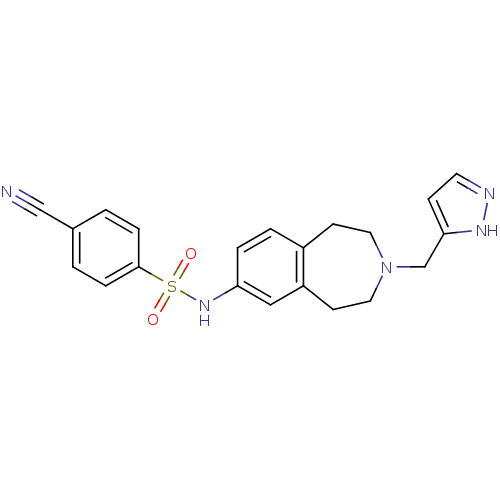
(CHEMBL1081860 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H21N5O2S/c22-14-16-1-5-21(6-2-16)29(27,28)25-19-4-3-17-8-11-26(12-9-18(17)13-19)15-20-7-10-23-24-20/h1-7,10,13,25H,8-9,11-12,15H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292984
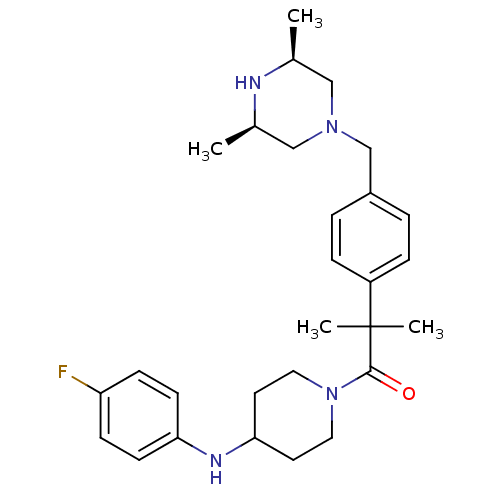
(1-[2-(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methy...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)C(C)(C)C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C28H39FN4O/c1-20-17-32(18-21(2)30-20)19-22-5-7-23(8-6-22)28(3,4)27(34)33-15-13-26(14-16-33)31-25-11-9-24(29)10-12-25/h5-12,20-21,26,30-31H,13-19H2,1-4H3/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292981
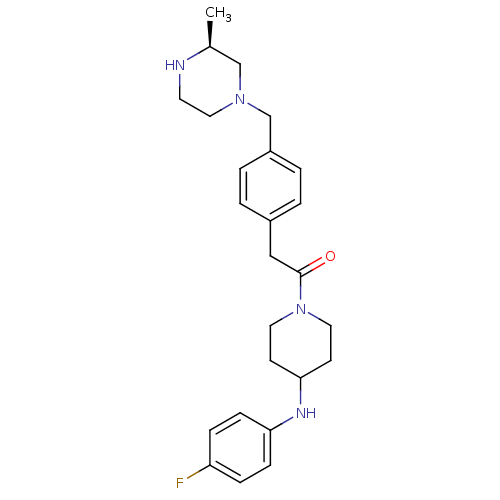
(CHEMBL489094 | N-(4-Fluorophenyl)-1-[(4-([(3S)-3-m...)Show SMILES C[C@H]1CN(Cc2ccc(CC(=O)N3CCC(CC3)Nc3ccc(F)cc3)cc2)CCN1 |r| Show InChI InChI=1S/C25H33FN4O/c1-19-17-29(15-12-27-19)18-21-4-2-20(3-5-21)16-25(31)30-13-10-24(11-14-30)28-23-8-6-22(26)7-9-23/h2-9,19,24,27-28H,10-18H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311417

(CHEMBL1081860 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H21N5O2S/c22-14-16-1-5-21(6-2-16)29(27,28)25-19-4-3-17-8-11-26(12-9-18(17)13-19)15-20-7-10-23-24-20/h1-7,10,13,25H,8-9,11-12,15H2,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using 7-{3-(4-phenylpiperazin-1-ylmethyl)benzyl}resorufin as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292983
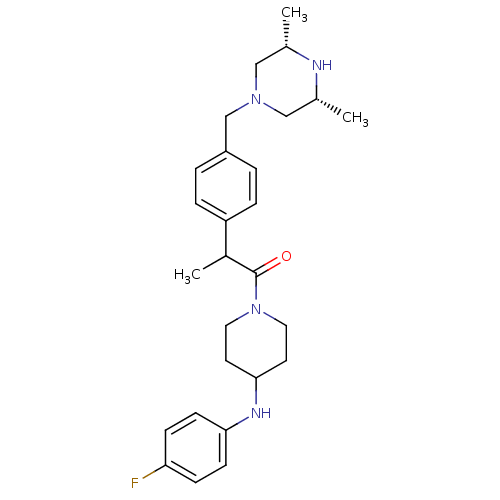
(1-[2-(4-([(3R,5S)-3,5-Dimethyl-1-piperazinyl]methy...)Show SMILES CC(C(=O)N1CCC(CC1)Nc1ccc(F)cc1)c1ccc(CN2C[C@H](C)N[C@H](C)C2)cc1 |r| Show InChI InChI=1S/C27H37FN4O/c1-19-16-31(17-20(2)29-19)18-22-4-6-23(7-5-22)21(3)27(33)32-14-12-26(13-15-32)30-25-10-8-24(28)9-11-25/h4-11,19-21,26,29-30H,12-18H2,1-3H3/t19-,20+,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292979
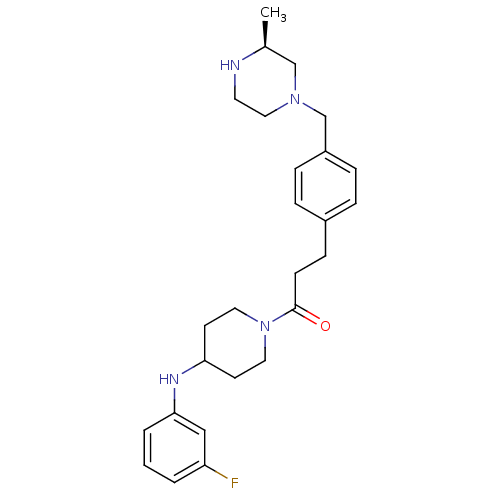
(CHEMBL474130 | N-(3-Fluorophenyl)-1-[3-(4-([(3S)-3...)Show SMILES C[C@H]1CN(Cc2ccc(CCC(=O)N3CCC(CC3)Nc3cccc(F)c3)cc2)CCN1 |r| Show InChI InChI=1S/C26H35FN4O/c1-20-18-30(16-13-28-20)19-22-7-5-21(6-8-22)9-10-26(32)31-14-11-24(12-15-31)29-25-4-2-3-23(27)17-25/h2-8,17,20,24,28-29H,9-16,18-19H2,1H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292981

(CHEMBL489094 | N-(4-Fluorophenyl)-1-[(4-([(3S)-3-m...)Show SMILES C[C@H]1CN(Cc2ccc(CC(=O)N3CCC(CC3)Nc3ccc(F)cc3)cc2)CCN1 |r| Show InChI InChI=1S/C25H33FN4O/c1-19-17-29(15-12-27-19)18-21-4-2-20(3-5-21)16-25(31)30-13-10-24(11-14-30)28-23-8-6-22(26)7-9-23/h2-9,19,24,27-28H,10-18H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50292978
(CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...)Show SMILES C[C@H]1CN(Cc2ccc(CC(=O)N3CCC(CC3)Nc3cccc(F)c3)cc2)CCN1 |r| Show InChI InChI=1S/C25H33FN4O/c1-19-17-29(14-11-27-19)18-21-7-5-20(6-8-21)15-25(31)30-12-9-23(10-13-30)28-24-4-2-3-22(26)16-24/h2-8,16,19,23,27-28H,9-15,17-18H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311426

(4-(5-((2,3,4,5-tetrahydro-1H-benzo[d]azepin-7-yl)m...)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C20H25N3O3S/c24-27(25,15-16-1-2-17-5-7-21-8-6-18(17)13-16)19-3-4-20(22-14-19)23-9-11-26-12-10-23/h1-4,13-14,21H,5-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311418

(CHEMBL1080047 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC(C)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C17H24N4O2S/c1-13(2)24(22,23)20-16-4-3-14-6-9-21(10-7-15(14)11-16)12-17-5-8-18-19-17/h3-5,8,11,13,20H,6-7,9-10,12H2,1-2H3,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292979

(CHEMBL474130 | N-(3-Fluorophenyl)-1-[3-(4-([(3S)-3...)Show SMILES C[C@H]1CN(Cc2ccc(CCC(=O)N3CCC(CC3)Nc3cccc(F)c3)cc2)CCN1 |r| Show InChI InChI=1S/C26H35FN4O/c1-20-18-30(16-13-28-20)19-22-7-5-21(6-8-22)9-10-26(32)31-14-11-24(12-15-31)29-25-4-2-3-23(27)17-25/h2-8,17,20,24,28-29H,9-16,18-19H2,1H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using 7BQ substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate measured in 0 to 5 mins by time dependent inhibition ass... |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292978

(CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...)Show SMILES C[C@H]1CN(Cc2ccc(CC(=O)N3CCC(CC3)Nc3cccc(F)c3)cc2)CCN1 |r| Show InChI InChI=1S/C25H33FN4O/c1-19-17-29(14-11-27-19)18-21-7-5-20(6-8-21)15-25(31)30-12-9-23(10-13-30)28-24-4-2-3-22(26)16-24/h2-8,16,19,23,27-28H,9-15,17-18H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50292982

(CHEMBL523933 | N-(3-Fluorophenyl)-1-[(4-([(3R)-3-m...)Show SMILES C[C@@H]1CN(Cc2ccc(CC(=O)N3CCC(CC3)Nc3cccc(F)c3)cc2)CCN1 |r| Show InChI InChI=1S/C25H33FN4O/c1-19-17-29(14-11-27-19)18-21-7-5-20(6-8-21)15-25(31)30-12-9-23(10-13-30)28-24-4-2-3-22(26)16-24/h2-8,16,19,23,27-28H,9-15,17-18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 expressed in Escherichia coli using diethoxyfluorescein substrate by time dependent inhibition assay |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50247157

((3-(4-(((3R,5S)-3,5-dimethylpiperazin-1-yl)methyl)...)Show SMILES C[C@H]1CN(Cc2ccc(cc2)-c2cccnc2C(=O)N2CCC(CC2)Nc2ccc(F)cc2)C[C@@H](C)N1 |r| Show InChI InChI=1S/C30H36FN5O/c1-21-18-35(19-22(2)33-21)20-23-5-7-24(8-6-23)28-4-3-15-32-29(28)30(37)36-16-13-27(14-17-36)34-26-11-9-25(31)10-12-26/h3-12,15,21-22,27,33-34H,13-14,16-20H2,1-2H3/t21-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50292978

(CHEMBL489095 | GSK-962040 | N-(3-Fluorophenyl)-1-[...)Show SMILES C[C@H]1CN(Cc2ccc(CC(=O)N3CCC(CC3)Nc3cccc(F)c3)cc2)CCN1 |r| Show InChI InChI=1S/C25H33FN4O/c1-19-17-29(14-11-27-19)18-21-7-5-20(6-8-21)15-25(31)30-12-9-23(10-13-30)28-24-4-2-3-22(26)16-24/h2-8,16,19,23,27-28H,9-15,17-18H2,1H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 52: 1180-9 (2009)
Article DOI: 10.1021/jm801332q
BindingDB Entry DOI: 10.7270/Q208666T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data