 Found 1173 hits with Last Name = 'mckittrick' and Initial = 'ba'
Found 1173 hits with Last Name = 'mckittrick' and Initial = 'ba' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306314

((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(C2CCC2)C(C)=O)cc1 |r| Show InChI InChI=1S/C24H29ClN2O2/c1-16(28)27(20-4-3-5-20)14-17-6-8-18(9-7-17)22-15-26(2)11-10-19-12-23(25)24(29)13-21(19)22/h6-9,12-13,20,22,29H,3-5,10-11,14-15H2,1-2H3/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306315
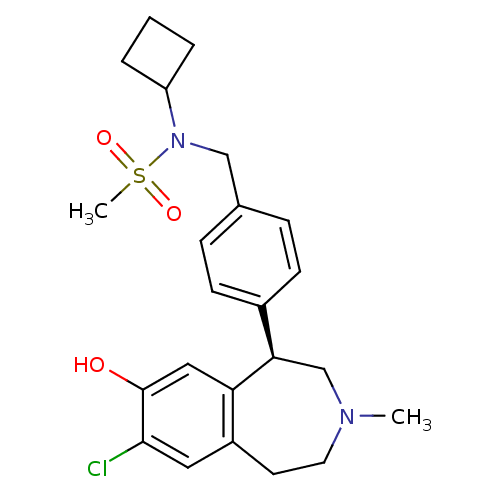
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(C2CCC2)S(C)(=O)=O)cc1 |r| Show InChI InChI=1S/C23H29ClN2O3S/c1-25-11-10-18-12-22(24)23(27)13-20(18)21(15-25)17-8-6-16(7-9-17)14-26(30(2,28)29)19-4-3-5-19/h6-9,12-13,19,21,27H,3-5,10-11,14-15H2,1-2H3/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306316
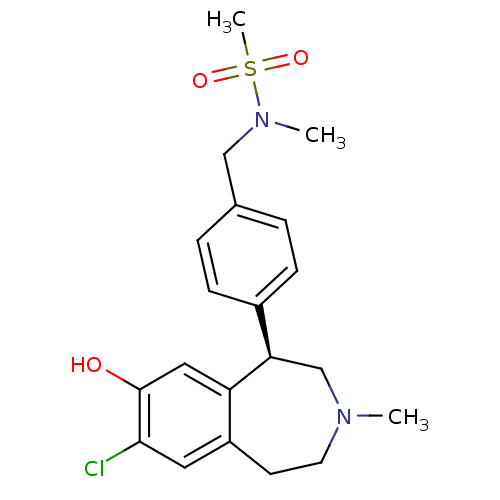
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(C)(=O)=O |r| Show InChI InChI=1S/C20H25ClN2O3S/c1-22-9-8-16-10-19(21)20(24)11-17(16)18(13-22)15-6-4-14(5-7-15)12-23(2)27(3,25)26/h4-7,10-11,18,24H,8-9,12-13H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306322
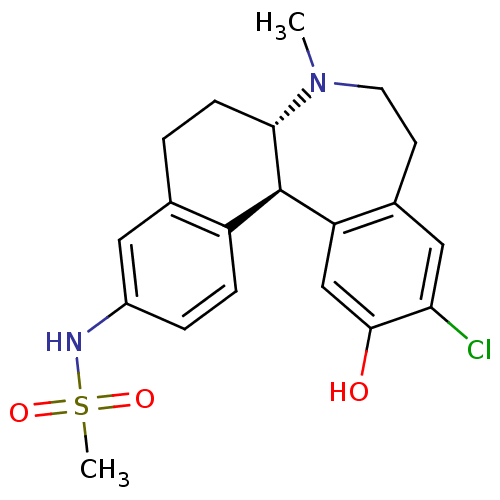
(CHEMBL597909 | N-((6aS,13bR)-11-chloro-12-hydroxy-...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1cc(NS(C)(=O)=O)ccc21 |r| Show InChI InChI=1S/C20H23ClN2O3S/c1-23-8-7-13-10-17(21)19(24)11-16(13)20-15-5-4-14(22-27(2,25)26)9-12(15)3-6-18(20)23/h4-5,9-11,18,20,22,24H,3,6-8H2,1-2H3/t18-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065428
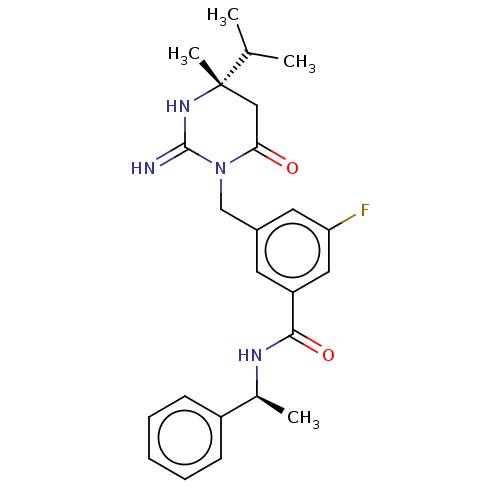
(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306317
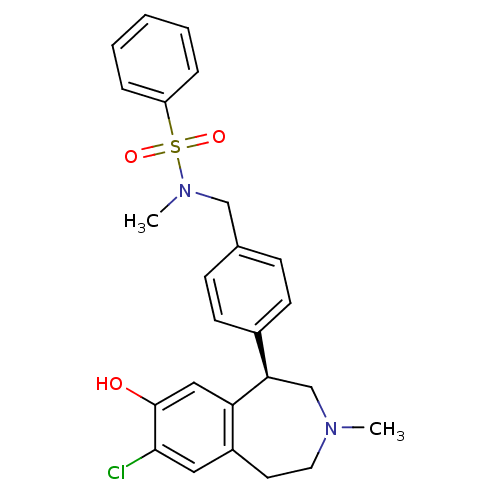
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C25H27ClN2O3S/c1-27-13-12-20-14-24(26)25(29)15-22(20)23(17-27)19-10-8-18(9-11-19)16-28(2)32(30,31)21-6-4-3-5-7-21/h3-11,14-15,23,29H,12-13,16-17H2,1-2H3/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306319
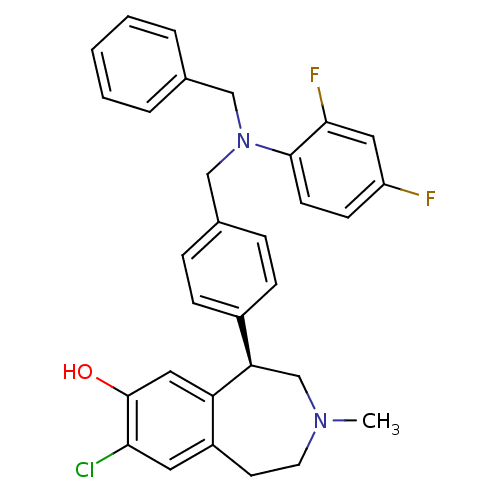
((R)-5-(4-((benzyl(2,4-difluorophenyl)amino)methyl)...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(Cc2ccccc2)c2ccc(F)cc2F)cc1 |r| Show InChI InChI=1S/C31H29ClF2N2O/c1-35-14-13-24-15-28(32)31(37)17-26(24)27(20-35)23-9-7-22(8-10-23)19-36(18-21-5-3-2-4-6-21)30-12-11-25(33)16-29(30)34/h2-12,15-17,27,37H,13-14,18-20H2,1H3/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50111336
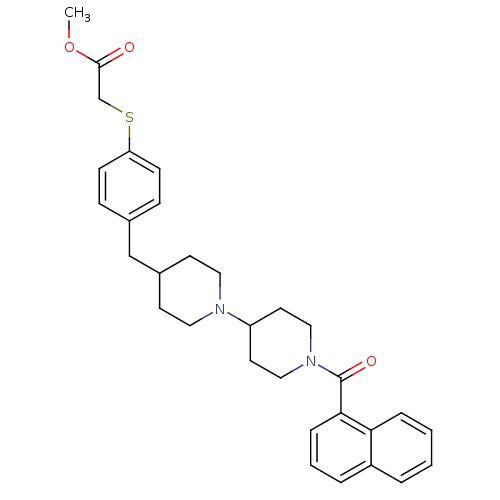
(CHEMBL11892 | {4-[1'-(Naphthalene-1-carbonyl)-[1,4...)Show SMILES COC(=O)CSc1ccc(CC2CCN(CC2)C2CCN(CC2)C(=O)c2cccc3ccccc23)cc1 Show InChI InChI=1S/C31H36N2O3S/c1-36-30(34)22-37-27-11-9-23(10-12-27)21-24-13-17-32(18-14-24)26-15-19-33(20-16-26)31(35)29-8-4-6-25-5-2-3-7-28(25)29/h2-12,24,26H,13-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand. |
Bioorg Med Chem Lett 12: 1087-91 (2002)
BindingDB Entry DOI: 10.7270/Q2MS3S2P |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306325

(1-((6aS,13bR)-11-chloro-12-hydroxy-7-methyl-6,6a,7...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1cc(NC(=O)Nc3c(Cl)cccc3Cl)ccc21 |r| Show InChI InChI=1S/C26H24Cl3N3O2/c1-32-10-9-15-12-21(29)23(33)13-18(15)24-17-7-6-16(11-14(17)5-8-22(24)32)30-26(34)31-25-19(27)3-2-4-20(25)28/h2-4,6-7,11-13,22,24,33H,5,8-10H2,1H3,(H2,30,31,34)/t22-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306323
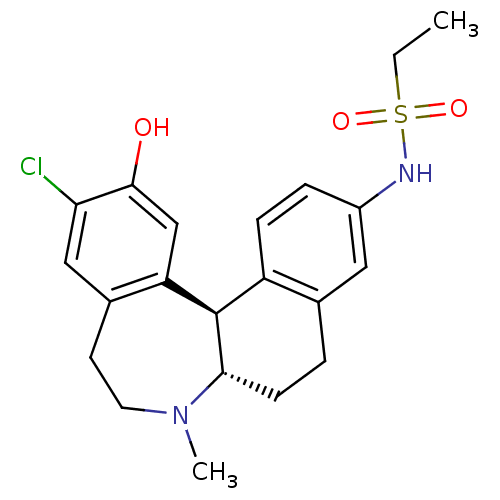
(CHEMBL600986 | N-((6aS,13bR)-11-chloro-12-hydroxy-...)Show SMILES CCS(=O)(=O)Nc1ccc2[C@H]3[C@H](CCc2c1)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C21H25ClN2O3S/c1-3-28(26,27)23-15-5-6-16-13(10-15)4-7-19-21(16)17-12-20(25)18(22)11-14(17)8-9-24(19)2/h5-6,10-12,19,21,23,25H,3-4,7-9H2,1-2H3/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50111346

((2-Amino-3-methyl-phenyl)-{4-[4-(3-chloro-benzenes...)Show SMILES Cc1cccc(C(=O)N2CCC(CC2)N2CCC(Cc3ccc(cc3)S(=O)(=O)c3cccc(Cl)c3)CC2)c1N Show InChI InChI=1S/C31H36ClN3O3S/c1-22-4-2-7-29(30(22)33)31(36)35-18-14-26(15-19-35)34-16-12-24(13-17-34)20-23-8-10-27(11-9-23)39(37,38)28-6-3-5-25(32)21-28/h2-11,21,24,26H,12-20,33H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand. |
Bioorg Med Chem Lett 12: 1087-91 (2002)
BindingDB Entry DOI: 10.7270/Q2MS3S2P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50111332

(CHEMBL276239 | [4-(4-Isopropylsulfanyl-benzyl)-[1,...)Show SMILES CC(C)Sc1ccc(CC2CCN(CC2)C2CCN(CC2)C(=O)c2cccc3ccccc23)cc1 Show InChI InChI=1S/C31H38N2OS/c1-23(2)35-28-12-10-24(11-13-28)22-25-14-18-32(19-15-25)27-16-20-33(21-17-27)31(34)30-9-5-7-26-6-3-4-8-29(26)30/h3-13,23,25,27H,14-22H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand. |
Bioorg Med Chem Lett 12: 1087-91 (2002)
BindingDB Entry DOI: 10.7270/Q2MS3S2P |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306313
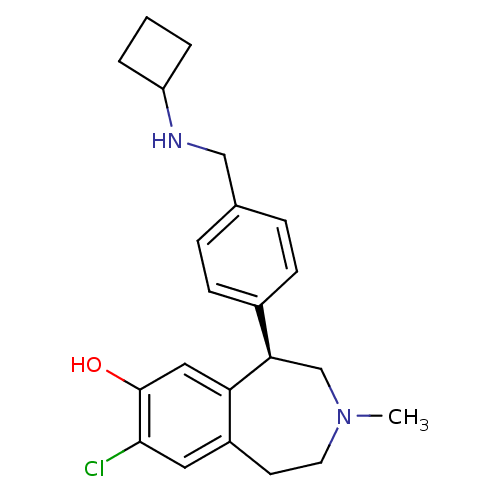
((R)-8-chloro-5-(4-((cyclobutylamino)methyl)phenyl)...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CNC2CCC2)cc1 |r| Show InChI InChI=1S/C22H27ClN2O/c1-25-10-9-17-11-21(23)22(26)12-19(17)20(14-25)16-7-5-15(6-8-16)13-24-18-3-2-4-18/h5-8,11-12,18,20,24,26H,2-4,9-10,13-14H2,1H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50111356
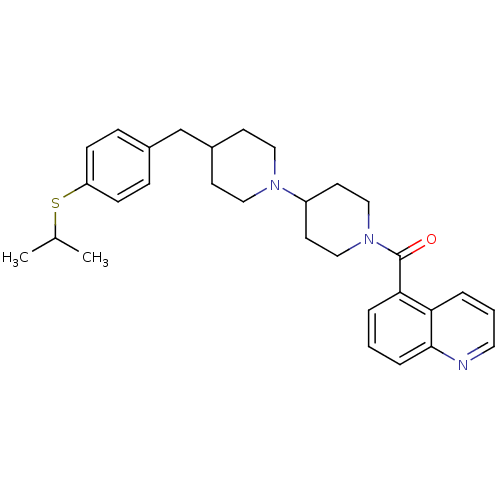
(CHEMBL11756 | [4-(4-Isopropylsulfanyl-benzyl)-[1,4...)Show SMILES CC(C)Sc1ccc(CC2CCN(CC2)C2CCN(CC2)C(=O)c2cccc3ncccc23)cc1 Show InChI InChI=1S/C30H37N3OS/c1-22(2)35-26-10-8-23(9-11-26)21-24-12-17-32(18-13-24)25-14-19-33(20-15-25)30(34)28-5-3-7-29-27(28)6-4-16-31-29/h3-11,16,22,24-25H,12-15,17-21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand. |
Bioorg Med Chem Lett 12: 1087-91 (2002)
BindingDB Entry DOI: 10.7270/Q2MS3S2P |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398688
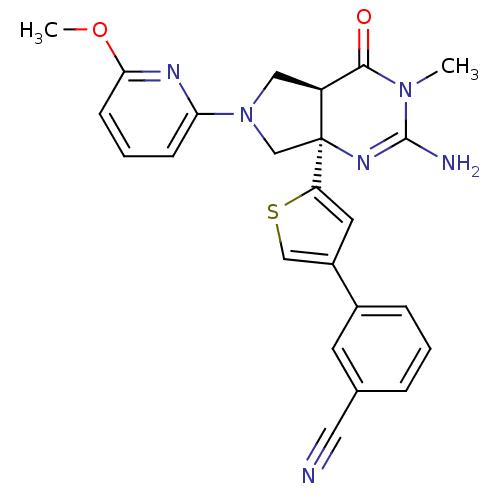
(CHEMBL2178715)Show SMILES COc1cccc(n1)N1C[C@H]2C(=O)N(C)C(N)=N[C@]2(C1)c1cc(cs1)-c1cccc(c1)C#N |r,c:17| Show InChI InChI=1S/C24H22N6O2S/c1-29-22(31)18-12-30(20-7-4-8-21(27-20)32-2)14-24(18,28-23(29)26)19-10-17(13-33-19)16-6-3-5-15(9-16)11-25/h3-10,13,18H,12,14H2,1-2H3,(H2,26,28)/t18-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 55: 9331-45 (2012)
Article DOI: 10.1021/jm301039c
BindingDB Entry DOI: 10.7270/Q2JH3NB7 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398683
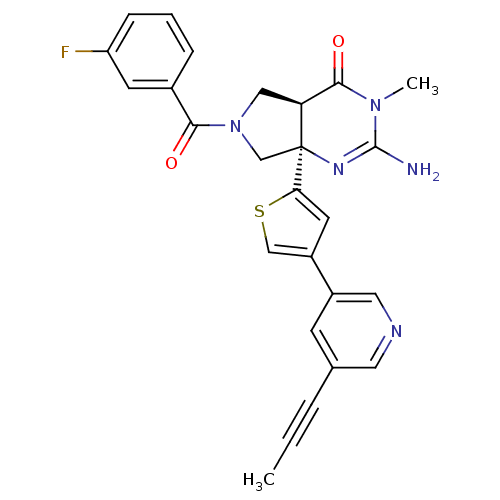
(CHEMBL2178147)Show SMILES CC#Cc1cncc(c1)-c1csc(c1)[C@]12CN(C[C@H]1C(=O)N(C)C(N)=N2)C(=O)c1cccc(F)c1 |r,c:27| Show InChI InChI=1S/C26H22FN5O2S/c1-3-5-16-8-18(12-29-11-16)19-10-22(35-14-19)26-15-32(23(33)17-6-4-7-20(27)9-17)13-21(26)24(34)31(2)25(28)30-26/h4,6-12,14,21H,13,15H2,1-2H3,(H2,28,30)/t21-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 55: 9331-45 (2012)
Article DOI: 10.1021/jm301039c
BindingDB Entry DOI: 10.7270/Q2JH3NB7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE2 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215615

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC1CC[C@@H](CO)N1CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1 |w:16.18,1.0| Show InChI InChI=1S/C28H33Cl2N5O2/c1-18-5-6-24(17-36)34(18)9-10-35(27(37)32-22-13-25(29)33-26(30)14-22)23-7-8-28(15-21(28)12-23)20-4-2-3-19(11-20)16-31/h2-4,11,13-14,18,21,23-24,36H,5-10,12,15,17H2,1H3,(H,32,33,37)/t18?,21?,23-,24+,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004823
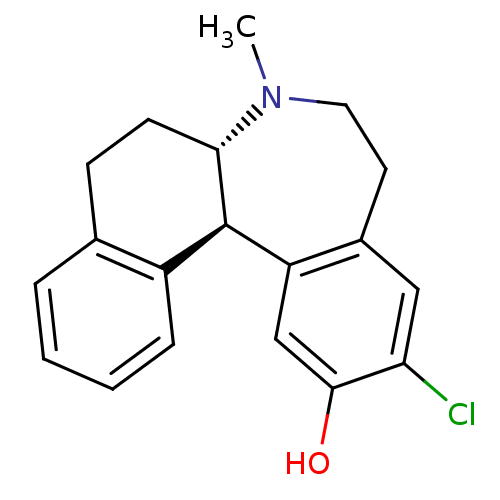
((6aS,13bR)-11-Chloro-7-methyl-5,6a,7,8,9,13b-hexah...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1ccccc21 Show InChI InChI=1S/C19H20ClNO/c1-21-9-8-13-10-16(20)18(22)11-15(13)19-14-5-3-2-4-12(14)6-7-17(19)21/h2-5,10-11,17,19,22H,6-9H2,1H3/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50306317

((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C25H27ClN2O3S/c1-27-13-12-20-14-24(26)25(29)15-22(20)23(17-27)19-10-8-18(9-11-19)16-28(2)32(30,31)21-6-4-3-5-7-21/h3-11,14-15,23,29H,12-13,16-17H2,1-2H3/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D5 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50306334
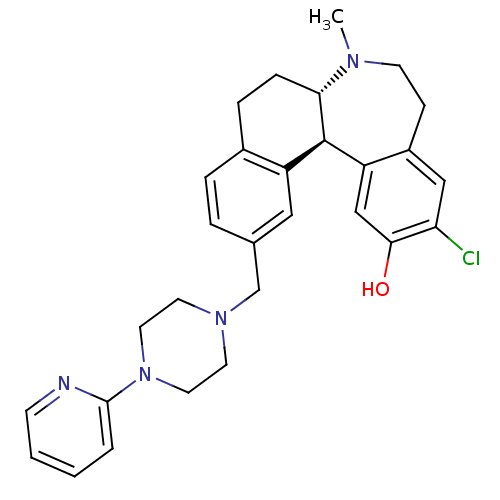
((6aS,13bR)-11-chloro-7-methyl-2-((4-(pyridin-2-yl)...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1ccc(CN3CCN(CC3)c3ccccn3)cc21 |r| Show InChI InChI=1S/C29H33ClN4O/c1-32-11-9-22-17-25(30)27(35)18-24(22)29-23-16-20(5-6-21(23)7-8-26(29)32)19-33-12-14-34(15-13-33)28-4-2-3-10-31-28/h2-6,10,16-18,26,29,35H,7-9,11-15,19H2,1H3/t26-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50164512
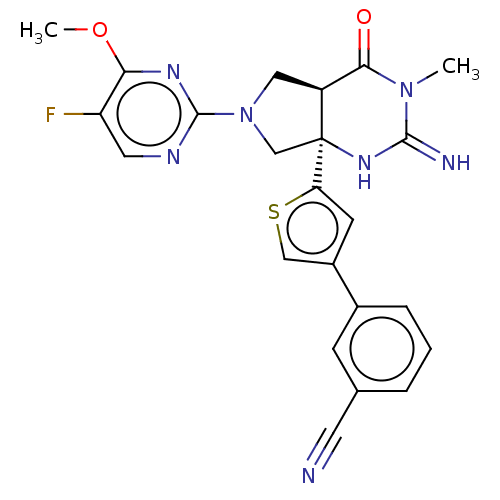
(CHEMBL3800286)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OC)n1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)16-10-31(22-27-9-17(24)19(28-22)33-2)12-23(16,29-21(30)26)18-7-15(11-34-18)14-5-3-4-13(6-14)8-25/h3-7,9,11,16H,10,12H2,1-2H3,(H2,26,29)/t16-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... |
J Med Chem 59: 3231-48 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01995
BindingDB Entry DOI: 10.7270/Q2CR5W8V |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306326

(CHEMBL603485 | ethyl(6aS,13bR)-11-chloro-12-hydrox...)Show SMILES CCOC(=O)Nc1ccc2[C@H]3[C@H](CCc2c1)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C22H25ClN2O3/c1-3-28-22(27)24-15-5-6-16-13(10-15)4-7-19-21(16)17-12-20(26)18(23)11-14(17)8-9-25(19)2/h5-6,10-12,19,21,26H,3-4,7-9H2,1-2H3,(H,24,27)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306324
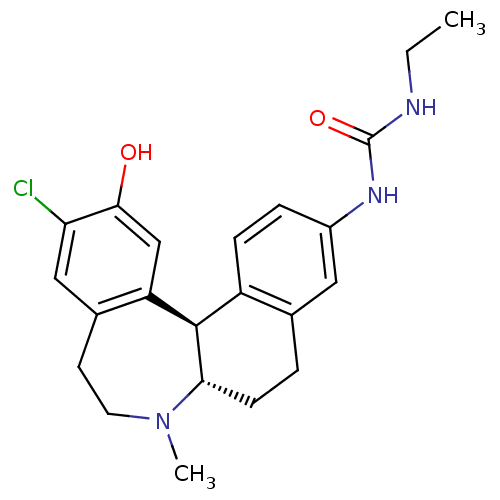
(1-((6aS,13bR)-11-chloro-12-hydroxy-7-methyl-6,6a,7...)Show SMILES CCNC(=O)Nc1ccc2[C@H]3[C@H](CCc2c1)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C22H26ClN3O2/c1-3-24-22(28)25-15-5-6-16-13(10-15)4-7-19-21(16)17-12-20(27)18(23)11-14(17)8-9-26(19)2/h5-6,10-12,19,21,27H,3-4,7-9H2,1-2H3,(H2,24,25,28)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50190059
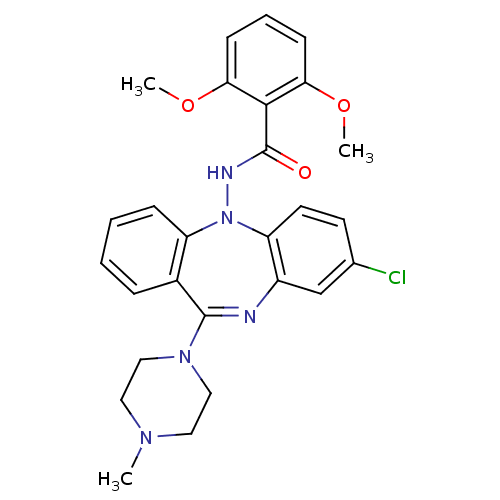
(CHEMBL425069 | N-[8-chloro-11-(4-methyl-piperazin-...)Show SMILES COc1cccc(OC)c1C(=O)NN1c2ccc(Cl)cc2N=C(N2CCN(C)CC2)c2ccccc12 |t:23| Show InChI InChI=1S/C27H28ClN5O3/c1-31-13-15-32(16-14-31)26-19-7-4-5-8-21(19)33(22-12-11-18(28)17-20(22)29-26)30-27(34)25-23(35-2)9-6-10-24(25)36-3/h4-12,17H,13-16H2,1-3H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH 23390 from dopamine D1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4548-53 (2006)
Checked by Author
Article DOI: 10.1016/j.bmcl.2006.06.034
BindingDB Entry DOI: 10.7270/Q2MG7P4R |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215655

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCO)CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1 |w:15.16| Show InChI InChI=1S/C27H33Cl2N5O2/c1-18(2)33(10-11-35)8-9-34(26(36)31-22-14-24(28)32-25(29)15-22)23-6-7-27(16-21(27)13-23)20-5-3-4-19(12-20)17-30/h3-5,12,14-15,18,21,23,35H,6-11,13,16H2,1-2H3,(H,31,32,36)/t21?,23-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215583
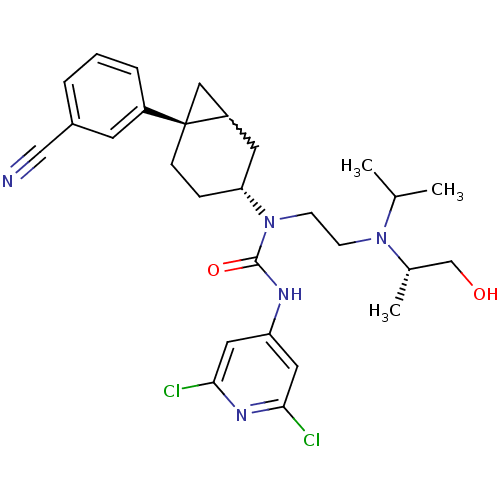
(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1)[C@@H](C)CO |w:12.13| Show InChI InChI=1S/C28H35Cl2N5O2/c1-18(2)34(19(3)17-36)9-10-35(27(37)32-23-13-25(29)33-26(30)14-23)24-7-8-28(15-22(28)12-24)21-6-4-5-20(11-21)16-31/h4-6,11,13-14,18-19,22,24,36H,7-10,12,15,17H2,1-3H3,(H,32,33,37)/t19-,22?,24+,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215645

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)NCCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1 |w:12.13| Show InChI InChI=1S/C25H29Cl2N5O/c1-16(2)29-8-9-32(24(33)30-20-12-22(26)31-23(27)13-20)21-6-7-25(14-19(25)11-21)18-5-3-4-17(10-18)15-28/h3-5,10,12-13,16,19,21,29H,6-9,11,14H2,1-2H3,(H,30,31,33)/t19?,21-,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306318
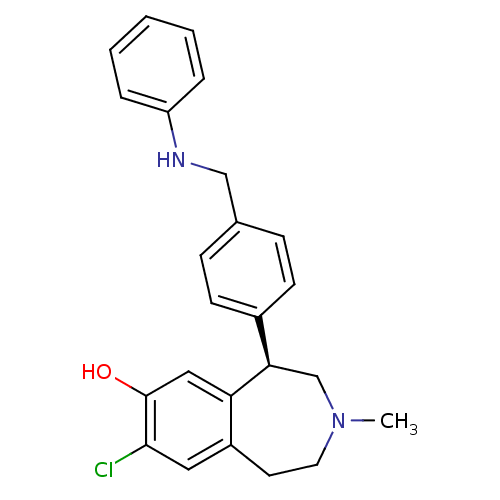
((R)-8-chloro-3-methyl-5-(4-((phenylamino)methyl)ph...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CNc2ccccc2)cc1 |r| Show InChI InChI=1S/C24H25ClN2O/c1-27-12-11-19-13-23(25)24(28)14-21(19)22(16-27)18-9-7-17(8-10-18)15-26-20-5-3-2-4-6-20/h2-10,13-14,22,26,28H,11-12,15-16H2,1H3/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50190082
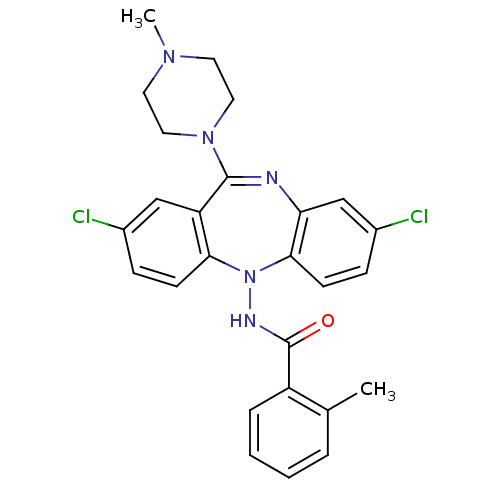
(CHEMBL379942 | N-[2,8-dichloro-11-(4-methyl-pipera...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2N(NC(=O)c2ccccc2C)c2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C26H25Cl2N5O/c1-17-5-3-4-6-20(17)26(34)30-33-23-9-7-18(27)15-21(23)25(32-13-11-31(2)12-14-32)29-22-16-19(28)8-10-24(22)33/h3-10,15-16H,11-14H2,1-2H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH 23390 from dopamine D1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4548-53 (2006)
Checked by Author
Article DOI: 10.1016/j.bmcl.2006.06.034
BindingDB Entry DOI: 10.7270/Q2MG7P4R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398684

(CHEMBL2178146)Show SMILES CN1C(N)=N[C@]2(CN(C[C@H]2C1=O)C(=O)c1cccc(F)c1)c1cc(cs1)-c1ccc(F)c(c1)C#N |r,c:3| Show InChI InChI=1S/C25H19F2N5O2S/c1-31-23(34)19-11-32(22(33)15-3-2-4-18(26)8-15)13-25(19,30-24(31)29)21-9-17(12-35-21)14-5-6-20(27)16(7-14)10-28/h2-9,12,19H,11,13H2,1H3,(H2,29,30)/t19-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 55: 9331-45 (2012)
Article DOI: 10.1021/jm301039c
BindingDB Entry DOI: 10.7270/Q2JH3NB7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398678
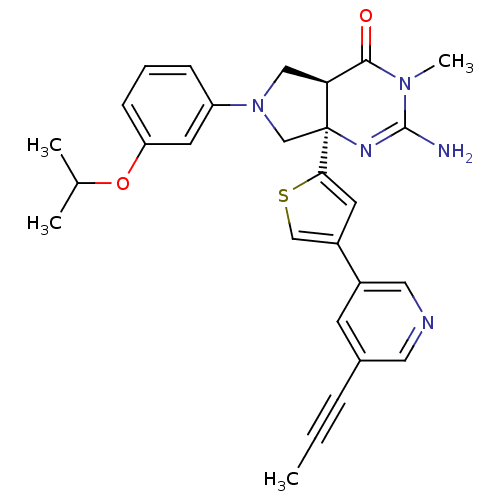
(CHEMBL2178152)Show SMILES CC#Cc1cncc(c1)-c1csc(c1)[C@]12CN(C[C@H]1C(=O)N(C)C(N)=N2)c1cccc(OC(C)C)c1 |r,c:27| Show InChI InChI=1S/C28H29N5O2S/c1-5-7-19-10-20(14-30-13-19)21-11-25(36-16-21)28-17-33(15-24(28)26(34)32(4)27(29)31-28)22-8-6-9-23(12-22)35-18(2)3/h6,8-14,16,18,24H,15,17H2,1-4H3,(H2,29,31)/t24-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 55: 9331-45 (2012)
Article DOI: 10.1021/jm301039c
BindingDB Entry DOI: 10.7270/Q2JH3NB7 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50004823

((6aS,13bR)-11-Chloro-7-methyl-5,6a,7,8,9,13b-hexah...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1ccccc21 Show InChI InChI=1S/C19H20ClNO/c1-21-9-8-13-10-16(20)18(22)11-15(13)19-14-5-3-2-4-12(14)6-7-17(19)21/h2-5,10-11,17,19,22H,6-9H2,1H3/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D5 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065395

(CHEMBL3401345)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2CC(CC2=O)c2cccc(Cl)c2)C(=N)N1 |r| Show InChI InChI=1S/C25H29ClN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-6-4-9-21(10-17)29-15-19(12-22(29)31)18-7-5-8-20(26)11-18/h4-11,16,19H,12-15H2,1-3H3,(H2,27,28)/t19?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215607

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1)C(C)C |w:12.13| Show InChI InChI=1S/C28H35Cl2N5O/c1-18(2)34(19(3)4)10-11-35(27(36)32-23-14-25(29)33-26(30)15-23)24-8-9-28(16-22(28)13-24)21-7-5-6-20(12-21)17-31/h5-7,12,14-15,18-19,22,24H,8-11,13,16H2,1-4H3,(H,32,33,36)/t22?,24-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215607

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1)C(C)C |w:12.13| Show InChI InChI=1S/C28H35Cl2N5O/c1-18(2)34(19(3)4)10-11-35(27(36)32-23-14-25(29)33-26(30)15-23)24-8-9-28(16-22(28)13-24)21-7-5-6-20(12-21)17-31/h5-7,12,14-15,18-19,22,24H,8-11,13,16H2,1-4H3,(H,32,33,36)/t22?,24-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215579

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cccc(F)c1)C(C)C |w:12.13| Show InChI InChI=1S/C29H37FN4O/c1-20(2)33(21(3)4)13-14-34(28(35)32-26-10-6-9-25(30)17-26)27-11-12-29(18-24(29)16-27)23-8-5-7-22(15-23)19-31/h5-10,15,17,20-21,24,27H,11-14,16,18H2,1-4H3,(H,32,35)/t24?,27-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065426
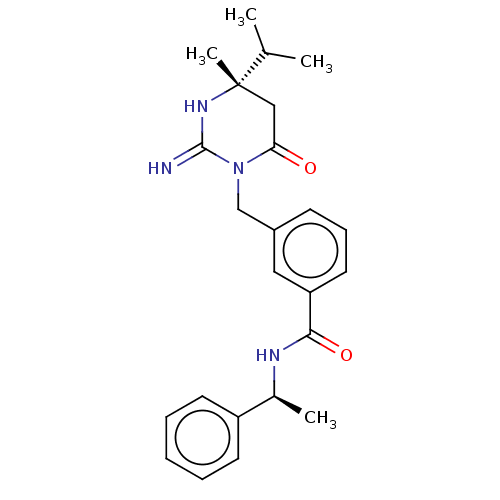
(CHEMBL3401348)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215575
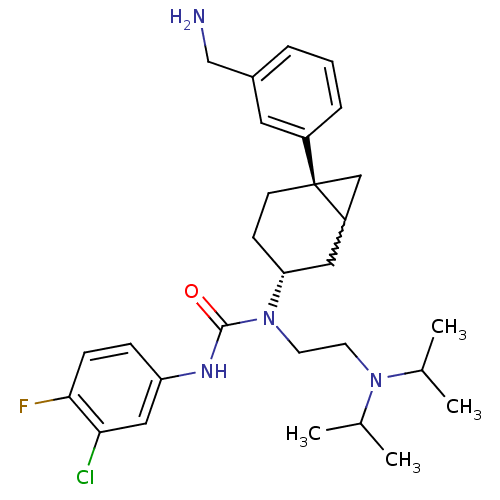
(1-((3R,6S)-6-(3-(aminomethyl)phenyl)bicyclo[4.1.0]...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(CN)c1)C(=O)Nc1ccc(F)c(Cl)c1)C(C)C |w:12.13| Show InChI InChI=1S/C29H40ClFN4O/c1-19(2)34(20(3)4)12-13-35(28(36)33-24-8-9-27(31)26(30)16-24)25-10-11-29(17-23(29)15-25)22-7-5-6-21(14-22)18-32/h5-9,14,16,19-20,23,25H,10-13,15,17-18,32H2,1-4H3,(H,33,36)/t23?,25-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306333
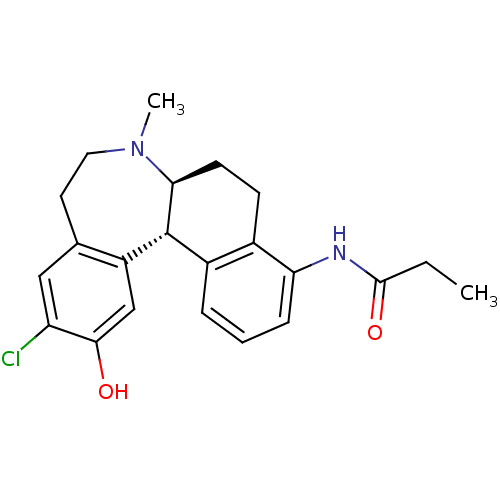
(CHEMBL601634 | N-((6aS,13bS)-11-chloro-12-hydroxy-...)Show SMILES CCC(=O)Nc1cccc2[C@H]3[C@H](CCc12)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C22H25ClN2O2/c1-3-21(27)24-18-6-4-5-15-14(18)7-8-19-22(15)16-12-20(26)17(23)11-13(16)9-10-25(19)2/h4-6,11-12,19,22,26H,3,7-10H2,1-2H3,(H,24,27)/t19-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306321
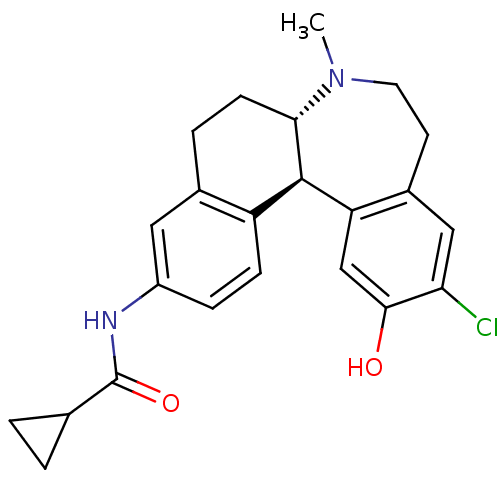
(CHEMBL598516 | N-((6aS,13bR)-11-chloro-12-hydroxy-...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1cc(NC(=O)C3CC3)ccc21 |r| Show InChI InChI=1S/C23H25ClN2O2/c1-26-9-8-15-11-19(24)21(27)12-18(15)22-17-6-5-16(25-23(28)13-2-3-13)10-14(17)4-7-20(22)26/h5-6,10-13,20,22,27H,2-4,7-9H2,1H3,(H,25,28)/t20-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306331
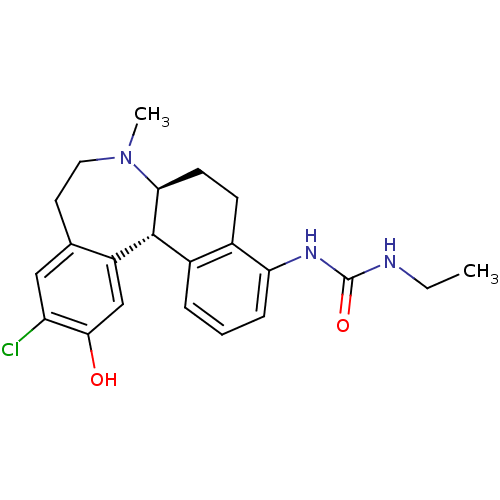
(1-((6aS,13bS)-11-chloro-12-hydroxy-7-methyl-6,6a,7...)Show SMILES CCNC(=O)Nc1cccc2[C@H]3[C@H](CCc12)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C22H26ClN3O2/c1-3-24-22(28)25-18-6-4-5-15-14(18)7-8-19-21(15)16-12-20(27)17(23)11-13(16)9-10-26(19)2/h4-6,11-12,19,21,27H,3,7-10H2,1-2H3,(H2,24,25,28)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215581
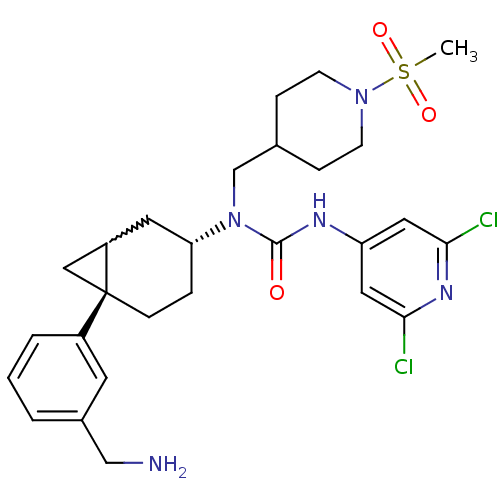
(1-((3R,6S)-6-(3-(aminomethyl)phenyl)bicyclo[4.1.0]...)Show SMILES CS(=O)(=O)N1CCC(CN([C@@H]2CC[C@@]3(CC3C2)c2cccc(CN)c2)C(=O)Nc2cc(Cl)nc(Cl)c2)CC1 |w:15.16| Show InChI InChI=1S/C27H35Cl2N5O3S/c1-38(36,37)33-9-6-18(7-10-33)17-34(26(35)31-22-13-24(28)32-25(29)14-22)23-5-8-27(15-21(27)12-23)20-4-2-3-19(11-20)16-30/h2-4,11,13-14,18,21,23H,5-10,12,15-17,30H2,1H3,(H,31,32,35)/t21?,23-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50111353
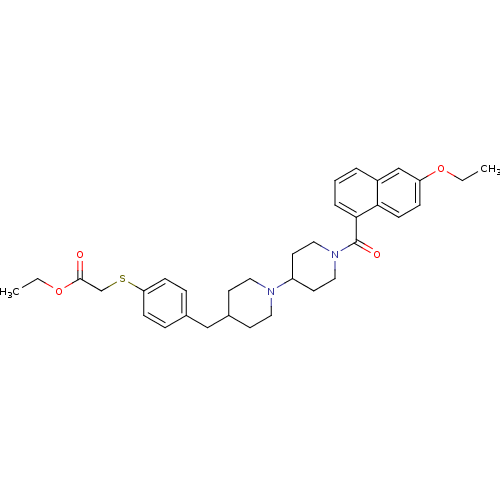
(CHEMBL418530 | {4-[1'-(6-Ethoxy-naphthalene-1-carb...)Show SMILES CCOC(=O)CSc1ccc(CC2CCN(CC2)C2CCN(CC2)C(=O)c2cccc3cc(OCC)ccc23)cc1 Show InChI InChI=1S/C34H42N2O4S/c1-3-39-29-10-13-31-27(23-29)6-5-7-32(31)34(38)36-20-16-28(17-21-36)35-18-14-26(15-19-35)22-25-8-11-30(12-9-25)41-24-33(37)40-4-2/h5-13,23,26,28H,3-4,14-22,24H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand. |
Bioorg Med Chem Lett 12: 1087-91 (2002)
BindingDB Entry DOI: 10.7270/Q2MS3S2P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215629
(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cccc(c1)[N+]([O-])=O)C(C)C |w:12.13| Show InChI InChI=1S/C29H37N5O3/c1-20(2)32(21(3)4)13-14-33(28(35)31-25-9-6-10-27(17-25)34(36)37)26-11-12-29(18-24(29)16-26)23-8-5-7-22(15-23)19-30/h5-10,15,17,20-21,24,26H,11-14,16,18H2,1-4H3,(H,31,35)/t24?,26-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHR1 expressed in CHO cells |
Bioorg Med Chem 15: 5369-85 (2007)
Article DOI: 10.1016/j.bmc.2007.05.068
BindingDB Entry DOI: 10.7270/Q25T3K5V |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D5 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data