

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354293 (1-(3-(tert-Butyl)-1-(4-(dimethylphosphoryl)phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50371784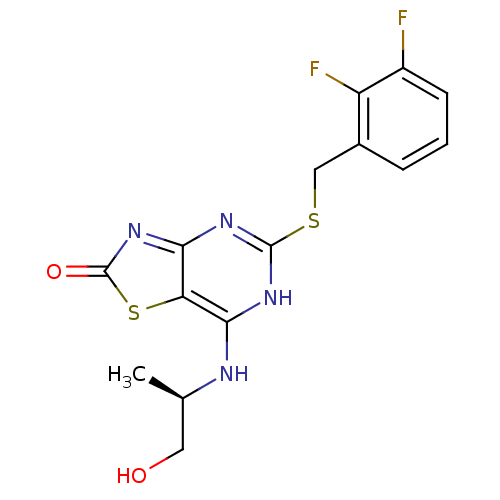 (CHEMBL446458) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354311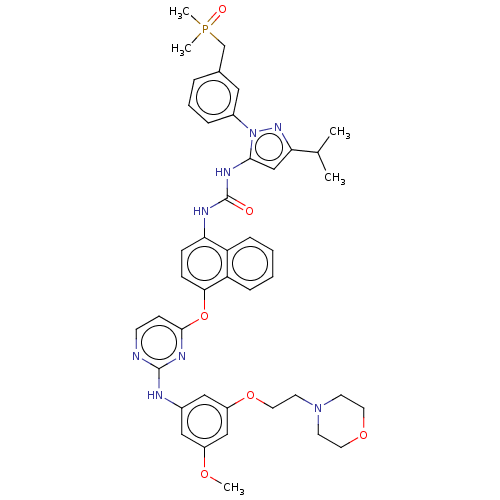 (1-(1-(3-((Dimethylphosphoryl)methyl)phenyl)-3-isop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354332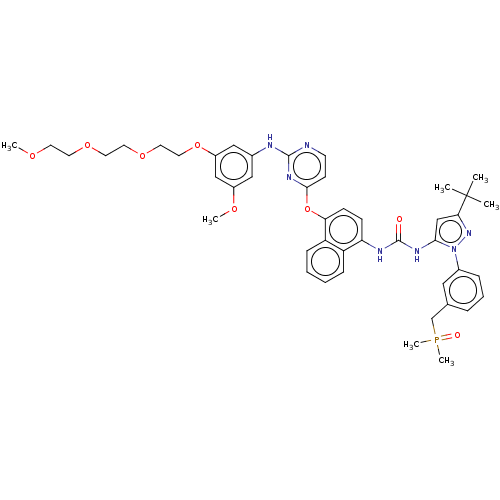 (1-(3-(tert-Butyl)-1-(3-((dimethylphosphoryl)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354293 (1-(3-(tert-Butyl)-1-(4-(dimethylphosphoryl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354310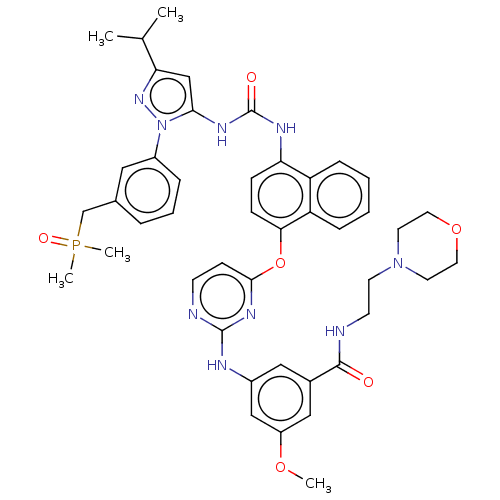 (3-((4-((4-(3-(1-(3-((Dimethylphosphoryl)methyl)phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/L-type calcium channel subunit beta-3/calcium channel subunit alpha-2/delta-1 (Homo sapiens (Human)) | BDBM327851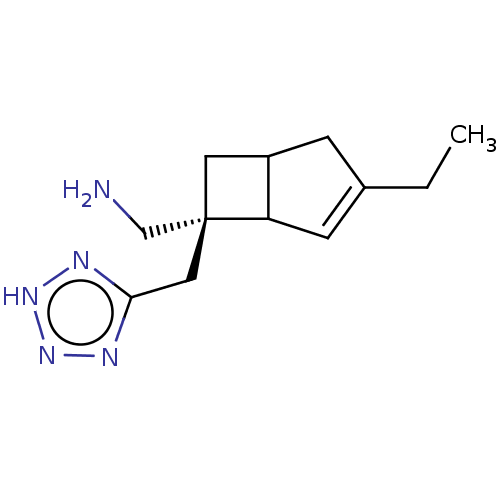 (US9663479, Formula 2 peak 2 (chiral)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novassay S.A. US Patent | Assay Description This section describes a scintillation proximity assay (SPA) to measure [3H] gabapentin ([3H]GBP) binding to membranes containing α2δ-1 and... | US Patent US9663479 (2017) BindingDB Entry DOI: 10.7270/Q2FT8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065327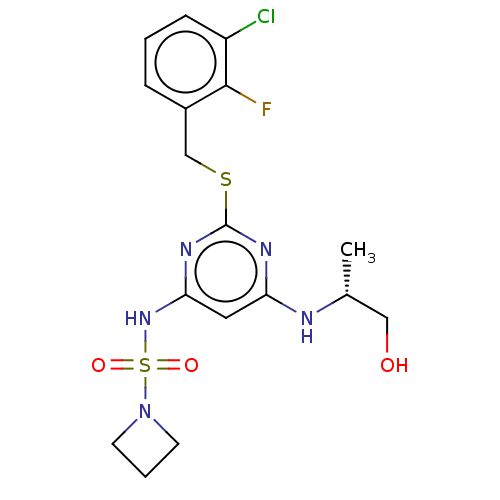 (CHEMBL3403851) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354311 (1-(1-(3-((Dimethylphosphoryl)methyl)phenyl)-3-isop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354324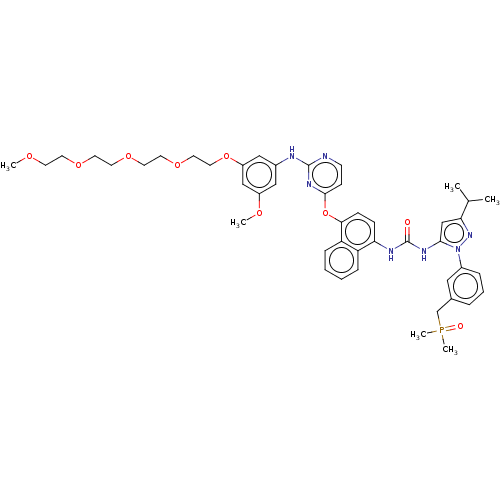 (1-(4-((2-((3-(2,5,8,11-Tetraoxatridecan-13-yloxy)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354310 (3-((4-((4-(3-(1-(3-((Dimethylphosphoryl)methyl)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM334348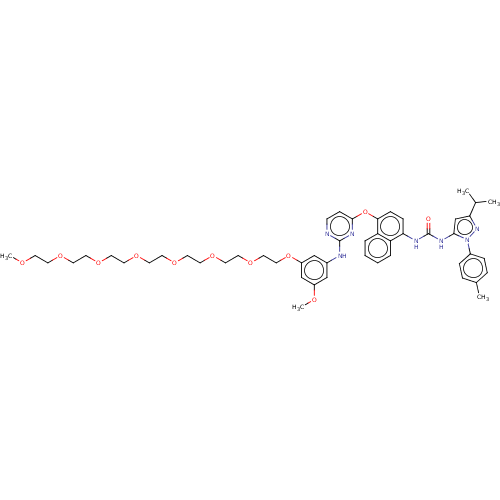 (1-(4-((2-((3-(2,5,8,11,14,17,20-Heptaoxadocosan-22...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM359614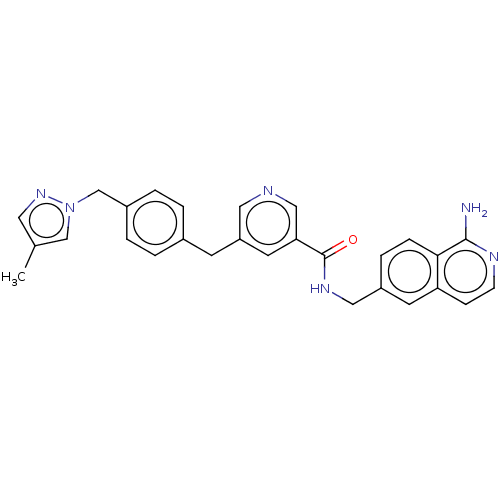 (N-[(1-Aminoisoquinolin-6-yl)methyl]-({4-[(4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalvista Pharmaceuticals Limited US Patent | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | US Patent US10221161 (2019) BindingDB Entry DOI: 10.7270/Q23B62DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM359614 (N-[(1-Aminoisoquinolin-6-yl)methyl]-({4-[(4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q23X89VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354324 (1-(4-((2-((3-(2,5,8,11-Tetraoxatridecan-13-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM334378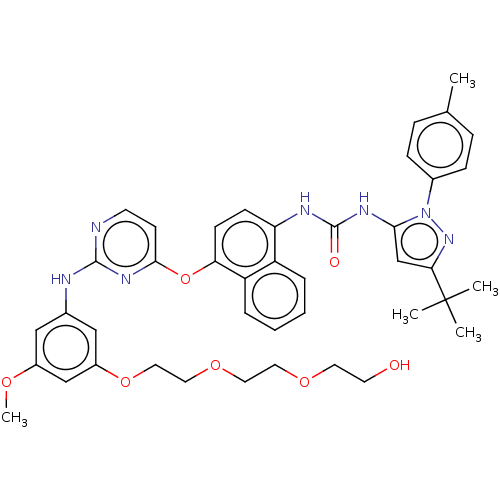 (1-(3-(tert-Butyl)-1-(P-tolyl)-1H-pyrazol-5-yl)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM334346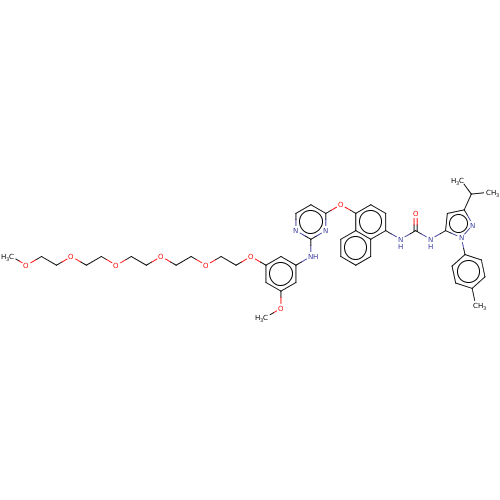 (1-(4-((2-((3-(2,5,8,11,14-Pentaoxahexadecan-16-ylo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50371783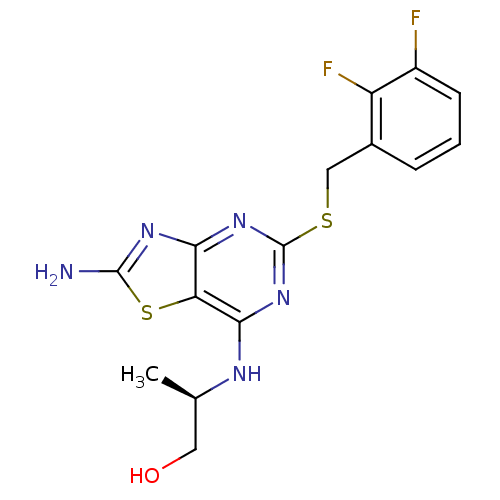 (CHEMBL397237) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065326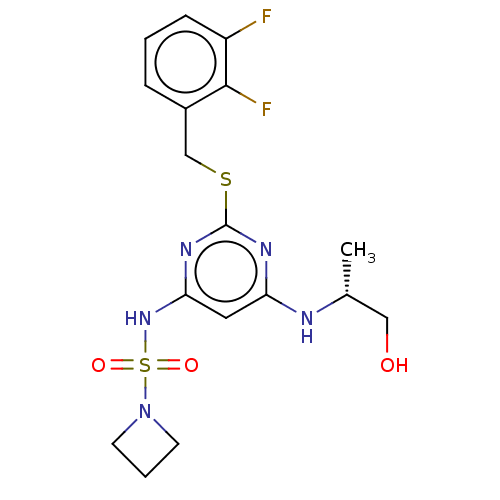 (CHEMBL3403850) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065330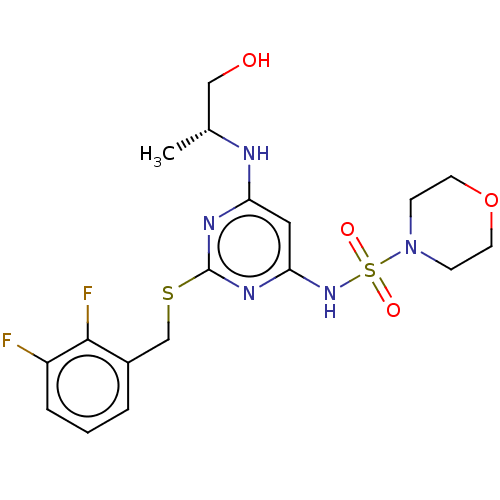 (CHEMBL3403854) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/L-type calcium channel subunit beta-3/calcium channel subunit alpha-2/delta-1 (Homo sapiens (Human)) | BDBM327848 (US9663479, Formula 2 (Racemic)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novassay S.A. US Patent | Assay Description This section describes a scintillation proximity assay (SPA) to measure [3H] gabapentin ([3H]GBP) binding to membranes containing α2δ-1 and... | US Patent US9663479 (2017) BindingDB Entry DOI: 10.7270/Q2FT8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354304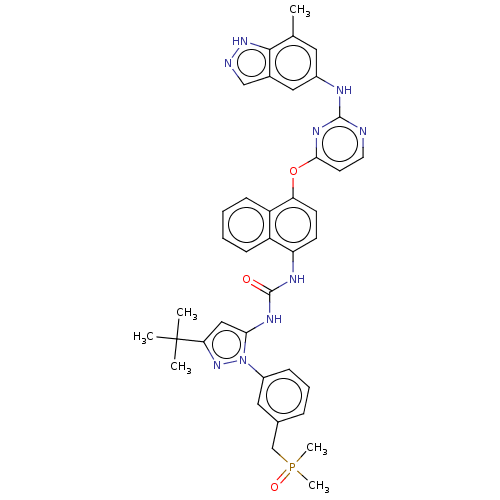 (1-(3-(tert-Butyl)-1-(3-((dimethylphosphoryl)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354304 (1-(3-(tert-Butyl)-1-(3-((dimethylphosphoryl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354309 (1-(1-(3-((Dimethylphosphoryl)methyl)phenyl)-3-isop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065331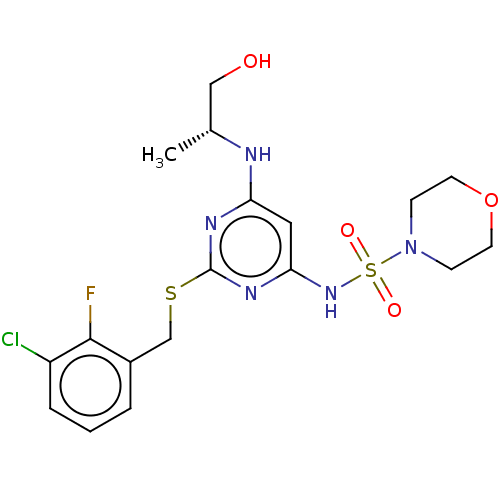 (CHEMBL3403855) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM334335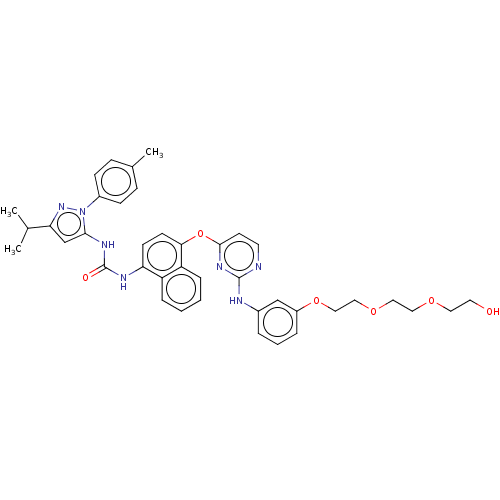 (1-(4-((2-((3-(2-(2-(2-Hydroxyethoxy)ethoxy)ethoxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065324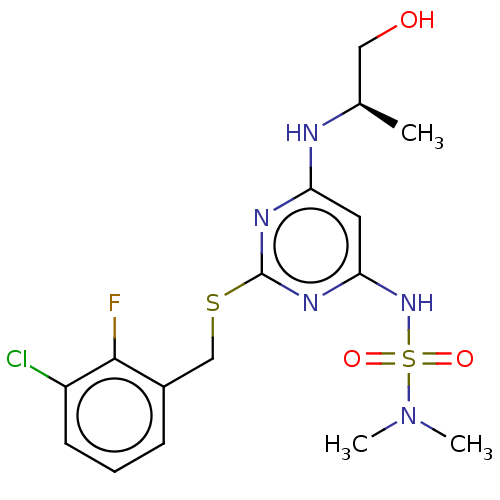 (CHEMBL3403848) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM345133 (1-(3-(tert-Butyldimethylsilyl)-1-(p-tolyl)-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against the GSK 3α enzyme isoform (Invitrogen), are evaluated by determining the level o... | US Patent US9783556 (2017) BindingDB Entry DOI: 10.7270/Q2833V5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM334378 (1-(3-(tert-Butyl)-1-(P-tolyl)-1H-pyrazol-5-yl)-3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256521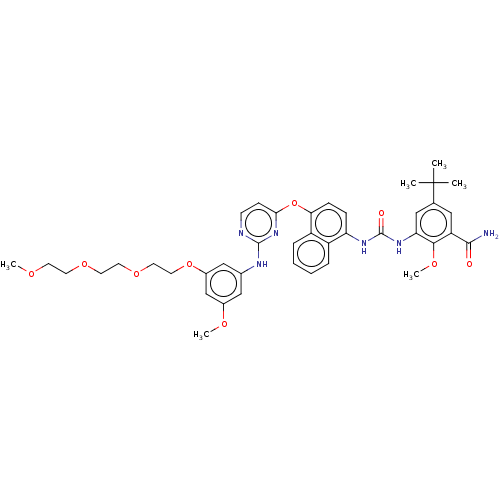 (US10435361, Example 20 | US9481648, 20 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM334345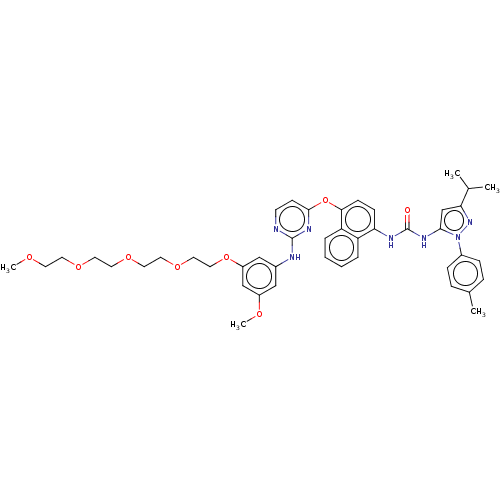 (1-(4-((2-((3-(2,5,8,11-Tetraoxatridecan-13-yloxy)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM345134 (1-(4-((2-((3-(2-Morpholinoethoxy)phenyl)amino)pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9783556 (2017) BindingDB Entry DOI: 10.7270/Q2833V5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM334373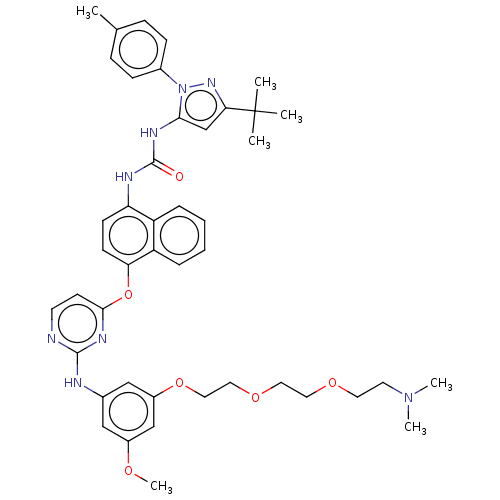 (1-(3-(tert-Butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM334348 (1-(4-((2-((3-(2,5,8,11,14,17,20-Heptaoxadocosan-22...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354331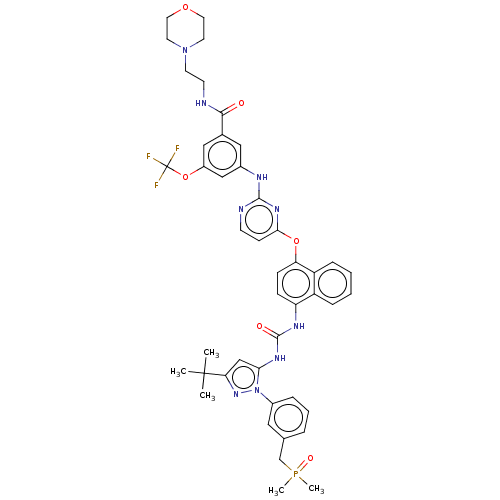 (3-((4-((4-(3-(3-(tert-Butyl)-1-(3-((dimethylphosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354330 (3-((4-((4-(3-(3-(tert-Butyl)-1-(3-((dimethylphosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354329 (1-(4-((2-((3-cyano-5-(2-morpholinoethoxy)phenyl)am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM359712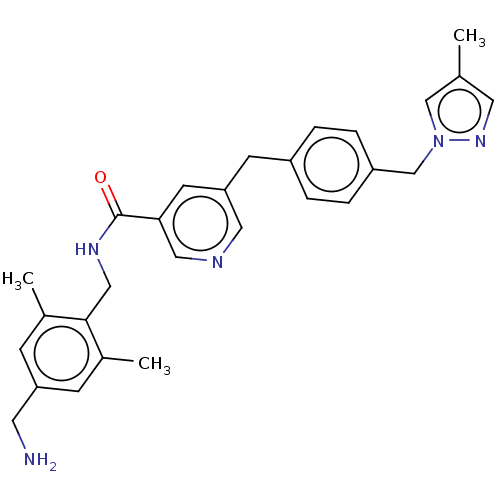 (N-{[4-(Aminomethyl)-2,6-dimethylphenyl]methyl}-5-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalvista Pharmaceuticals Limited US Patent | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | US Patent US10221161 (2019) BindingDB Entry DOI: 10.7270/Q23B62DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM535979 (N-{[4-(Aminomethyl)-2,6-dimethylphenyl]methyl}-5-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q23X89VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065328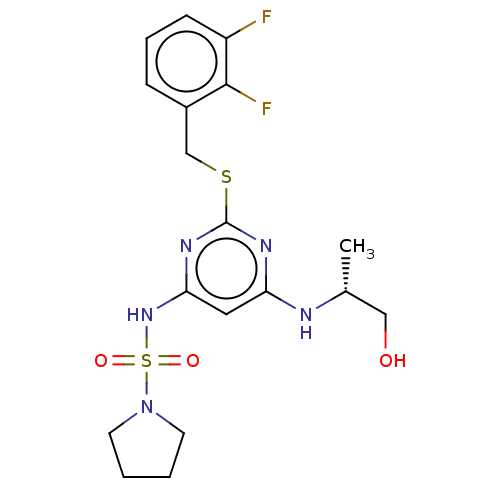 (CHEMBL3403852) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065325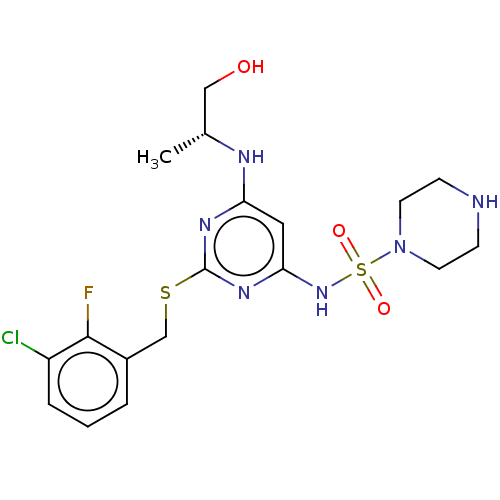 (CHEMBL3403849) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50065329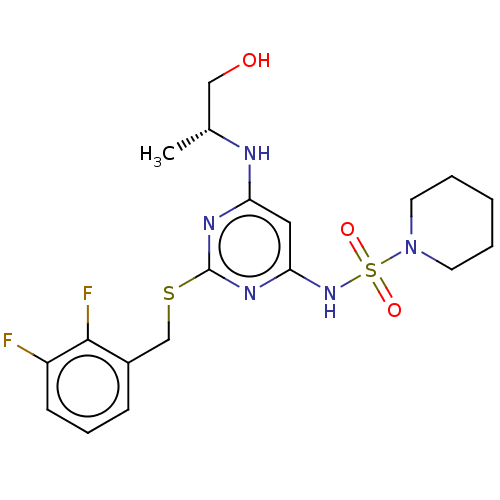 (CHEMBL3403853) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human recombinant CXCR2 receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 1616-20 (2015) Article DOI: 10.1016/j.bmcl.2015.01.067 BindingDB Entry DOI: 10.7270/Q2F47QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM354332 (1-(3-(tert-Butyl)-1-(3-((dimethylphosphoryl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM354273 (1-(3-(tert-Butyl)-1-(4-(dimethylphosphoryl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determining the leve... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354308 (1-(3-(tert-Butyl)-1-(3-((diethylphosphoryl)methyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM354323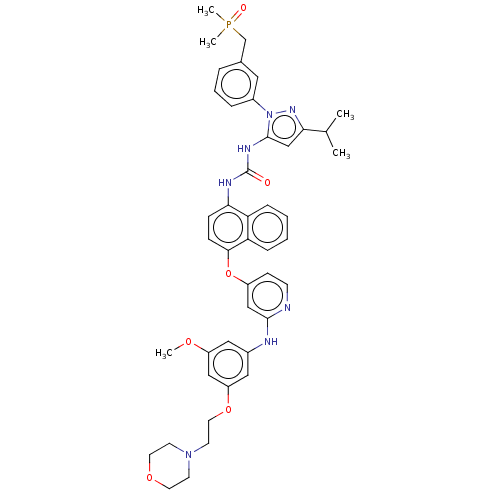 (1-(1-(3-((Dimethylphosphoryl)methyl)phenyl)-3-isop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9796742 (2017) BindingDB Entry DOI: 10.7270/Q2KW5J5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256511 (US10435361, Example 9 | US9481648, 9 | US9790174, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM334336 (1-(4-((2-((3-(2-(2-(2-Hydroxyethoxy)ethoxy)ethoxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM334336 (1-(4-((2-((3-(2-(2-(2-Hydroxyethoxy)ethoxy)ethoxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM334373 (1-(3-(tert-Butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)-3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description c-Src and Syk Enzyme InhibitionThe inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a... | US Patent US9732063 (2017) BindingDB Entry DOI: 10.7270/Q2PV6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1248 total ) | Next | Last >> |