

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101929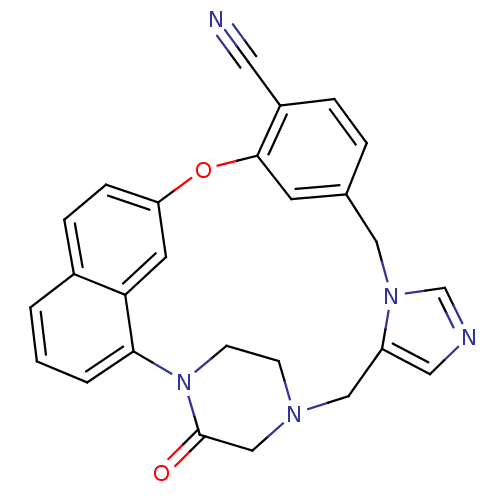 (3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50219021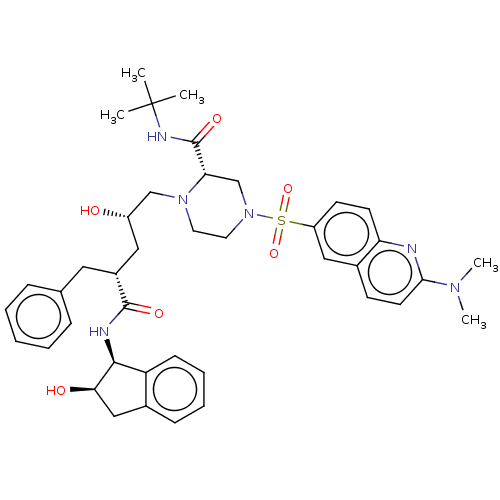 (CHEMBL1203459) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against mutant HIV-1 (A-44)protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50219020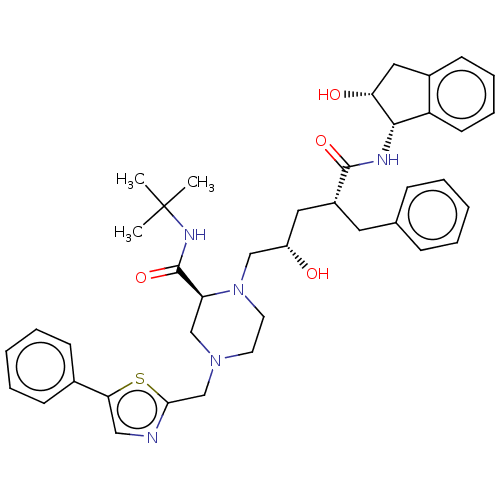 (CHEMBL421786) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested in vitro for the ability to inhibit cleavage of a substrate by the wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50219019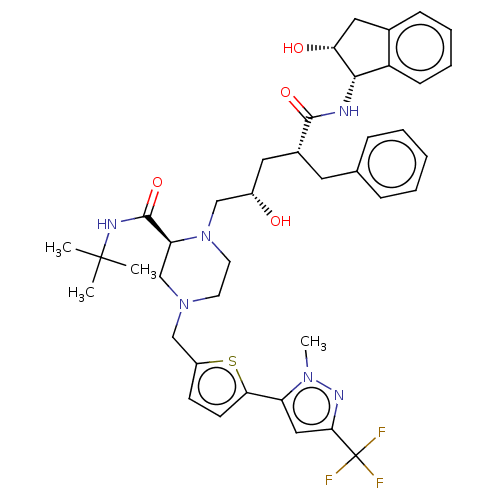 (CHEMBL422323) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested in vitro for the ability to inhibit cleavage of a substrate by the wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109710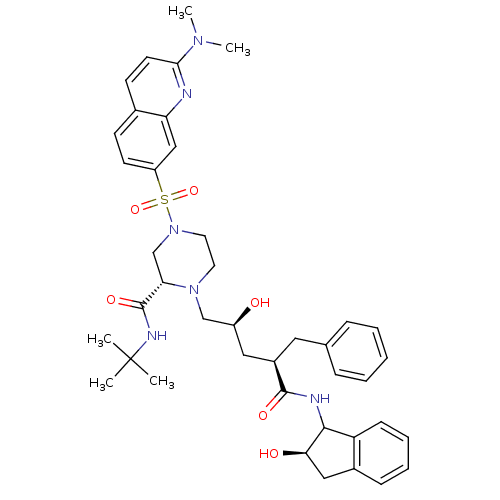 ((S)-4-(2-Dimethylamino-quinoline-7-sulfonyl)-1-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101925 (16-benzyl-17-oxo-(16R)-2-oxa-9,11,15,18-tetraazape...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109714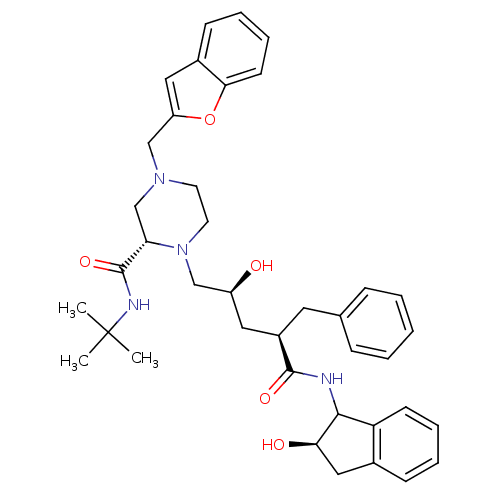 ((S)-4-Benzofuran-2-ylmethyl-1-[(2S,4R)-2-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109714 ((S)-4-Benzofuran-2-ylmethyl-1-[(2S,4R)-2-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested in vitro for the ability to inhibit cleavage of a substrate by the wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound to prevent cleavage of a substrate by the protease enzyme | Bioorg Med Chem Lett 10: 1527-30 (2000) BindingDB Entry DOI: 10.7270/Q2542MTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50219021 (CHEMBL1203459) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109706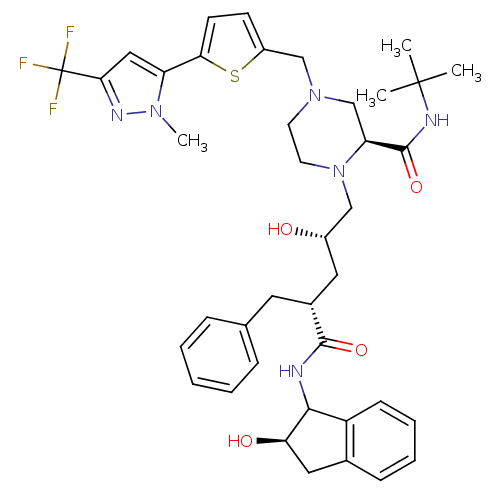 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against mutant HIV-1 (A-44)protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109706 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50219020 (CHEMBL421786) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested in vitro for the ability to inhibit cleavage of a substrate by the wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50219019 (CHEMBL422323) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against mutant HIV-1 (A-44)protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109705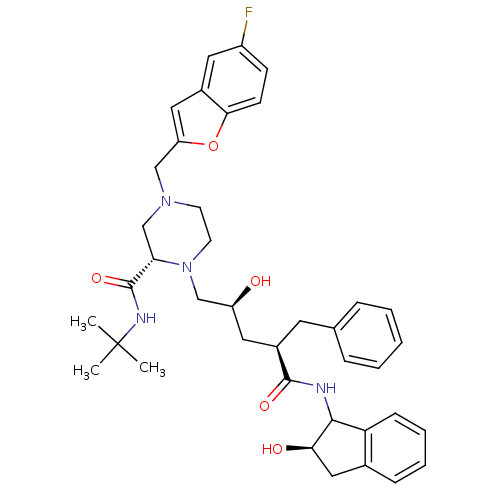 ((S)-4-(5-Fluoro-benzofuran-2-ylmethyl)-1-[(2S,4R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50090147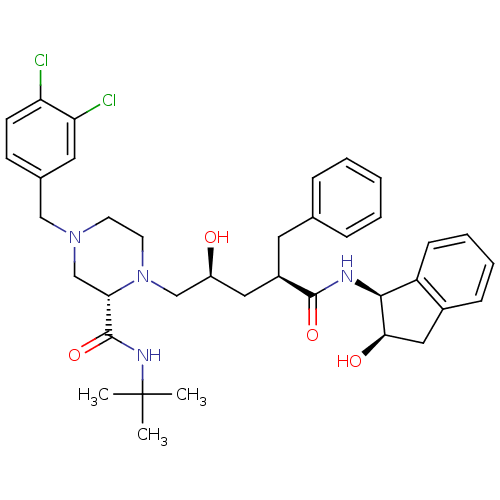 ((S)-4-(3,4-Dichloro-benzyl)-1-[(2S,4R)-2-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound to prevent cleavage of a substrate by the protease enzyme | Bioorg Med Chem Lett 10: 1527-30 (2000) BindingDB Entry DOI: 10.7270/Q2542MTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101923 (16-ethyl-17-oxo-(16R)-2-oxa-9,11,15,18-tetraazapen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109708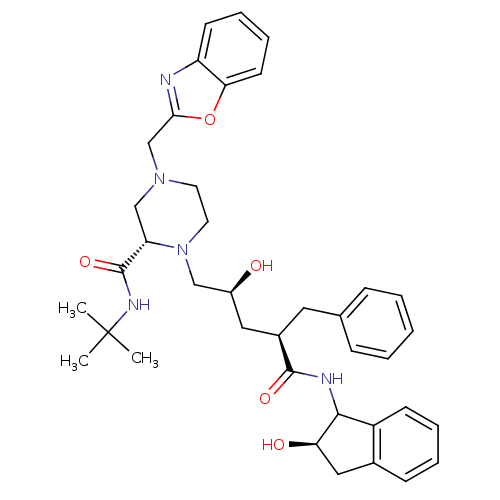 ((S)-4-Benzooxazol-2-ylmethyl-1-[(2S,4R)-2-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109708 ((S)-4-Benzooxazol-2-ylmethyl-1-[(2S,4R)-2-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109709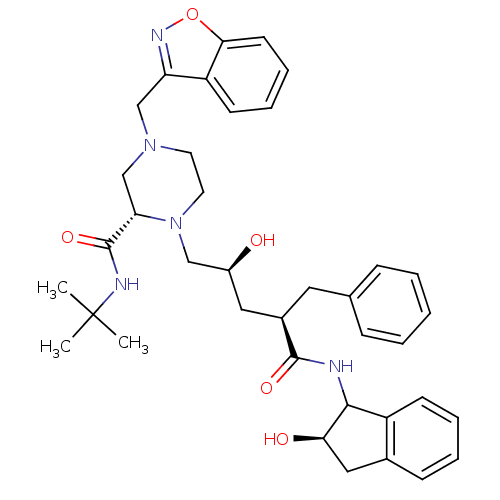 ((S)-4-Benzo[d]isoxazol-3-ylmethyl-1-[(2S,4R)-2-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101911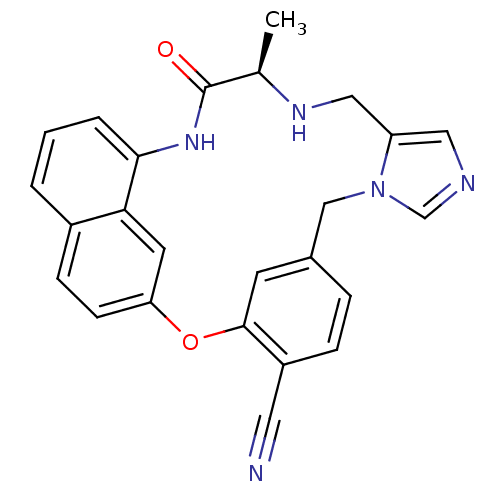 (16-methyl-17-oxo-(16R)-2-oxa-9,11,15,18-tetraazape...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50090146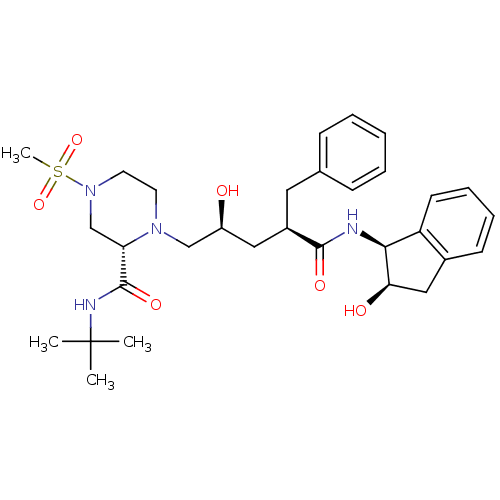 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound to prevent cleavage of a substrate by the protease enzyme | Bioorg Med Chem Lett 10: 1527-30 (2000) BindingDB Entry DOI: 10.7270/Q2542MTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101916 (17-oxo-16-(3-pyridylmethyl)-(16R)-2-oxa-9,11,15,18...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5284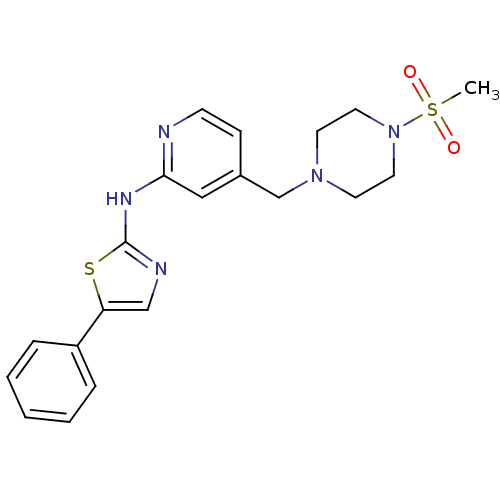 (4-{[4-(Methylsulfonyl)piperazin-1-yl]methyl}-N-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109712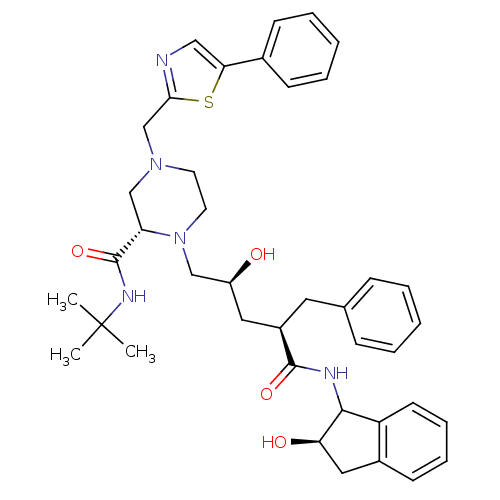 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against mutant HIV-1 (A-44)protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109712 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101918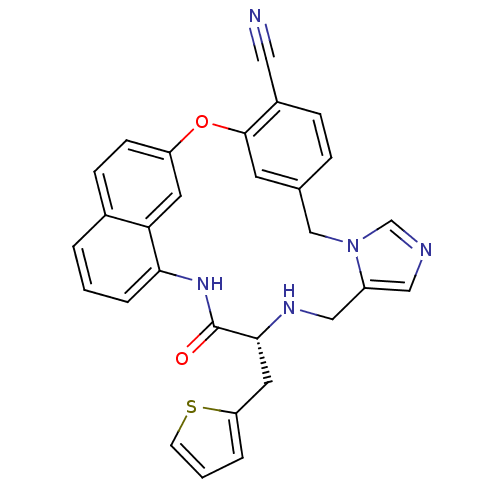 (17-oxo-16-(2-thienylmethyl)-(16R)-2-oxa-9,11,15,18...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109698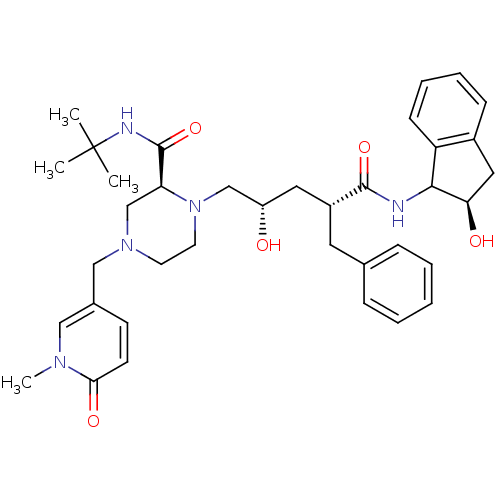 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against mutant HIV-1 (A-44)protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109710 ((S)-4-(2-Dimethylamino-quinoline-7-sulfonyl)-1-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against mutant HIV-1 (A-44)protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101917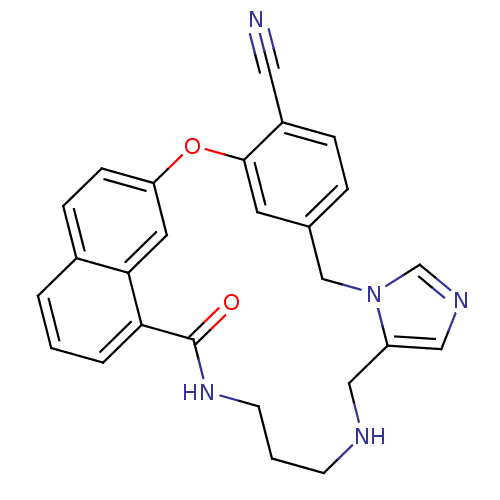 (20-oxo-2-oxa-9,11,15,19-tetraazapentacyclo[19.6.2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101910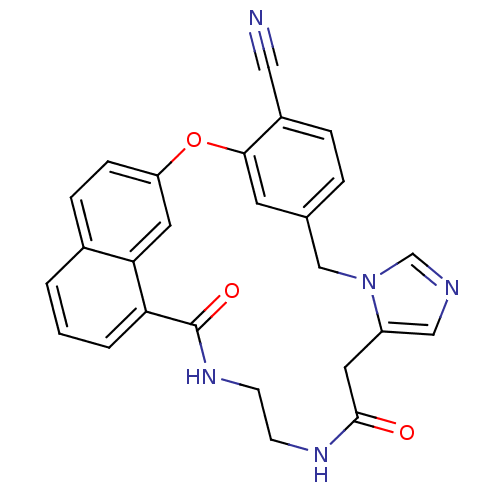 (15,20-dioxo-2-oxa-9,11,16,19-tetraazapentacyclo[19...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101924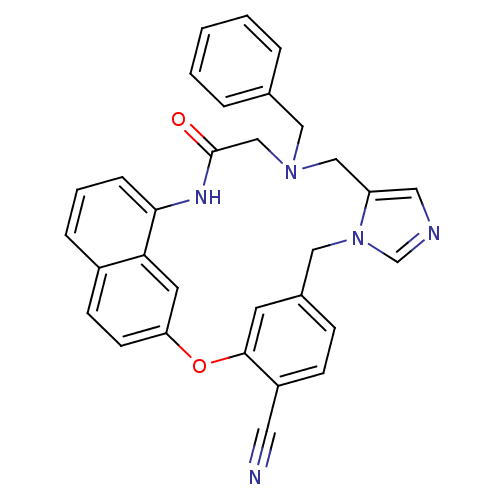 (15-benzyl-17-oxo-2-oxa-9,11,15,18-tetraazapentacyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101922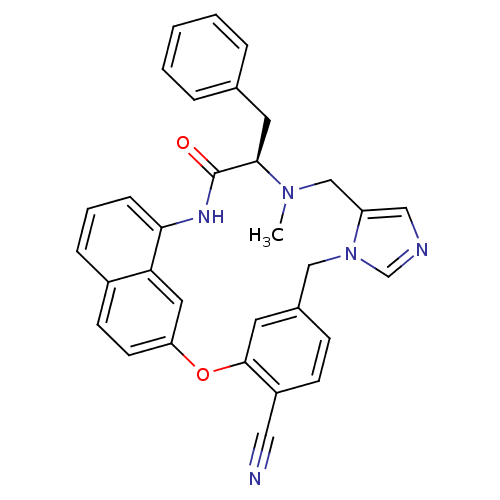 (16-benzyl-15-methyl-17-oxo-(16R)-2-oxa-9,11,15,18-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5282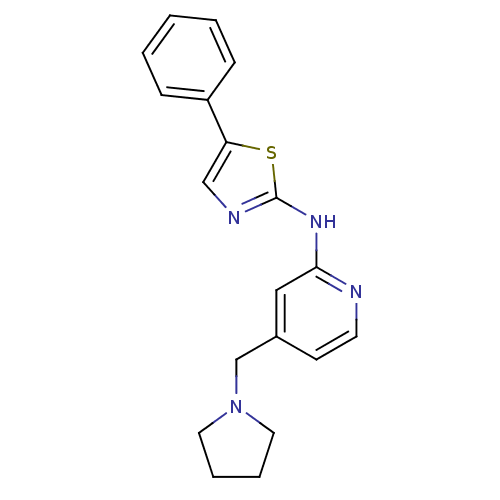 ((5-Phenylthiazol-2-yl)(4-pyrrolidin-1-ylmethylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101915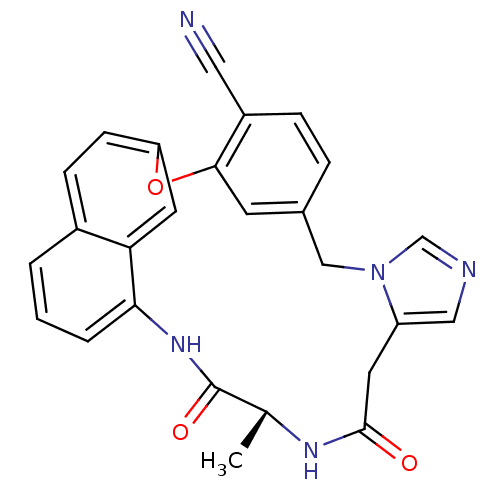 (17-methyl-15,18-dioxo-(17R)-2-oxa-9,11,16,19-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM14009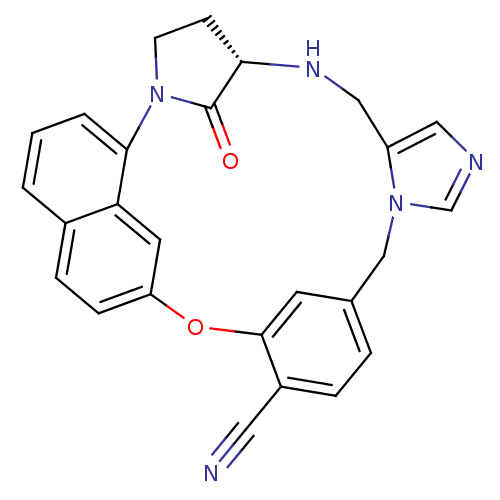 ((20S)-19,20,21,22-Tetrahydro-19-oxo-5H-18,20-ethan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101928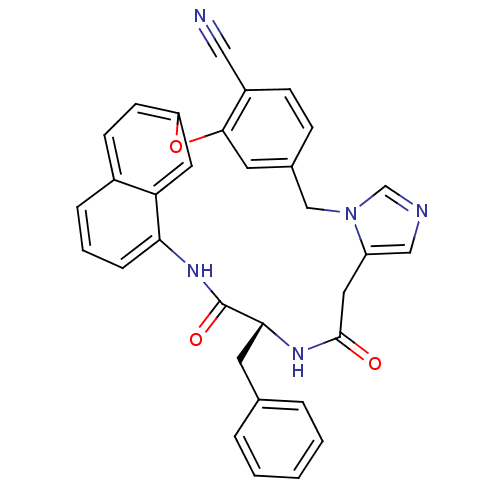 (17-benzyl-15,18-dioxo-(17R)-2-oxa-9,11,16,19-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101908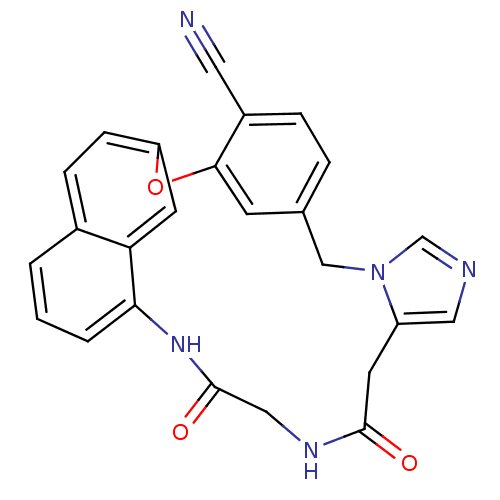 (15,18-dioxo-2-oxa-9,11,16,19-tetraazapentacyclo[18...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101926 (17-oxo-2-oxa-9,11,15,18-tetraazapentacyclo[17.6.2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101912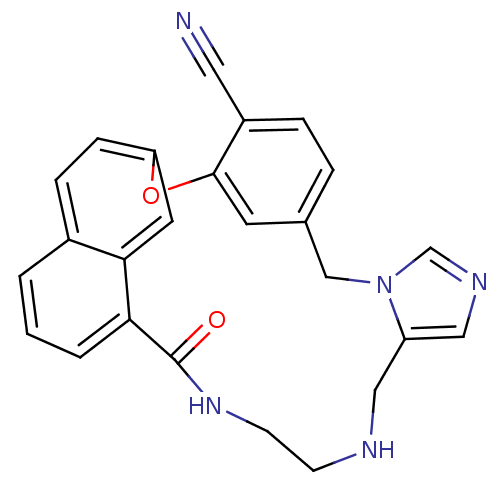 (19-oxo-2-oxa-9,11,15,18-tetraazapentacyclo[18.6.2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101914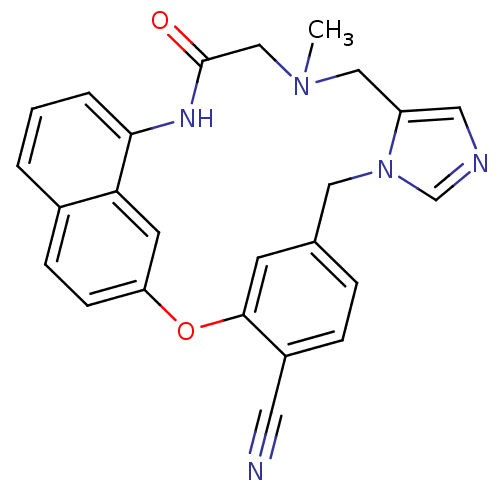 (15-methyl-17-oxo-2-oxa-9,11,15,18-tetraazapentacyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5281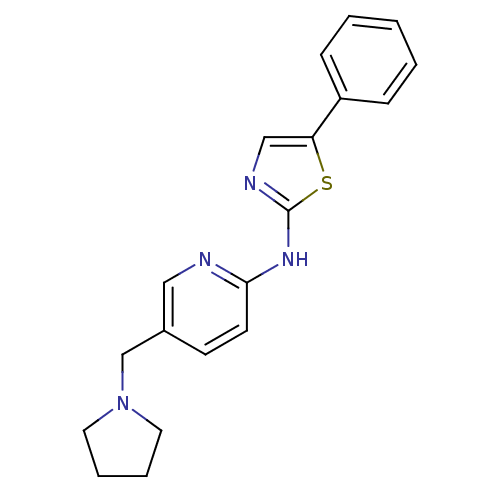 (5-phenyl-N-[5-(pyrrolidin-1-ylmethyl)pyridin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101921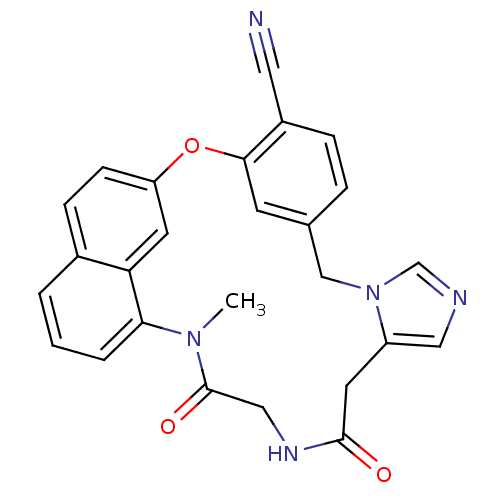 (19-methyl-15,18-dioxo-2-oxa-9,11,16,19-tetraazapen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109711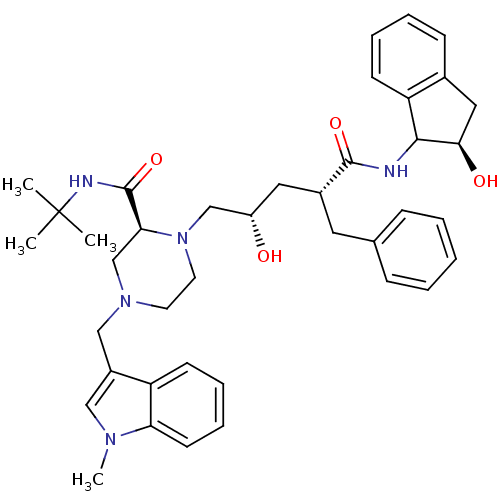 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50109699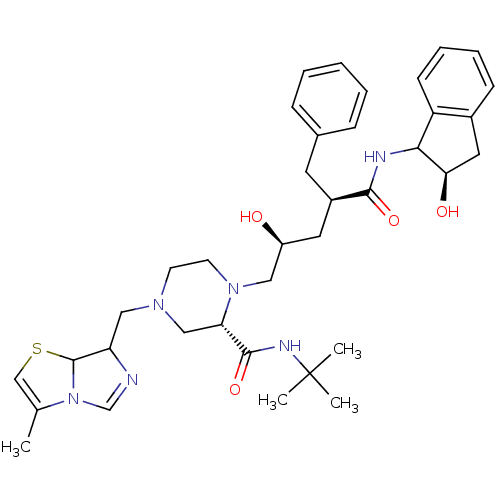 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against wild type HIV-1 protease | Bioorg Med Chem Lett 12: 529-32 (2002) BindingDB Entry DOI: 10.7270/Q2348JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50101931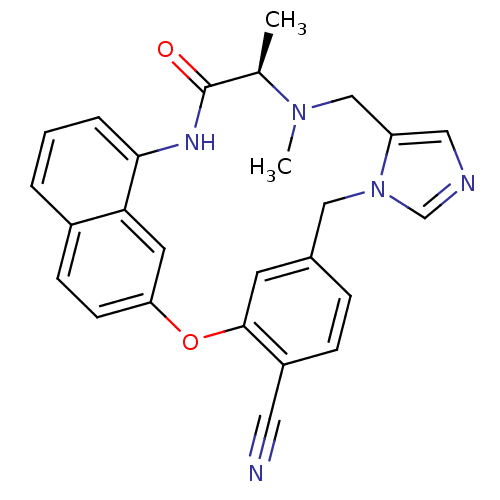 (15,16-dimethyl-17-oxo-(16R)-2-oxa-9,11,15,18-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM | Bioorg Med Chem Lett 11: 1817-21 (2001) BindingDB Entry DOI: 10.7270/Q2R210PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5292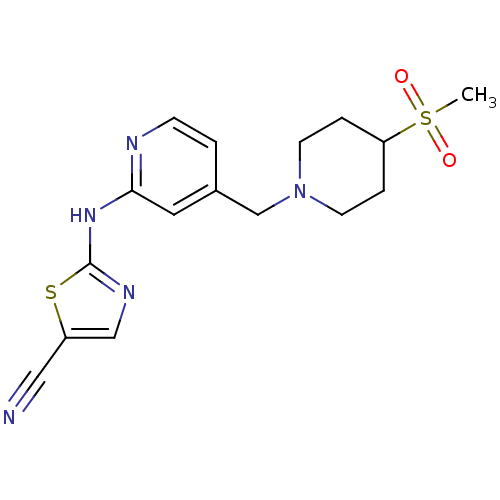 (2-({4-[(4-methanesulfonylpiperidin-1-yl)methyl]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5280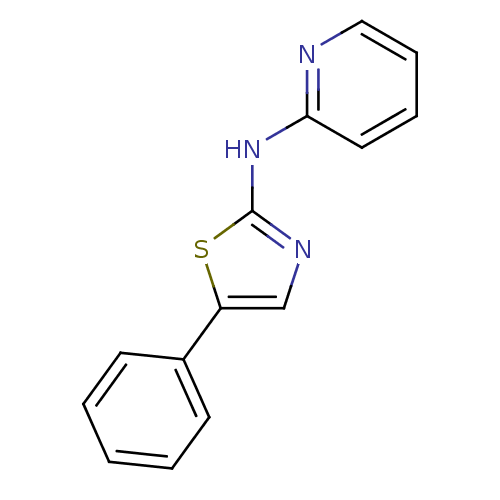 (5-phenyl-N-(pyridin-2-yl)-1,3-thiazol-2-amine | N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 281 total ) | Next | Last >> |