

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014156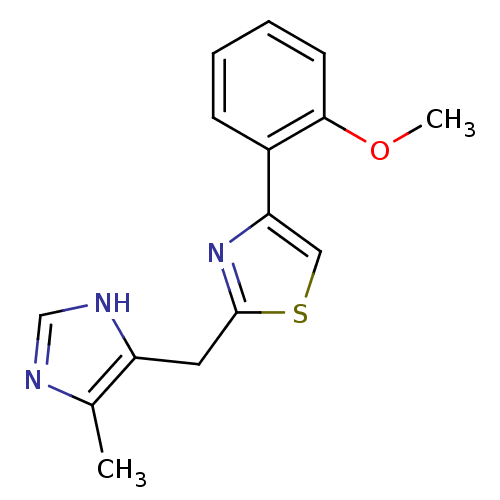 (4-(2-Methoxy-phenyl)-2-(5-methyl-1H-imidazol-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350987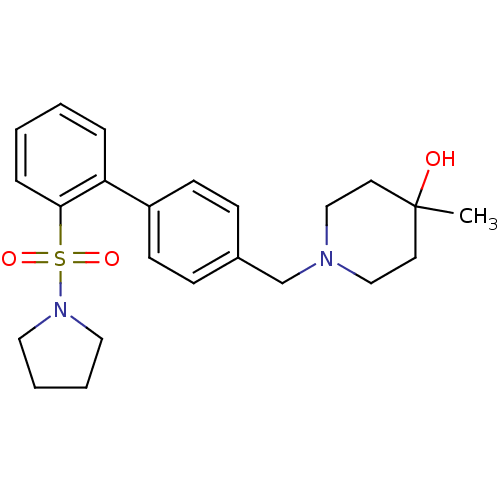 (CHEMBL1818233) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306288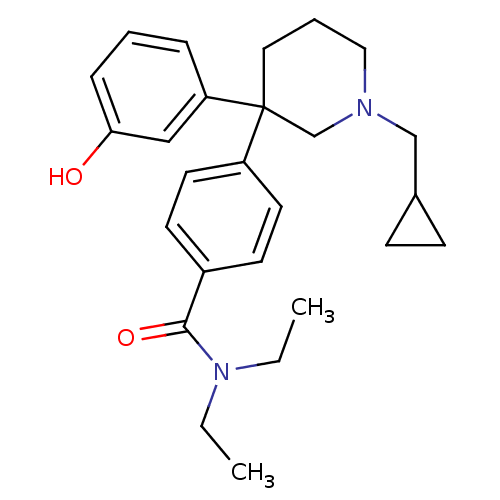 (4-(1-(cyclopropylmethyl)-3-(3-hydroxyphenyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306298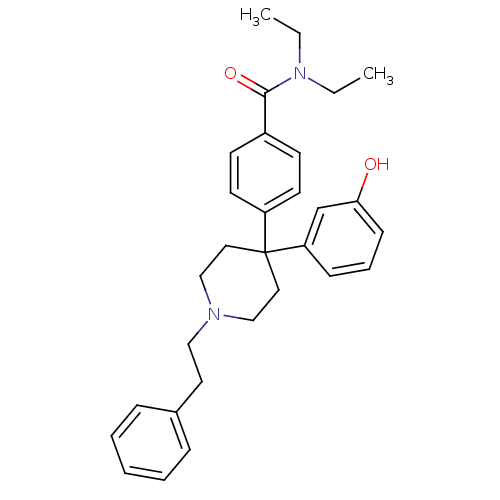 (CHEMBL595472 | N,N-diethyl-4-(4-(3-hydroxyphenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014174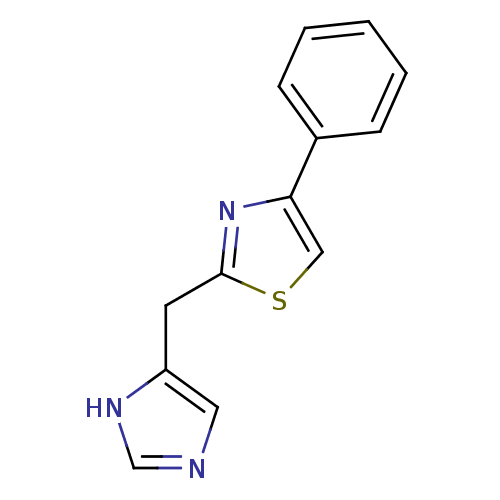 (2-(1H-Imidazol-4-ylmethyl)-4-phenyl-thiazole | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309873 (4-(6-Chlorooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013046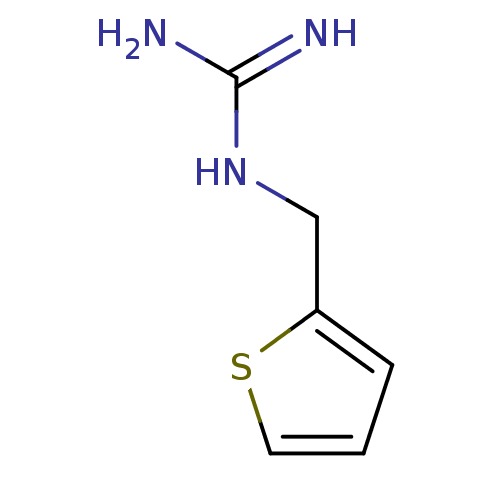 (1-(Thiophen-2-ylmethyl)guanidine | CHEMBL93064 | N...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014164 (8-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013040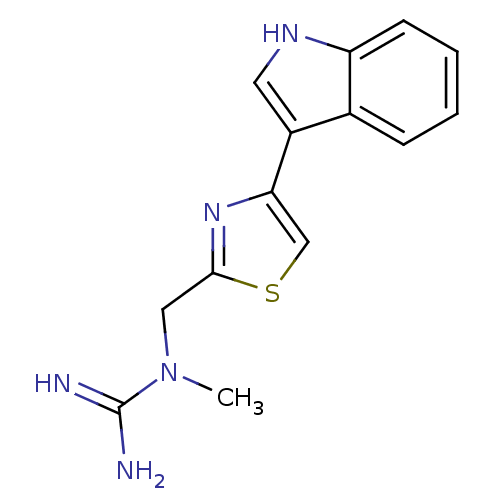 (CHEMBL93244 | N-[4-(1H-Indol-3-yl)-thiazol-2-ylmet...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013044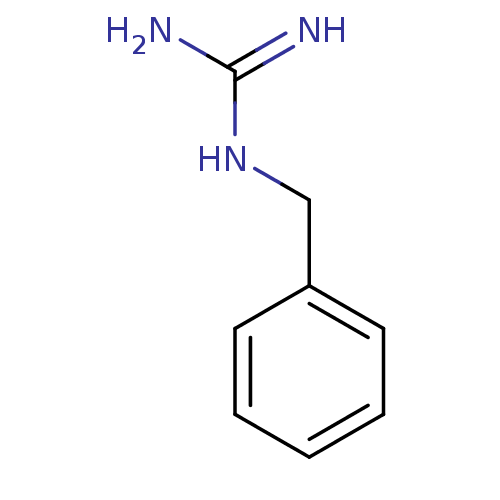 (1-Benzylguanidine | CHEMBL288640 | N-Benzyl-guanid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013045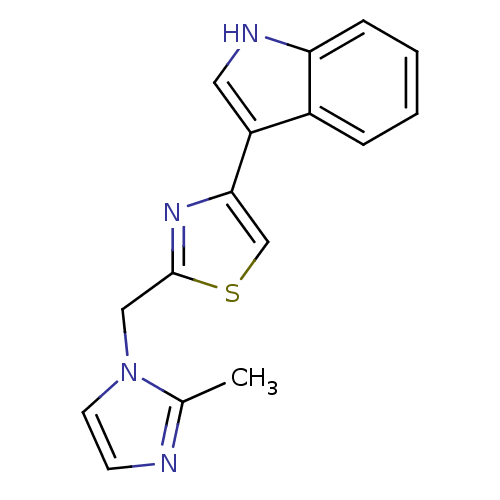 (3-[2-(2-Methyl-imidazol-1-ylmethyl)-thiazol-4-yl]-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350958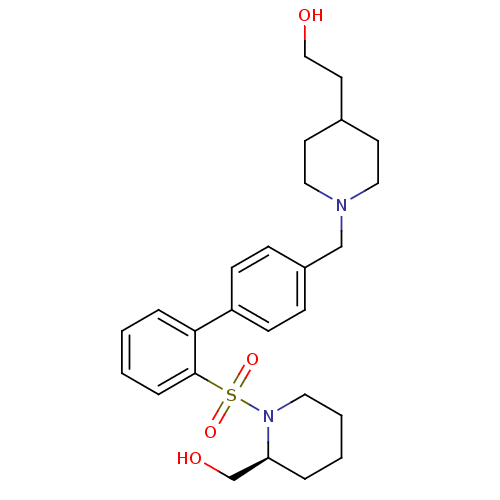 (CHEMBL1818236) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350976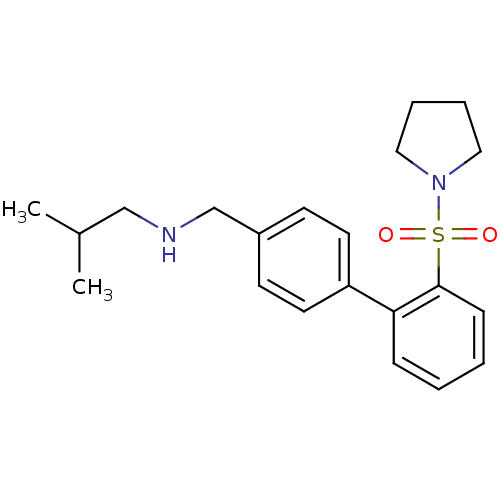 (CHEMBL1818341) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor by GTPgamma S binding assay | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306293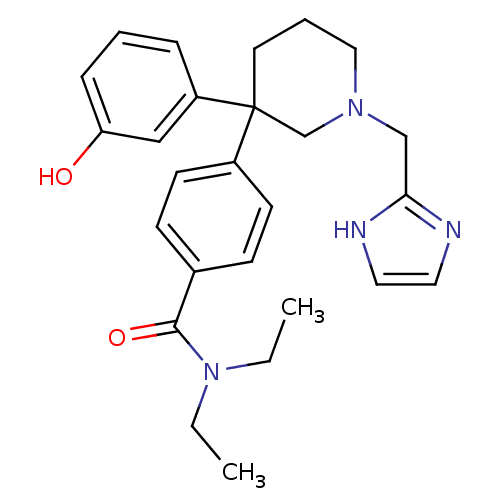 (4-(1-((1H-imidazol-2-yl)methyl)-3-(3-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of [3H]U-69,593 binding to human Opioid receptor kappa 1 expressed in HEK 293 cells | Bioorg Med Chem Lett 10: 523-6 (2000) BindingDB Entry DOI: 10.7270/Q2BG2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50086359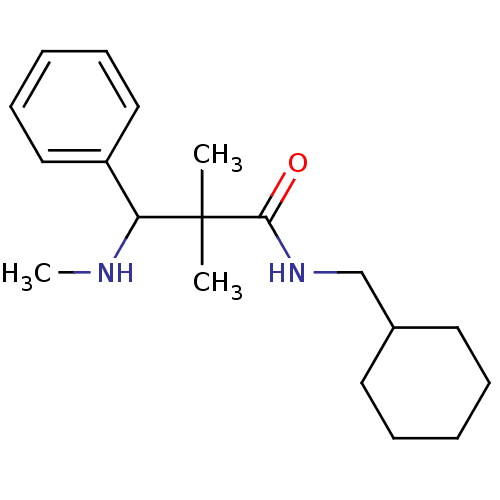 (CHEMBL147191 | N-Cyclohexylmethyl-2,2-dimethyl-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of [3H]U-69,593 binding to human Opioid receptor kappa 1 expressed in HEK 293 cells | Bioorg Med Chem Lett 10: 523-6 (2000) BindingDB Entry DOI: 10.7270/Q2BG2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309864 (4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50086361 (3-(3-Hydroxy-phenyl)-2,2-dimethyl-3-methylamino-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of [3H]U-69,593 binding to human Opioid receptor kappa 1 expressed in HEK 293 cells | Bioorg Med Chem Lett 10: 523-6 (2000) BindingDB Entry DOI: 10.7270/Q2BG2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Mus musculus (house mouse)) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50086367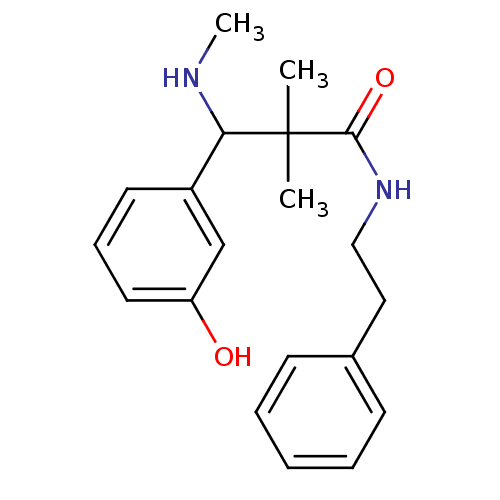 (3-(3-Hydroxy-phenyl)-2,2-dimethyl-3-methylamino-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to rat Opioid receptor mu 1 | Bioorg Med Chem Lett 10: 523-6 (2000) BindingDB Entry DOI: 10.7270/Q2BG2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to rat Opioid receptor mu 1 | Bioorg Med Chem Lett 10: 523-6 (2000) BindingDB Entry DOI: 10.7270/Q2BG2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50086361 (3-(3-Hydroxy-phenyl)-2,2-dimethyl-3-methylamino-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to rat Opioid receptor mu 1 | Bioorg Med Chem Lett 10: 523-6 (2000) BindingDB Entry DOI: 10.7270/Q2BG2N79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Antibacterial activity against Escherichia coli DHFR | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity of the compound towards human NK-1 receptor in IM-9 cells using [3H]-SP of substance P antagonist | Bioorg Med Chem Lett 4: 1865-1868 (1994) Article DOI: 10.1016/S0960-894X(01)80386-3 BindingDB Entry DOI: 10.7270/Q2X34XD3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50350990 (CHEMBL1818334) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor by GTPgamma S binding assay | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350956 (CHEMBL1818234) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306299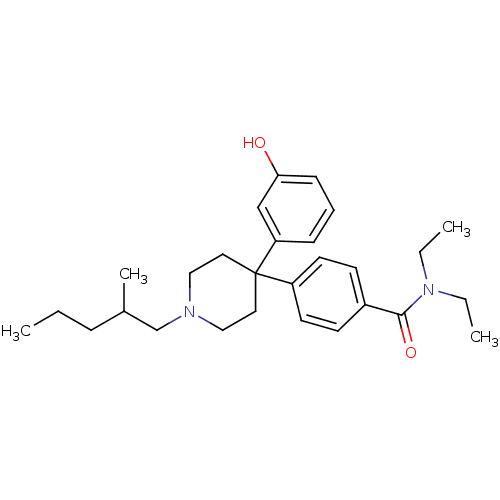 (CHEMBL595473 | N,N-diethyl-4-(4-(3-hydroxyphenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM81980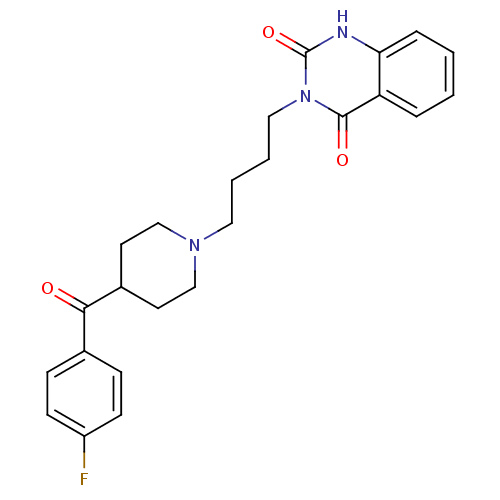 (Butanserin | CAS_87051-46-5 | NSC_65652) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oregon Health Sciences University Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 83: 8784-8 (1986) Article DOI: 10.1073/pnas.83.22.8784 BindingDB Entry DOI: 10.7270/Q2V122XD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014159 (4-(2-Fluoro-phenyl)-2-(5-methyl-1H-imidazol-4-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306297 (4-(1-allyl-4-(3-hydroxyphenyl)piperidin-4-yl)-N,N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309897 (2-(1,4-Diazabicyclo[3.2.2]nonan-4-yl)-5-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309864 (4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306287 (3-(4-(4-(3-hydroxypentan-3-yl)phenyl)-1-(2-methylp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309872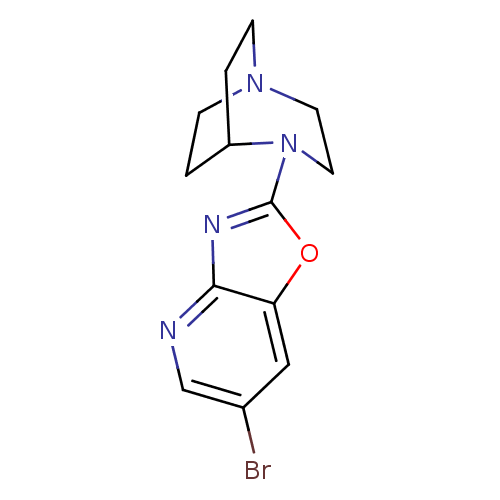 (4-(6-Bromooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309865 (4-(5-Bromobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350983 (CHEMBL1818330) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306290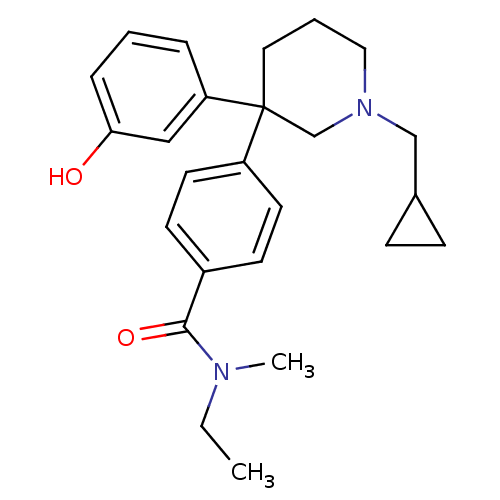 (4-(1-(cyclopropylmethyl)-3-(3-hydroxyphenyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50306301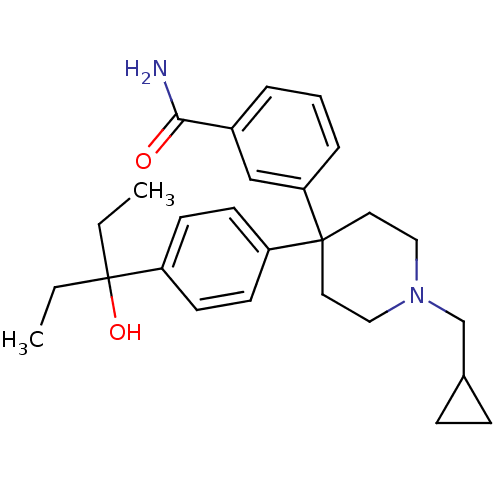 (3-(1-(cyclopropylmethyl)-4-(4-(3-hydroxypentan-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]diprenorphine from human delta opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 20: 503-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.113 BindingDB Entry DOI: 10.7270/Q2C53KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309880 (4-(6-Phenyloxazolo[5,4-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50225898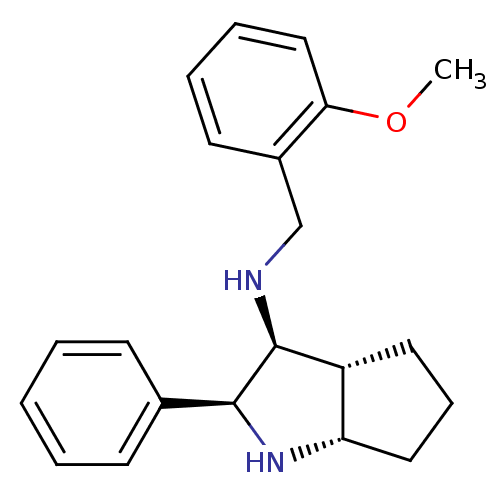 ((2-Methoxy-benzyl)-((2S,3S,3aR,6aS)-2-phenyl-octah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity of the compound towards human NK-1 receptor in IM-9 cells using [3H]-SP of substance P antagonist | Bioorg Med Chem Lett 4: 1865-1868 (1994) Article DOI: 10.1016/S0960-894X(01)80386-3 BindingDB Entry DOI: 10.7270/Q2X34XD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350990 (CHEMBL1818334) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor by GTPgamma S binding assay | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309879 (2-(6-Chlorooxazolo[5,4-b]pyridin-2-yl)-2,5-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350985 (CHEMBL1818332) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr by liquid scintillation counting | J Med Chem 54: 5868-77 (2011) Article DOI: 10.1021/jm2006035 BindingDB Entry DOI: 10.7270/Q2X63NBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 538 total ) | Next | Last >> |