 Found 778 hits with Last Name = 'mclay' and Initial = 'im'
Found 778 hits with Last Name = 'mclay' and Initial = 'im' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339718
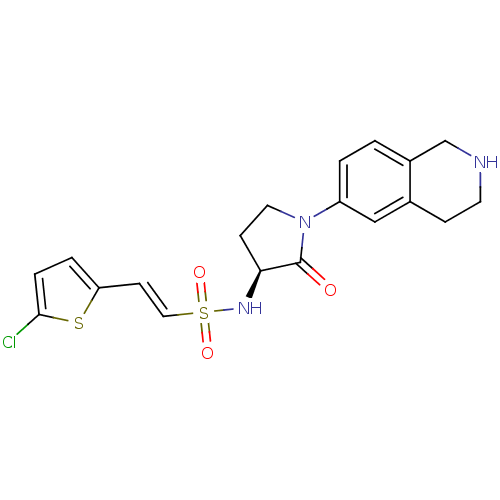
((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CNCCc3c2)s1 |r| Show InChI InChI=1S/C19H20ClN3O3S2/c20-18-4-3-16(27-18)7-10-28(25,26)22-17-6-9-23(19(17)24)15-2-1-14-12-21-8-5-13(14)11-15/h1-4,7,10-11,17,21-22H,5-6,8-9,12H2/b10-7+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339708

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(5-fluoro-1,2,3...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C19H19ClFN3O3S2/c20-17-4-2-13(28-17)7-10-29(26,27)23-15-6-9-24(19(15)25)16-3-1-12-11-22-8-5-14(12)18(16)21/h1-4,7,10,15,22-23H,5-6,8-9,11H2/b10-7+/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339713
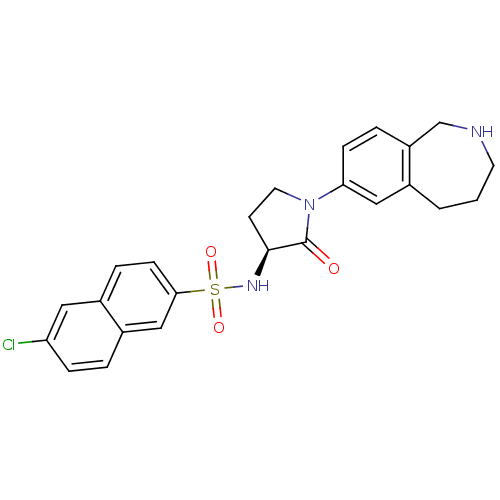
((S)-6-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCCc2c1 |r| Show InChI InChI=1S/C24H24ClN3O3S/c25-20-6-3-18-14-22(8-5-17(18)12-20)32(30,31)27-23-9-11-28(24(23)29)21-7-4-19-15-26-10-1-2-16(19)13-21/h3-8,12-14,23,26-27H,1-2,9-11,15H2/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339716

((S)-6-chloro-N-(1-(6-fluoro-2,3,4,5-tetrahydro-1H-...)Show SMILES Fc1c2CCCNCc2ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-21-9-11-29(24(21)30)22-8-5-17-14-27-10-1-2-20(17)23(22)26/h3-8,12-13,21,27-28H,1-2,9-11,14H2/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339714
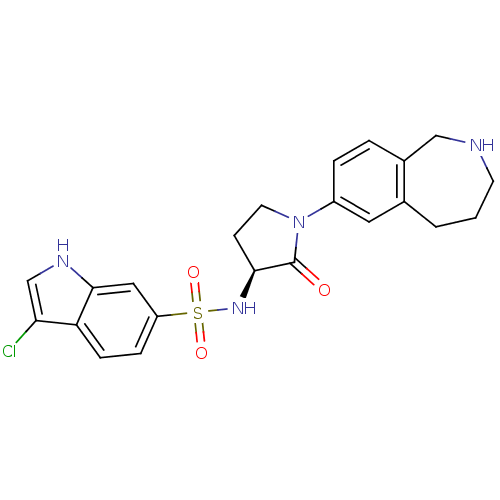
((S)-3-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...)Show SMILES Clc1c[nH]c2cc(ccc12)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCCc2c1 |r| Show InChI InChI=1S/C22H23ClN4O3S/c23-19-13-25-21-11-17(5-6-18(19)21)31(29,30)26-20-7-9-27(22(20)28)16-4-3-15-12-24-8-1-2-14(15)10-16/h3-6,10-11,13,20,24-26H,1-2,7-9,12H2/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339711

((S)-3-chloro-N-(1-(7-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1cc2CNCCc2cc1N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C21H20ClFN4O3S/c22-16-11-25-19-9-14(1-2-15(16)19)31(29,30)26-18-4-6-27(21(18)28)20-8-12-3-5-24-10-13(12)7-17(20)23/h1-2,7-9,11,18,24-26H,3-6,10H2/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339712

((S,E)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(2,3,4,...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CNCCCc3c2)s1 |r| Show InChI InChI=1S/C20H22ClN3O3S2/c21-19-6-5-17(28-19)8-11-29(26,27)23-18-7-10-24(20(18)25)16-4-3-15-13-22-9-1-2-14(15)12-16/h3-6,8,11-12,18,22-23H,1-2,7,9-10,13H2/b11-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339719
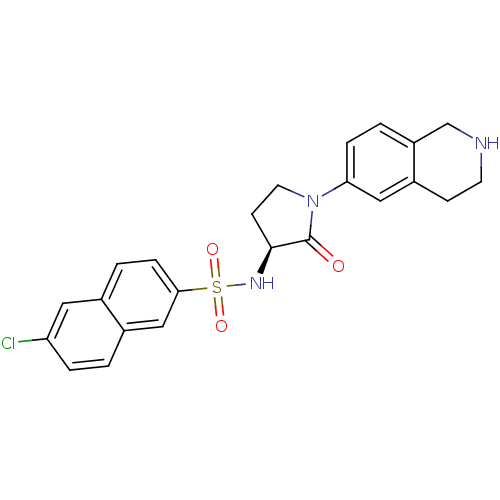
((S)-6-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCc2c1 |r| Show InChI InChI=1S/C23H22ClN3O3S/c24-19-4-1-16-13-21(6-3-15(16)11-19)31(29,30)26-22-8-10-27(23(22)28)20-5-2-18-14-25-9-7-17(18)12-20/h1-6,11-13,22,25-26H,7-10,14H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339717

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(2-methyl-1,2,3...)Show SMILES CN1CCc2cc(ccc2C1)N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C20H22ClN3O3S2/c1-23-9-6-14-12-16(3-2-15(14)13-23)24-10-7-18(20(24)25)22-29(26,27)11-8-17-4-5-19(21)28-17/h2-5,8,11-12,18,22H,6-7,9-10,13H2,1H3/b11-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339720
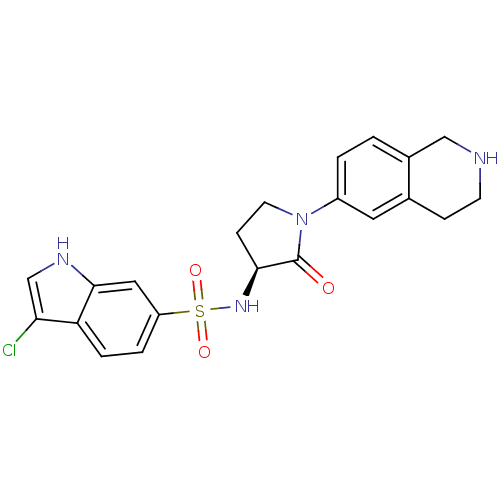
((S)-3-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1c[nH]c2cc(ccc12)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCc2c1 |r| Show InChI InChI=1S/C21H21ClN4O3S/c22-18-12-24-20-10-16(3-4-17(18)20)30(28,29)25-19-6-8-26(21(19)27)15-2-1-14-11-23-7-5-13(14)9-15/h1-4,9-10,12,19,23-25H,5-8,11H2/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339706

((S)-6-chloro-N-(1-(5-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C23H21ClFN3O3S/c24-17-4-1-15-12-18(5-2-14(15)11-17)32(30,31)27-20-8-10-28(23(20)29)21-6-3-16-13-26-9-7-19(16)22(21)25/h1-6,11-12,20,26-27H,7-10,13H2/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339715
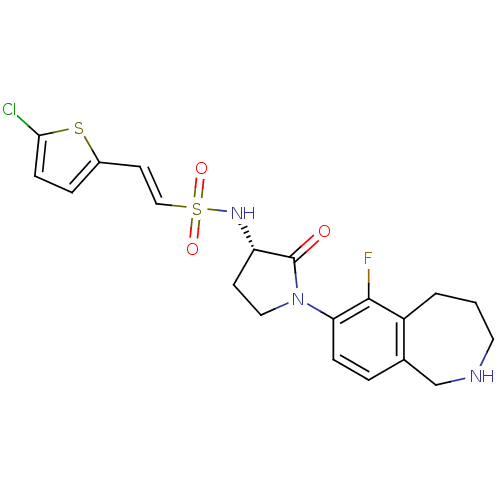
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(6-fluoro-2,3,4...)Show SMILES Fc1c2CCCNCc2ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C20H21ClFN3O3S2/c21-18-6-4-14(29-18)8-11-30(27,28)24-16-7-10-25(20(16)26)17-5-3-13-12-23-9-1-2-15(13)19(17)22/h3-6,8,11,16,23-24H,1-2,7,9-10,12H2/b11-8+/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339710
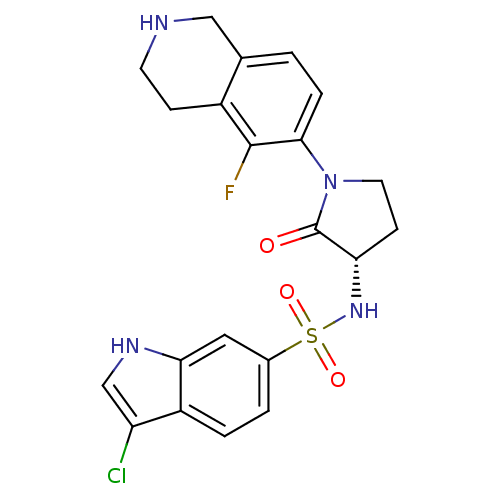
((S)-3-chloro-N-(1-(5-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C21H20ClFN4O3S/c22-16-11-25-18-9-13(2-3-15(16)18)31(29,30)26-17-6-8-27(21(17)28)19-4-1-12-10-24-7-5-14(12)20(19)23/h1-4,9,11,17,24-26H,5-8,10H2/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339709
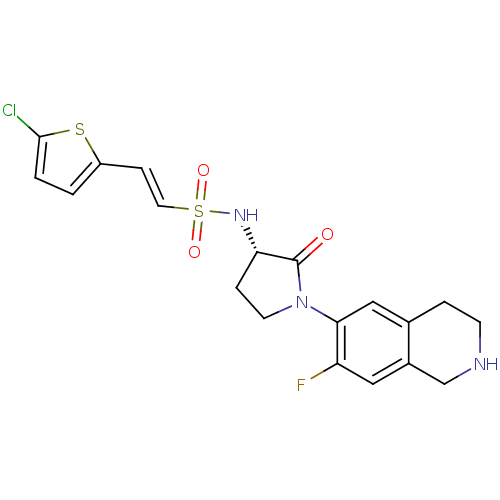
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(7-fluoro-1,2,3...)Show SMILES Fc1cc2CNCCc2cc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C19H19ClFN3O3S2/c20-18-2-1-14(28-18)5-8-29(26,27)23-16-4-7-24(19(16)25)17-10-12-3-6-22-11-13(12)9-15(17)21/h1-2,5,8-10,16,22-23H,3-4,6-7,11H2/b8-5+/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306134
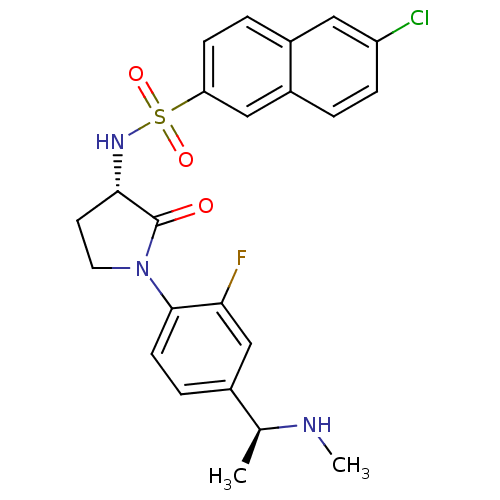
(6-chloro-N-((S)-1-(2-fluoro-4-((S)-1-(methylamino)...)Show SMILES CN[C@@H](C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C23H23ClFN3O3S/c1-14(26-2)15-5-8-22(20(25)13-15)28-10-9-21(23(28)29)27-32(30,31)19-7-4-16-11-18(24)6-3-17(16)12-19/h3-8,11-14,21,26-27H,9-10H2,1-2H3/t14-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339707
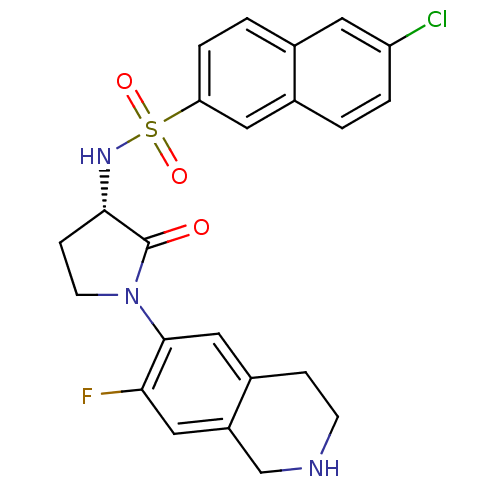
((S)-6-chloro-N-(1-(7-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1cc2CNCCc2cc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C23H21ClFN3O3S/c24-18-3-1-15-10-19(4-2-14(15)9-18)32(30,31)27-21-6-8-28(23(21)29)22-12-16-5-7-26-13-17(16)11-20(22)25/h1-4,9-12,21,26-27H,5-8,13H2/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339721

((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CCNCc3c2)s1 |r| Show InChI InChI=1S/C19H20ClN3O3S2/c20-18-4-3-16(27-18)7-10-28(25,26)22-17-6-9-23(19(17)24)15-2-1-13-5-8-21-12-14(13)11-15/h1-4,7,10-11,17,21-22H,5-6,8-9,12H2/b10-7+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339722
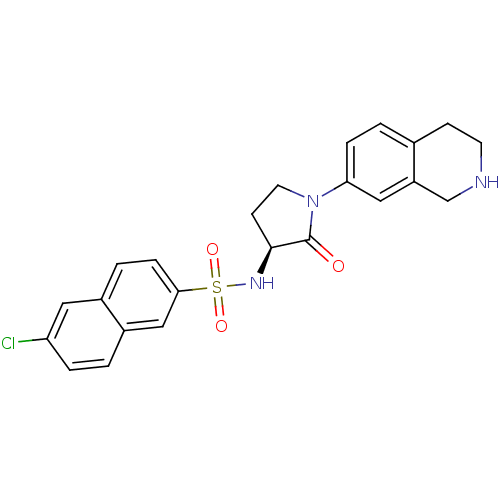
((S)-6-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CCNCc2c1 |r| Show InChI InChI=1S/C23H22ClN3O3S/c24-19-4-1-17-13-21(6-3-16(17)11-19)31(29,30)26-22-8-10-27(23(22)28)20-5-2-15-7-9-25-14-18(15)12-20/h1-6,11-13,22,25-26H,7-10,14H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339723

((S)-3-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1c[nH]c2cc(ccc12)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CCNCc2c1 |r| Show InChI InChI=1S/C21H21ClN4O3S/c22-18-12-24-20-10-16(3-4-17(18)20)30(28,29)25-19-6-8-26(21(19)27)15-2-1-13-5-7-23-11-14(13)9-15/h1-4,9-10,12,19,23-25H,5-8,11H2/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50354851
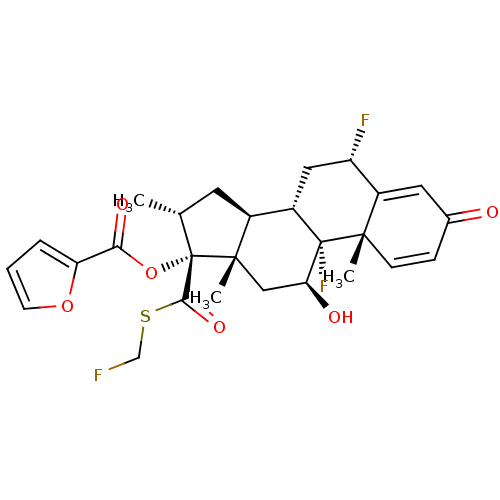
(FLUTICASONE FUROATE | Veramyst)Show SMILES C[C@@H]1C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)c1ccco1)C(=O)SCF |r,c:12,t:8| Show InChI InChI=1S/C27H29F3O6S/c1-14-9-16-17-11-19(29)18-10-15(31)6-7-24(18,2)26(17,30)21(32)12-25(16,3)27(14,23(34)37-13-28)36-22(33)20-5-4-8-35-20/h4-8,10,14,16-17,19,21,32H,9,11-13H2,1-3H3/t14-,16+,17+,19+,21+,24+,25+,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414604

(CHEMBL551816)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H22Cl2F4N4O2/c1-2-35(24(37)23-19(27)5-3-6-20(23)28)15-25(38,26(30,31)32)14-33-21-7-4-8-22-18(21)13-34-36(22)17-11-9-16(29)10-12-17/h3-13,33,38H,2,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417875
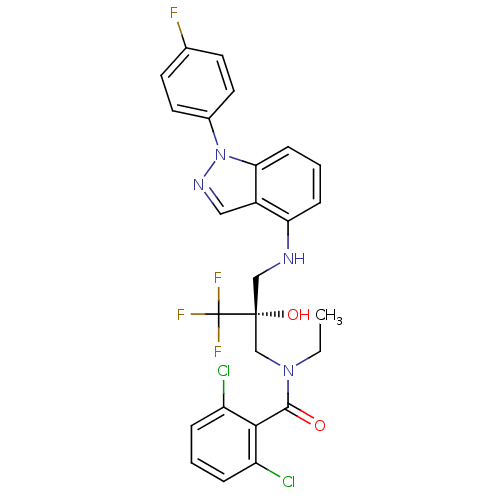
(CHEMBL1668063)Show SMILES CCN(C[C@](O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(Cl)cccc1Cl |r| Show InChI InChI=1S/C26H22Cl2F4N4O2/c1-2-35(24(37)23-19(27)5-3-6-20(23)28)15-25(38,26(30,31)32)14-33-21-7-4-8-22-18(21)13-34-36(22)17-11-9-16(29)10-12-17/h3-13,33,38H,2,14-15H2,1H3/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417887
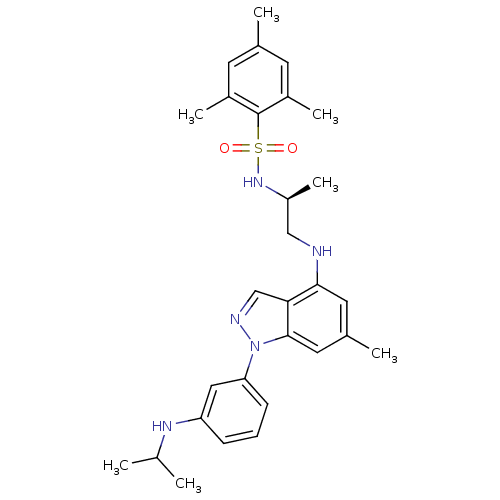
(CHEMBL1668077)Show SMILES CC(C)Nc1cccc(c1)-n1ncc2c(NC[C@H](C)NS(=O)(=O)c3c(C)cc(C)cc3C)cc(C)cc12 |r| Show InChI InChI=1S/C29H37N5O2S/c1-18(2)32-24-9-8-10-25(15-24)34-28-14-20(4)13-27(26(28)17-31-34)30-16-23(7)33-37(35,36)29-21(5)11-19(3)12-22(29)6/h8-15,17-18,23,30,32-33H,16H2,1-7H3/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414609

(CHEMBL550730)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C26H23F5N4O2/c1-2-34(24(36)19-6-3-4-7-21(19)28)16-25(37,26(29,30)31)15-32-22-8-5-9-23-20(22)14-33-35(23)18-12-10-17(27)11-13-18/h3-14,32,37H,2,15-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50473625

(CHEMBL69319)Show SMILES CN(C)C(=O)[C@@]1(C)CO[C@@H](OC1)c1nc(c([nH]1)-c1ccnc(NCC2CC2)n1)-c1ccc(F)cc1 |wU:9.12,5.5,wD:5.4,(23.19,-11.78,;22.43,-10.44,;23.2,-9.1,;20.89,-10.44,;20.11,-11.77,;20.12,-9.1,;20.89,-7.77,;19.36,-7.77,;17.82,-7.77,;17.05,-9.1,;17.82,-10.44,;19.34,-10.44,;15.51,-9.1,;14.6,-10.34,;13.12,-9.87,;13.14,-8.31,;14.61,-7.84,;11.81,-7.54,;11.81,-5.99,;10.46,-5.22,;9.13,-5.99,;9.13,-7.54,;7.79,-8.31,;6.46,-7.54,;5.12,-8.31,;4.36,-9.64,;3.59,-8.31,;10.46,-8.31,;11.89,-10.76,;12.04,-12.28,;10.8,-13.2,;9.38,-12.56,;8.04,-13.33,;9.22,-11.02,;10.48,-10.12,)| Show InChI InChI=1S/C25H29FN6O3/c1-25(23(33)32(2)3)13-34-22(35-14-25)21-30-19(16-6-8-17(26)9-7-16)20(31-21)18-10-11-27-24(29-18)28-12-15-4-5-15/h6-11,15,22H,4-5,12-14H2,1-3H3,(H,30,31)(H,27,28,29)/t22-,25- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma S.A.
Curated by ChEMBL
| Assay Description
Inhibition of p38-related TNF alpha release by human monocyte cell line (THP-1) |
J Med Chem 45: 2173-84 (2002)
Article DOI: 10.1021/jm011132l
BindingDB Entry DOI: 10.7270/Q2WW7MFK |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414610
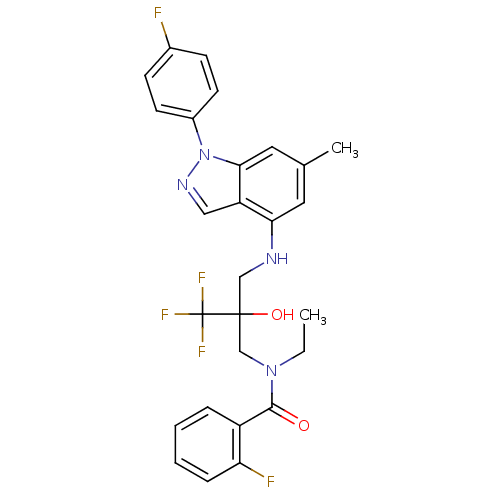
(CHEMBL564160)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C27H25F5N4O2/c1-3-35(25(37)20-6-4-5-7-22(20)29)16-26(38,27(30,31)32)15-33-23-12-17(2)13-24-21(23)14-34-36(24)19-10-8-18(28)9-11-19/h4-14,33,38H,3,15-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417890

(CHEMBL1668080)Show SMILES C[C@@H](CNc1cc(C)cc2n(ncc12)-c1cccc(N[C@H](C)C(N)=O)c1)NS(=O)(=O)c1c(C)cc(C)cc1C |r| Show InChI InChI=1S/C29H36N6O3S/c1-17-10-19(3)28(20(4)11-17)39(37,38)34-21(5)15-31-26-12-18(2)13-27-25(26)16-32-35(27)24-9-7-8-23(14-24)33-22(6)29(30)36/h7-14,16,21-22,31,33-34H,15H2,1-6H3,(H2,30,36)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417889
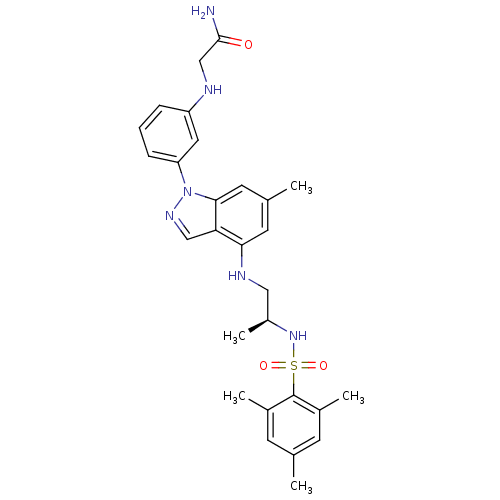
(CHEMBL1668079)Show SMILES C[C@@H](CNc1cc(C)cc2n(ncc12)-c1cccc(NCC(N)=O)c1)NS(=O)(=O)c1c(C)cc(C)cc1C |r| Show InChI InChI=1S/C28H34N6O3S/c1-17-9-19(3)28(20(4)10-17)38(36,37)33-21(5)14-31-25-11-18(2)12-26-24(25)15-32-34(26)23-8-6-7-22(13-23)30-16-27(29)35/h6-13,15,21,30-31,33H,14,16H2,1-5H3,(H2,29,35)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
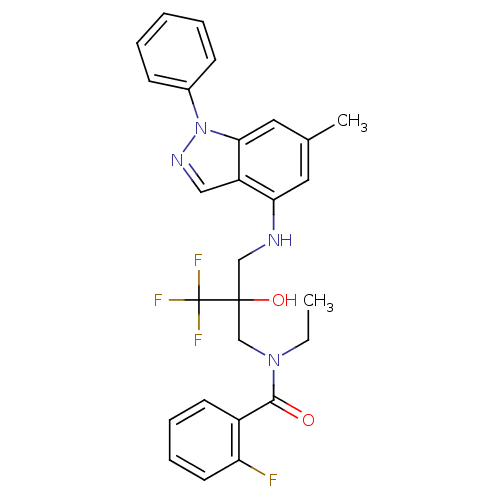
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414611
(CHEMBL560797)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C27H26F4N4O2/c1-3-34(25(36)20-11-7-8-12-22(20)28)17-26(37,27(29,30)31)16-32-23-13-18(2)14-24-21(23)15-33-35(24)19-9-5-4-6-10-19/h4-15,32,37H,3,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
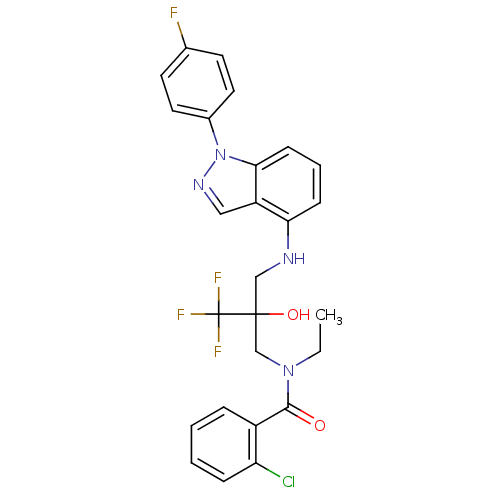
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414607
(CHEMBL563812)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1Cl Show InChI InChI=1S/C26H23ClF4N4O2/c1-2-34(24(36)19-6-3-4-7-21(19)27)16-25(37,26(29,30)31)15-32-22-8-5-9-23-20(22)14-33-35(23)18-12-10-17(28)11-13-18/h3-14,32,37H,2,15-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
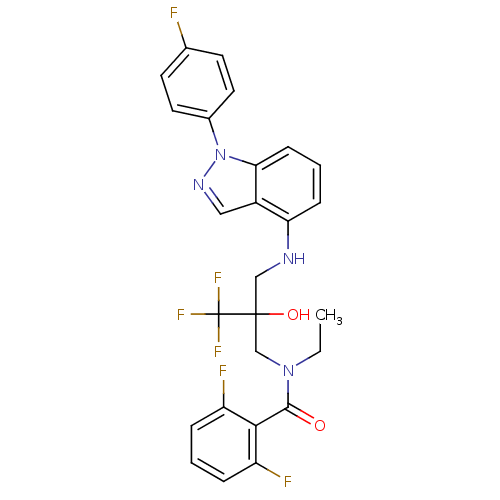
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414605
(CHEMBL556231)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(F)cccc1F Show InChI InChI=1S/C26H22F6N4O2/c1-2-35(24(37)23-19(28)5-3-6-20(23)29)15-25(38,26(30,31)32)14-33-21-7-4-8-22-18(21)13-34-36(22)17-11-9-16(27)10-12-17/h3-13,33,38H,2,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417875

(CHEMBL1668063)Show SMILES CCN(C[C@](O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(Cl)cccc1Cl |r| Show InChI InChI=1S/C26H22Cl2F4N4O2/c1-2-35(24(37)23-19(27)5-3-6-20(23)28)15-25(38,26(30,31)32)14-33-21-7-4-8-22-18(21)13-34-36(22)17-11-9-16(29)10-12-17/h3-13,33,38H,2,14-15H2,1H3/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
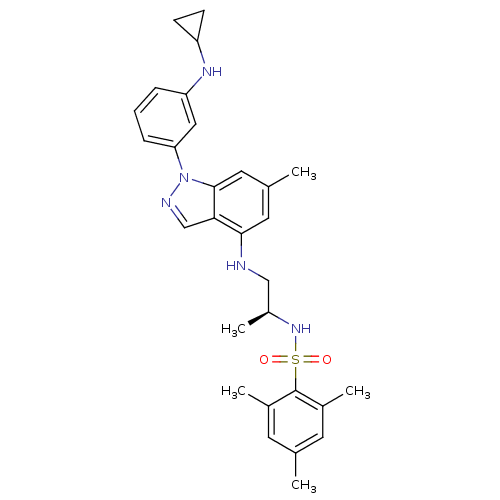
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417888
(CHEMBL1668078)Show SMILES C[C@@H](CNc1cc(C)cc2n(ncc12)-c1cccc(NC2CC2)c1)NS(=O)(=O)c1c(C)cc(C)cc1C |r| Show InChI InChI=1S/C29H35N5O2S/c1-18-11-20(3)29(21(4)12-18)37(35,36)33-22(5)16-30-27-13-19(2)14-28-26(27)17-31-34(28)25-8-6-7-24(15-25)32-23-9-10-23/h6-8,11-15,17,22-23,30,32-33H,9-10,16H2,1-5H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414612
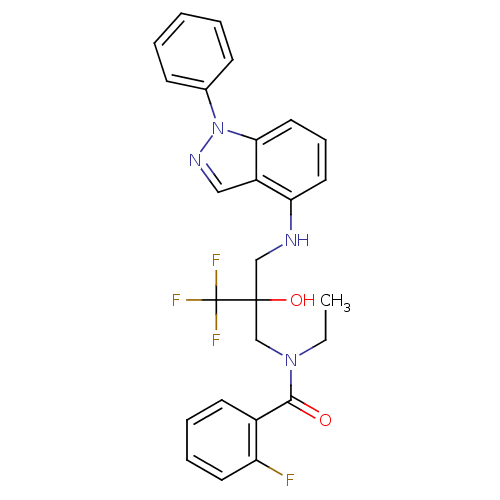
(CHEMBL559115)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1F Show InChI InChI=1S/C26H24F4N4O2/c1-2-33(24(35)19-11-6-7-12-21(19)27)17-25(36,26(28,29)30)16-31-22-13-8-14-23-20(22)15-32-34(23)18-9-4-3-5-10-18/h3-15,31,36H,2,16-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414608

(CHEMBL550933)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1C Show InChI InChI=1S/C27H26F4N4O2/c1-3-34(25(36)21-8-5-4-7-18(21)2)17-26(37,27(29,30)31)16-32-23-9-6-10-24-22(23)15-33-35(24)20-13-11-19(28)12-14-20/h4-15,32,37H,3,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414615

(CHEMBL559942)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C27H27F3N4O2/c1-3-33(25(35)20-10-6-4-7-11-20)18-26(36,27(28,29)30)17-31-23-14-19(2)15-24-22(23)16-32-34(24)21-12-8-5-9-13-21/h4-16,31,36H,3,17-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414613
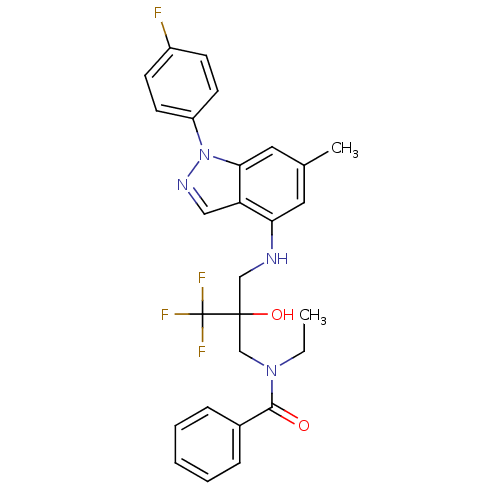
(CHEMBL561475)Show SMILES CCN(CC(O)(CNc1cc(C)cc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C27H26F4N4O2/c1-3-34(25(36)19-7-5-4-6-8-19)17-26(37,27(29,30)31)16-32-23-13-18(2)14-24-22(23)15-33-35(24)21-11-9-20(28)10-12-21/h4-15,32,37H,3,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417891
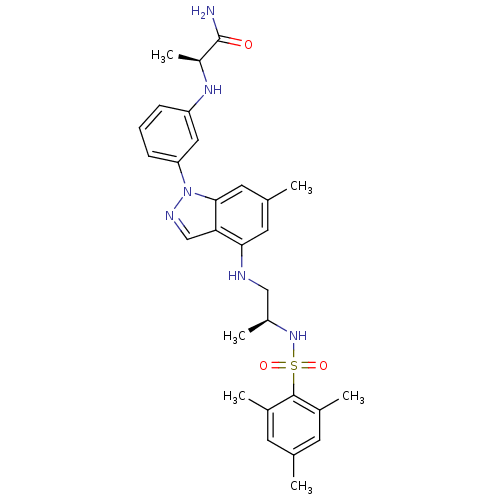
(CHEMBL1668081)Show SMILES C[C@@H](CNc1cc(C)cc2n(ncc12)-c1cccc(N[C@@H](C)C(N)=O)c1)NS(=O)(=O)c1c(C)cc(C)cc1C |r| Show InChI InChI=1S/C29H36N6O3S/c1-17-10-19(3)28(20(4)11-17)39(37,38)34-21(5)15-31-26-12-18(2)13-27-25(26)16-32-35(27)24-9-7-8-23(14-24)33-22(6)29(30)36/h7-14,16,21-22,31,33-34H,15H2,1-6H3,(H2,30,36)/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417887

(CHEMBL1668077)Show SMILES CC(C)Nc1cccc(c1)-n1ncc2c(NC[C@H](C)NS(=O)(=O)c3c(C)cc(C)cc3C)cc(C)cc12 |r| Show InChI InChI=1S/C29H37N5O2S/c1-18(2)32-24-9-8-10-25(15-24)34-28-14-20(4)13-27(26(28)17-31-34)30-16-23(7)33-37(35,36)29-21(5)11-19(3)12-22(29)6/h8-15,17-18,23,30,32-33H,16H2,1-7H3/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414606
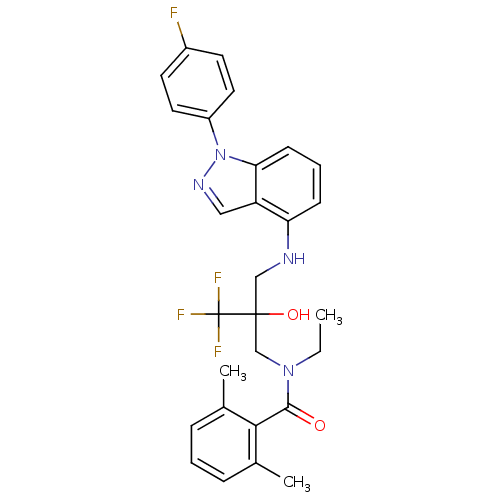
(CHEMBL550258)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C28H28F4N4O2/c1-4-35(26(37)25-18(2)7-5-8-19(25)3)17-27(38,28(30,31)32)16-33-23-9-6-10-24-22(23)15-34-36(24)21-13-11-20(29)12-14-21/h5-15,33,38H,4,16-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414618

(CHEMBL563595)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccccc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C26H25F3N4O2/c1-2-32(24(34)19-10-5-3-6-11-19)18-25(35,26(27,28)29)17-30-22-14-9-15-23-21(22)16-31-33(23)20-12-7-4-8-13-20/h3-16,30,35H,2,17-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50414614
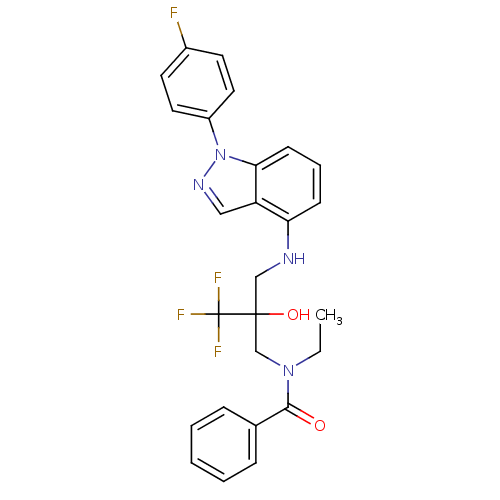
(CHEMBL561276)Show SMILES CCN(CC(O)(CNc1cccc2n(ncc12)-c1ccc(F)cc1)C(F)(F)F)C(=O)c1ccccc1 Show InChI InChI=1S/C26H24F4N4O2/c1-2-33(24(35)18-7-4-3-5-8-18)17-25(36,26(28,29)30)16-31-22-9-6-10-23-21(22)15-32-34(23)20-13-11-19(27)12-14-20/h3-15,31,36H,2,16-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207
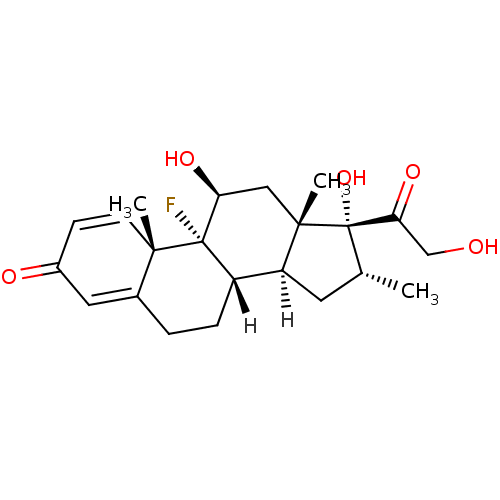
((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 17: 4737-45 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.066
BindingDB Entry DOI: 10.7270/Q2KD206G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at GR in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 19: 4846-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.020
BindingDB Entry DOI: 10.7270/Q2RR20HH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047
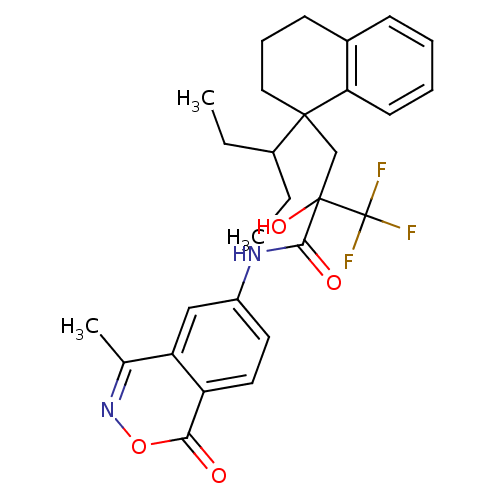
(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50411047

(CHEMBL211441)Show SMILES CCC(CC)C1(CC(O)(C(=O)Nc2ccc3c(c2)c(C)noc3=O)C(F)(F)F)CCCc2ccccc12 Show InChI InChI=1S/C28H31F3N2O4/c1-4-19(5-2)26(14-8-10-18-9-6-7-11-23(18)26)16-27(36,28(29,30)31)25(35)32-20-12-13-21-22(15-20)17(3)33-37-24(21)34/h6-7,9,11-13,15,19,36H,4-5,8,10,14,16H2,1-3H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
J Med Chem 50: 6519-34 (2007)
Article DOI: 10.1021/jm070778w
BindingDB Entry DOI: 10.7270/Q21R6RRR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at GR assessed as NF-kappaB-mediated transrepression of secreted placental alkaline phosphatase gene in human A549 cells |
J Med Chem 49: 4216-31 (2006)
Checked by Author
Article DOI: 10.1021/jm060302x
BindingDB Entry DOI: 10.7270/Q2BC40SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50417876
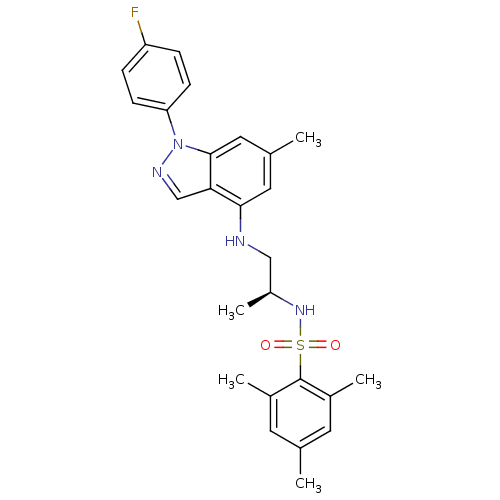
(CHEMBL1668064)Show SMILES C[C@@H](CNc1cc(C)cc2n(ncc12)-c1ccc(F)cc1)NS(=O)(=O)c1c(C)cc(C)cc1C |r| Show InChI InChI=1S/C26H29FN4O2S/c1-16-10-18(3)26(19(4)11-16)34(32,33)30-20(5)14-28-24-12-17(2)13-25-23(24)15-29-31(25)22-8-6-21(27)7-9-22/h6-13,15,20,28,30H,14H2,1-5H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Center
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in human A549 cells by NF-kappaB transrepression assay |
Bioorg Med Chem Lett 21: 1126-33 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.121
BindingDB Entry DOI: 10.7270/Q2571D8H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data