

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591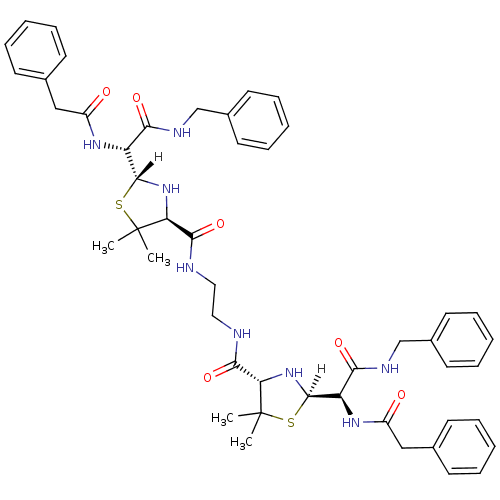 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM591 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041945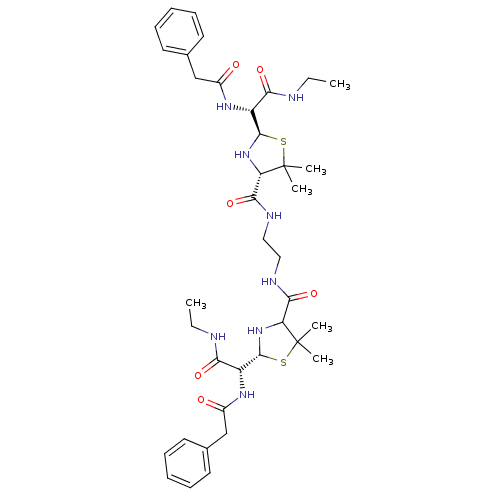 (1N-ethyl-2-benzylcarboxamido-2-[4-{2-[2-benzylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM658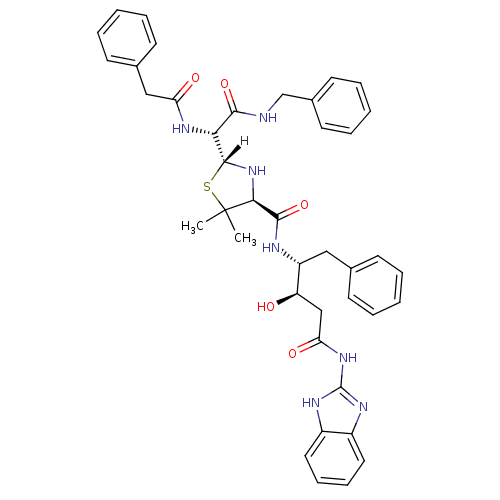 ((3R,4R)-N-(1H-1,3-benzodiazol-2-yl)-4-{[(2R,4S)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041946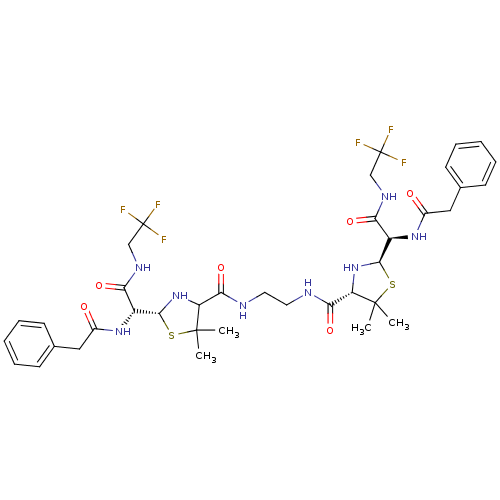 (1N-(2,2,2-trifluoroethyl)-2-benzylcarboxamido-2-[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM656 ((3R,4R)-N-benzyl-4-{[(2R,4S)-2-[(R)-(benzylcarbamo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM636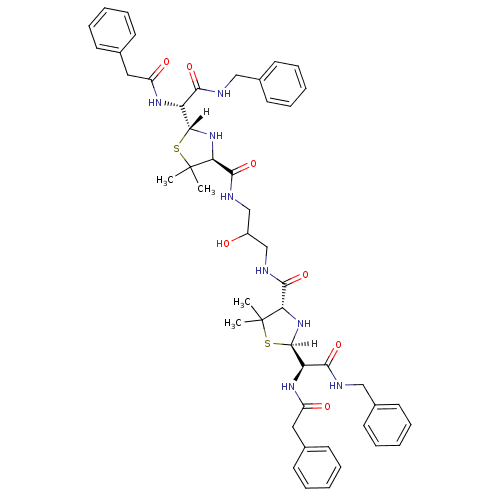 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041944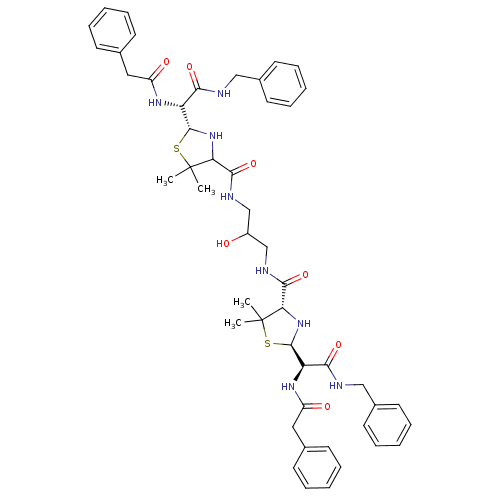 (1N-benzyl-2-[4-{3-[2-benzylcarbamoyl(benzylcarboxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037825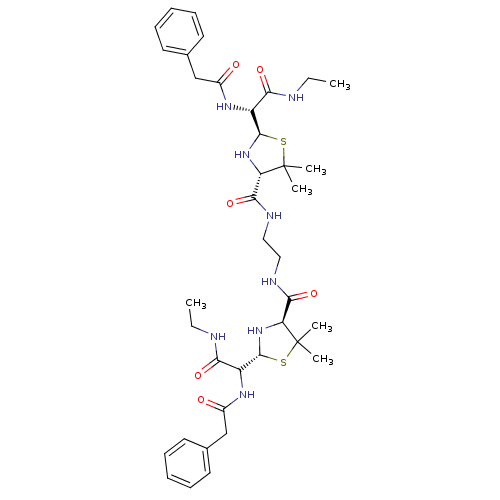 (1N-ethyl-2-benzylcarboxamido-2-[4-{2-[2-benzylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM636 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041949 (4N-{2-[5-ethylcarbamoyl(5-methyl-3-phenyl-4-isoxaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041948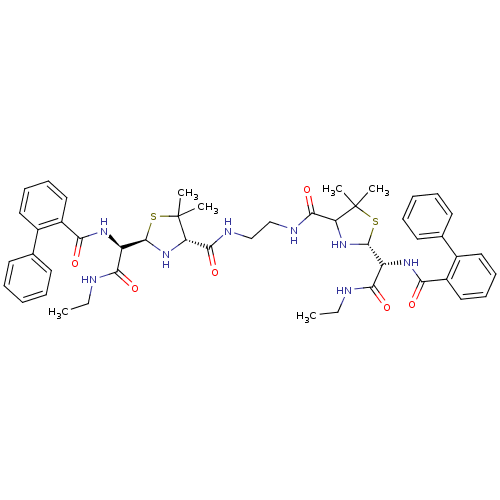 (4N-{2-[5-ethylcarbamoyl(2-phenylphenylcarboxamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037842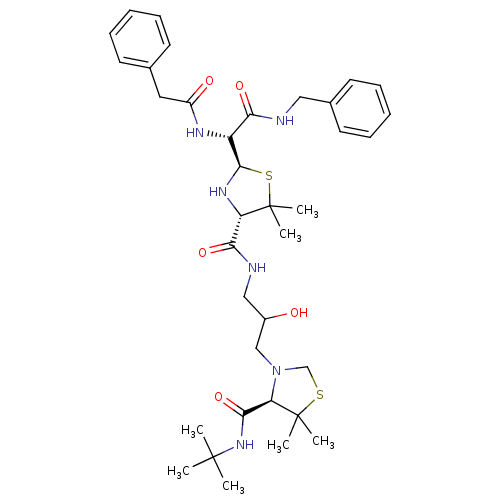 (4N-(tert-butyl)-3-{3-[2-benzylcarbamoyl(benzylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037817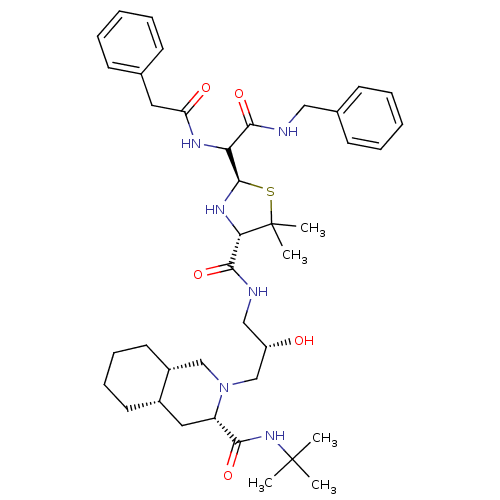 ((3S,4aS,8aS)-2-[(S)-3-({(2R,4S)-2-[Benzylcarbamoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037832 ((3S,4aS,8aS)-2-[(S)-3-({(2R,4S)-2-[Carbamoyl-((S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037833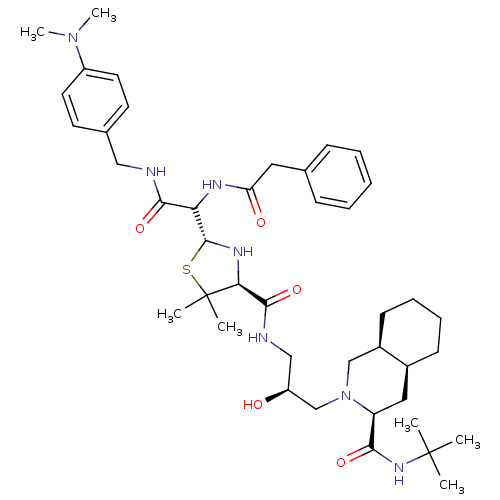 ((3S,4aS,8aS)-2-[(S)-3-({(2R,4S)-2-[(4-Dimethylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037819 ((3S,4aS,8aS)-2-[3-({(2R,4S)-2-[Ethylcarbamoyl-((S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037824 (CHEMBL122641 | {(2R,4S)-4-[(S)-3-((3S,4aS,8aS)-3-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407257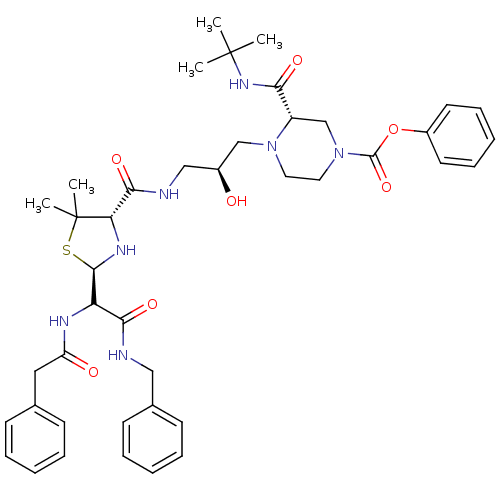 (CHEMBL2112601) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM654 ((3R,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407258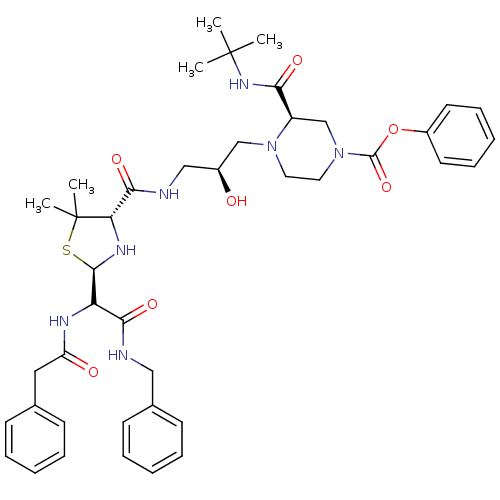 (CHEMBL2111802) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM652 ((3R,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037828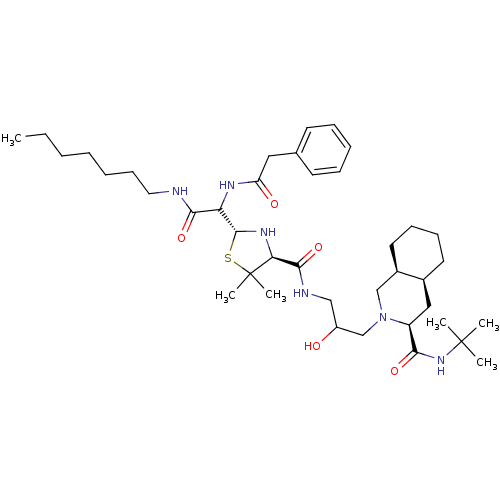 ((3S,4aS,8aS)-2-[3-({(2R,4S)-2-[Heptylcarbamoyl-((S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037835 (3-[(R)-3-({(2R,4S)-2-[Benzylcarbamoyl-((S)-phenyla...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037818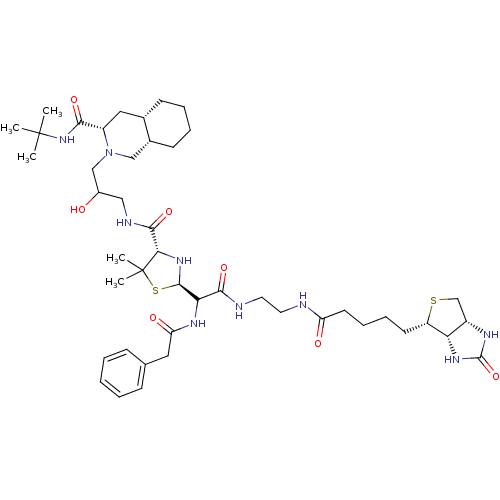 (2-(3-{[5,5-Dimethyl-2-({2-[5-(2-oxo-hexahydro-thie...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM640 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037841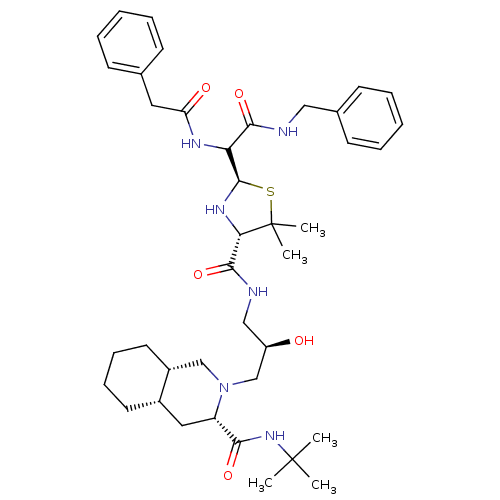 ((3S,4aS,8aS)-2-[(R)-3-({(2R,4S)-2-[Benzylcarbamoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037827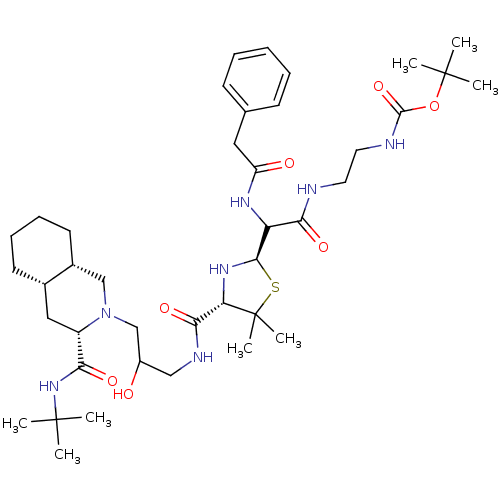 (CHEMBL125827 | {2-[2-{(2R,4S)-4-[3-((3S,4aS,8aS)-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM661 ((3R,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM660 ((3R,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM659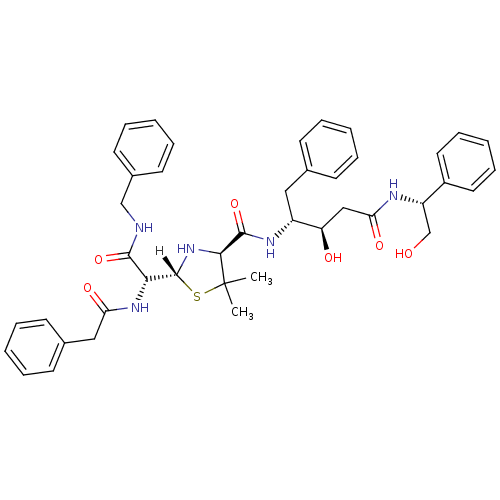 ((3R,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037822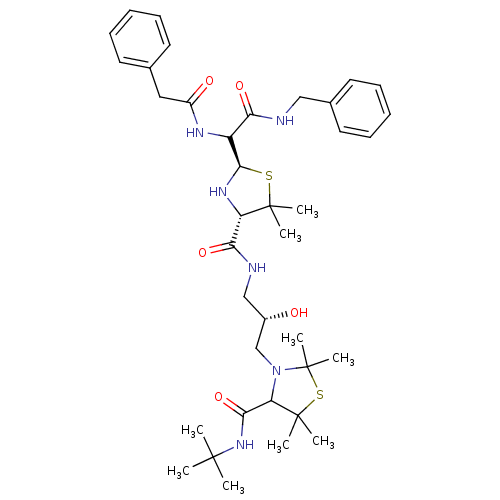 (3-[(S)-3-({(2R,4S)-2-[Benzylcarbamoyl-((S)-phenyla...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM662 ((3R,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM646 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037834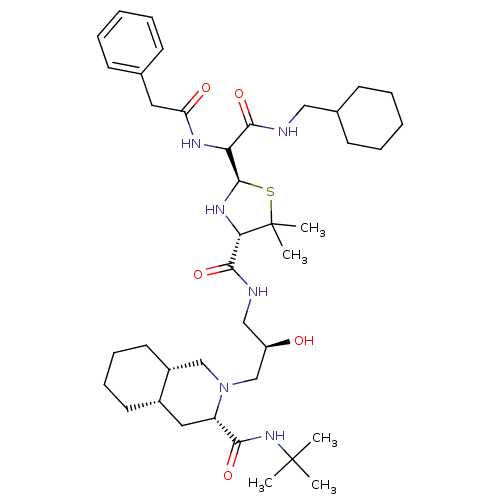 ((3S,4aS,8aS)-2-[(R)-3-({(2R,4S)-2-[(Cyclohexylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM663 ((3R,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041947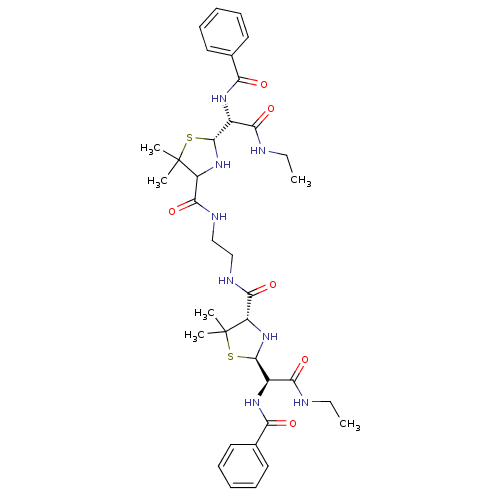 (4N-{2-[5-ethylcarbamoyl(phenylcarboxamido)methyl-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037830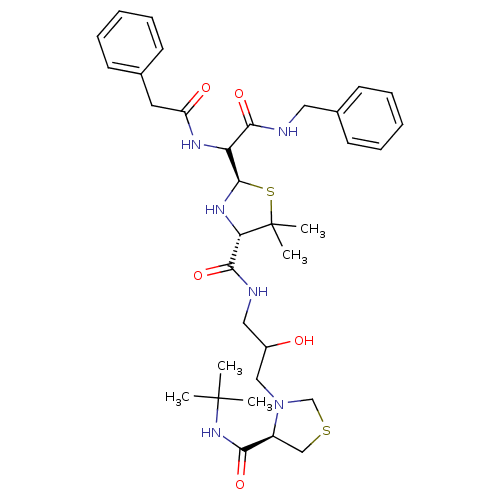 ((2R,4S)-2-[Benzylcarbamoyl-((S)-phenylacetylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM635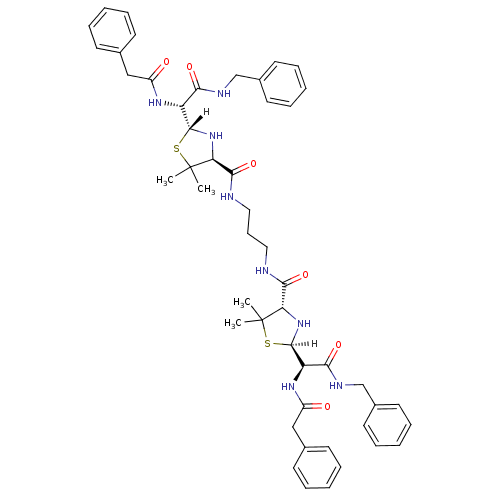 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041950 (1N-benzyl-2-[4-{3-[2-benzylcarbamoyl(benzylcarboxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease | J Med Chem 36: 3113-9 (1993) BindingDB Entry DOI: 10.7270/Q2057F0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM647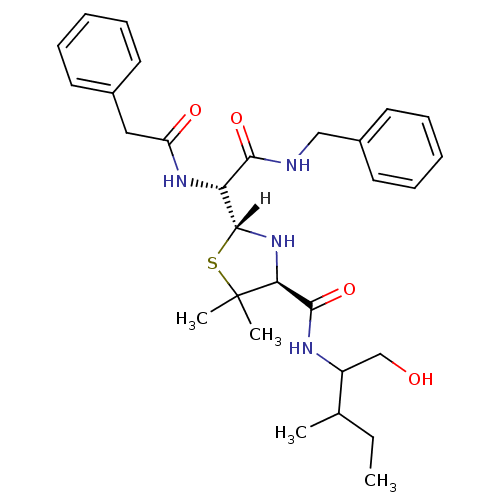 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >540 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM635 ((2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phenylacetamido...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037836 ((2R,4S)-2-[Benzylcarbamoyl-((S)-phenylacetylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037839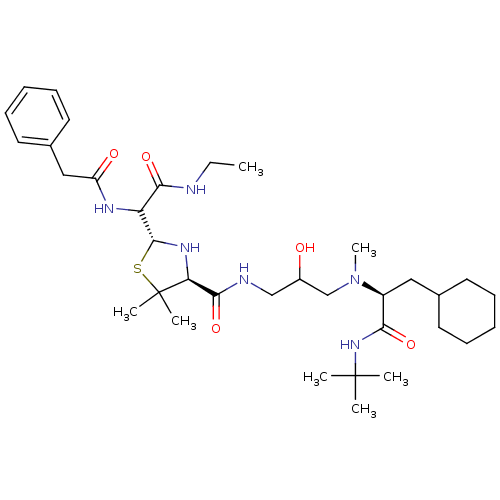 ((2R,4S)-2-[Ethylcarbamoyl-((S)-phenylacetylamino)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037821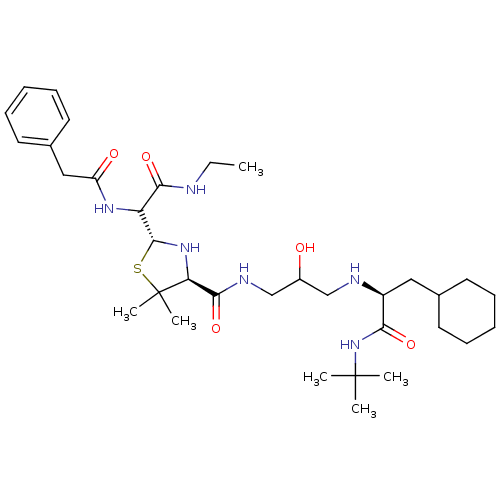 ((2R,4S)-2-[Ethylcarbamoyl-((S)-phenylacetylamino)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037829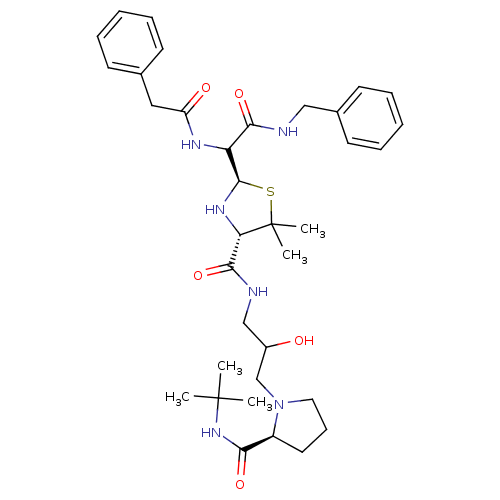 ((2R,4S)-2-[Benzylcarbamoyl-((S)-phenylacetylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037826 ((2R,4S)-2-[Benzylcarbamoyl-((S)-phenylacetylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM651 ((3S,4R)-4-{[(2R,4S)-2-[(R)-(benzylcarbamoyl)(1-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Glaxo Group Research Ltd. | Assay Description IC50 values were obtained by assaying the enzyme against the synthetic substrate peptide KQGTVSFNFPQIT, which was tritiated at the proline residue, a... | J Med Chem 36: 3129-36 (1993) Article DOI: 10.1021/jm00073a012 BindingDB Entry DOI: 10.7270/Q20P0X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037840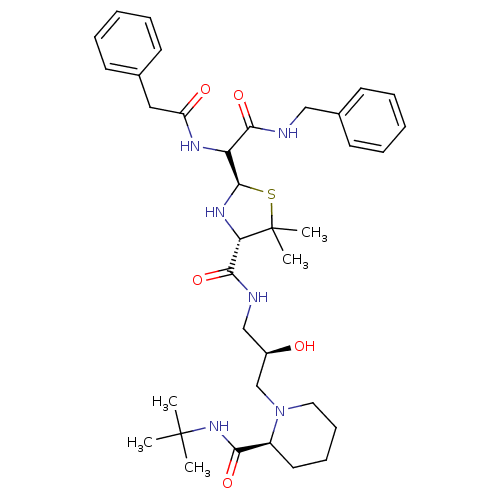 ((S)-1-[(R)-3-({(2R,4S)-2-[Benzylcarbamoyl-((S)-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |