

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM197336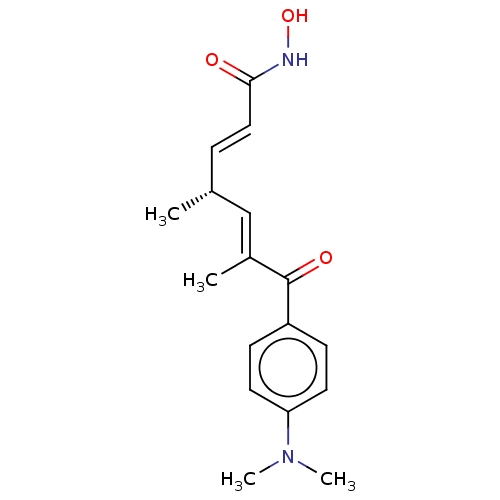 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 [25-831] (Danio rerio (Zebrafish)) | BDBM197336 (R-TSA) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50243109 (5-(2-m-tolylethynyl)pyrimidine | 5-(m-tolylethynyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulation activity at mGlu5 receptor | J Med Chem 55: 1445-64 (2012) Article DOI: 10.1021/jm201139r BindingDB Entry DOI: 10.7270/Q25D8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 [25-831] (Danio rerio (Zebrafish)) | BDBM197337 (S-TSA) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024253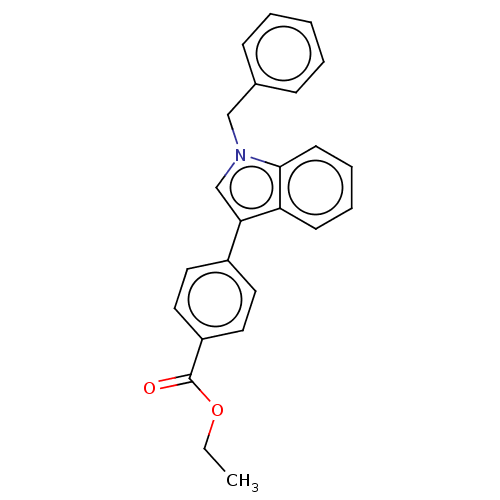 (CHEMBL3334887) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50518702 (CHEMBL4576513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | Bioorg Med Chem Lett 29: 362-366 (2019) Article DOI: 10.1016/j.bmcl.2018.12.039 BindingDB Entry DOI: 10.7270/Q2C53Q7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50518710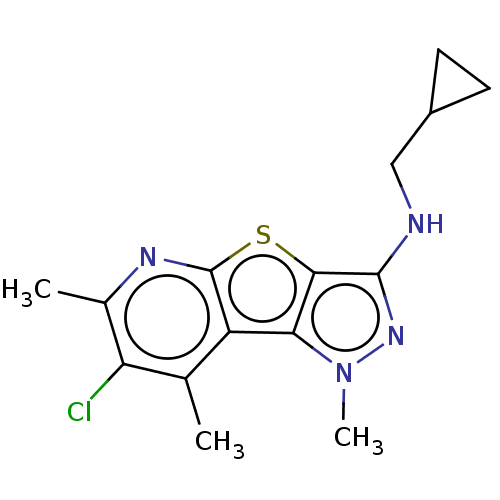 (CHEMBL4522777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | Bioorg Med Chem Lett 29: 362-366 (2019) Article DOI: 10.1016/j.bmcl.2018.12.039 BindingDB Entry DOI: 10.7270/Q2C53Q7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387763 (CHEMBL2057775) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387762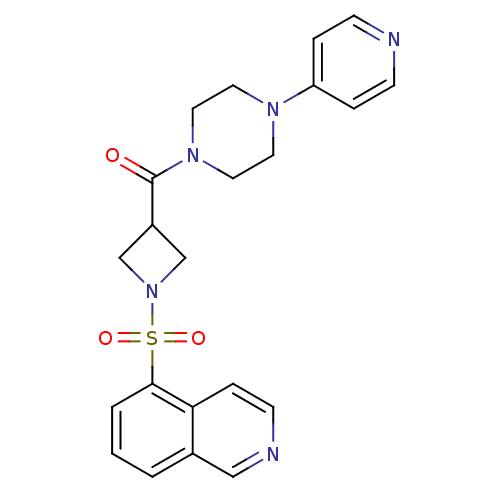 (CHEMBL2057513) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387763 (CHEMBL2057775) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387762 (CHEMBL2057513) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387764 (CHEMBL2057512) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024254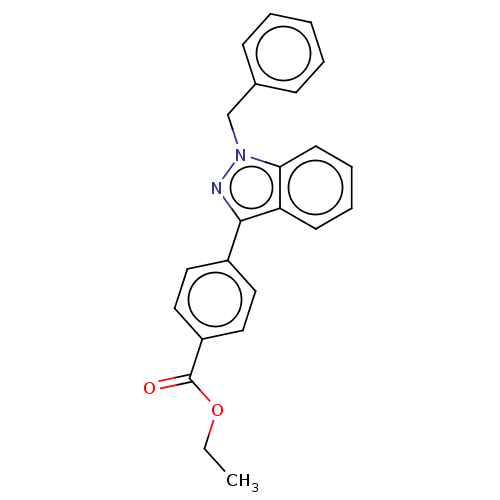 (CHEMBL125021) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387764 (CHEMBL2057512) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024252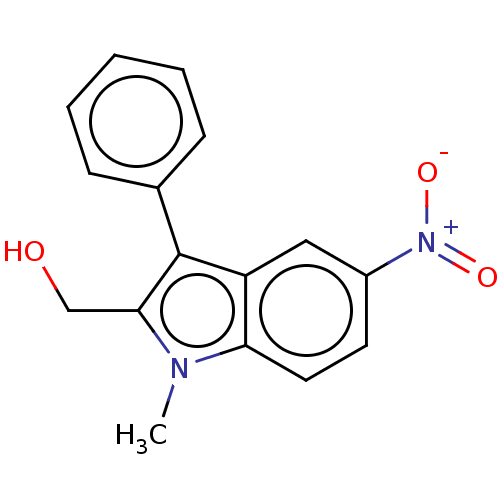 (CHEMBL1609104) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50207516 (CHEMBL3942511) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [125I]-RTI-55 from recombinant human DAT expressed in CHO-S cells | Bioorg Med Chem Lett 27: 171-175 (2017) Article DOI: 10.1016/j.bmcl.2016.11.086 BindingDB Entry DOI: 10.7270/Q2WM1GC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50207463 (CHEMBL3963788) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [125I]-RTI-55 from recombinant human DAT expressed in CHO-S cells | Bioorg Med Chem Lett 27: 171-175 (2017) Article DOI: 10.1016/j.bmcl.2016.11.086 BindingDB Entry DOI: 10.7270/Q2WM1GC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387765 (CHEMBL2057772) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50362394 (CHEMBL1940125) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at rat muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fluo... | Bioorg Med Chem Lett 22: 1044-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.110 BindingDB Entry DOI: 10.7270/Q2XS5VVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387765 (CHEMBL2057772) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387760 (CHEMBL2057776) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387760 (CHEMBL2057776) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50362367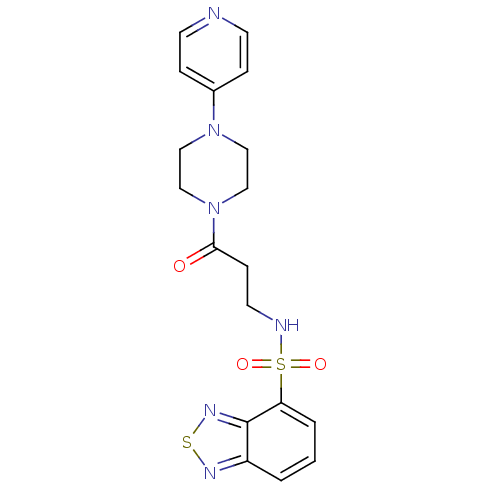 (CHEMBL1628667) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at rat muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fluo... | Bioorg Med Chem Lett 22: 1044-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.110 BindingDB Entry DOI: 10.7270/Q2XS5VVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024255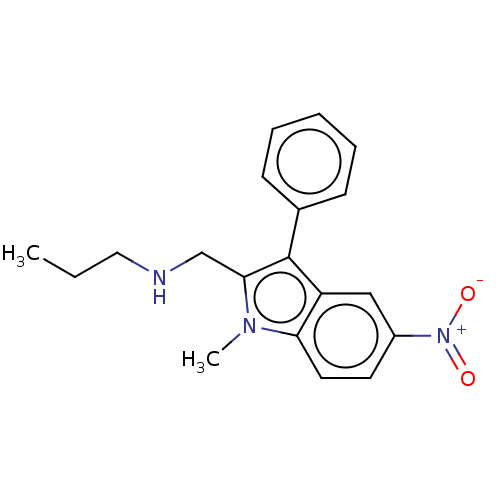 (CHEMBL3334944) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387766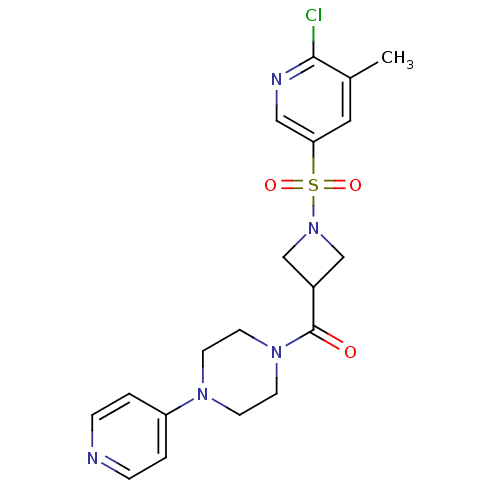 (CHEMBL2057508) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387766 (CHEMBL2057508) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50362377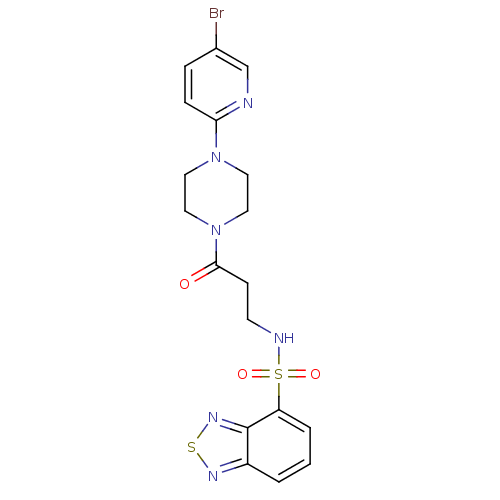 (CHEMBL1939962) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 1044-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.110 BindingDB Entry DOI: 10.7270/Q2XS5VVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024258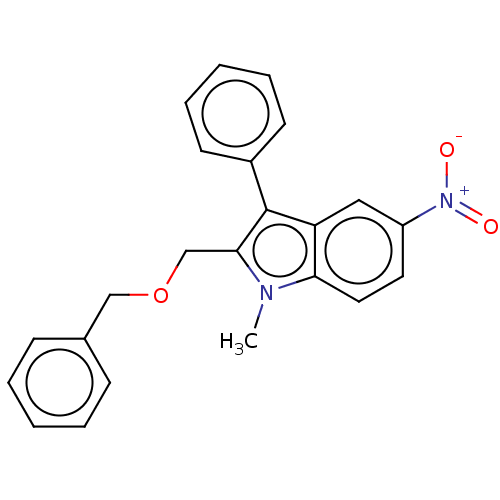 (CHEMBL3334941) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50249435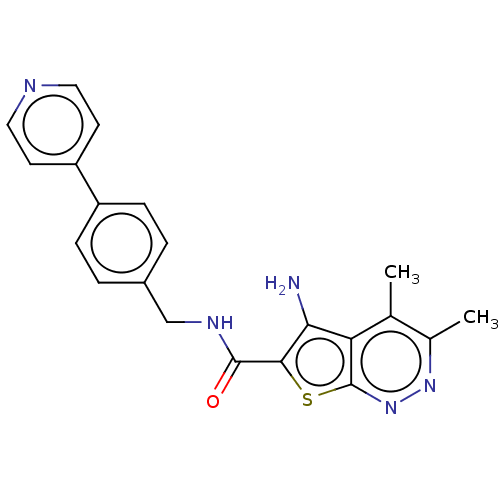 (CHEMBL4070692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Induction of CYP3A4 in cryopreserved human hepatocytes measured after 48 hrs | Bioorg Med Chem Lett 27: 2296-2301 (2017) Article DOI: 10.1016/j.bmcl.2017.04.043 BindingDB Entry DOI: 10.7270/Q23B62J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387767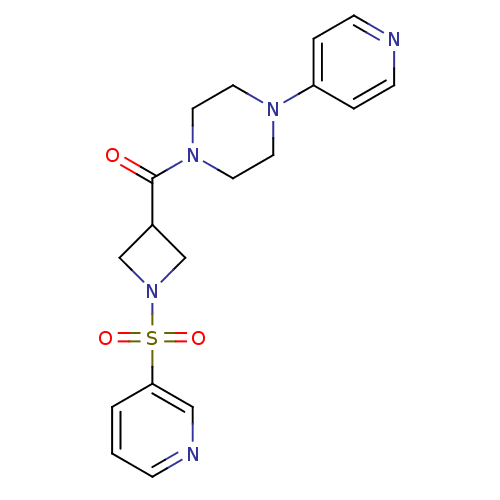 (CHEMBL2057506) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387767 (CHEMBL2057506) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50362394 (CHEMBL1940125) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 1044-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.110 BindingDB Entry DOI: 10.7270/Q2XS5VVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50362394 (CHEMBL1940125) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387803 (CHEMBL2058898) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50243108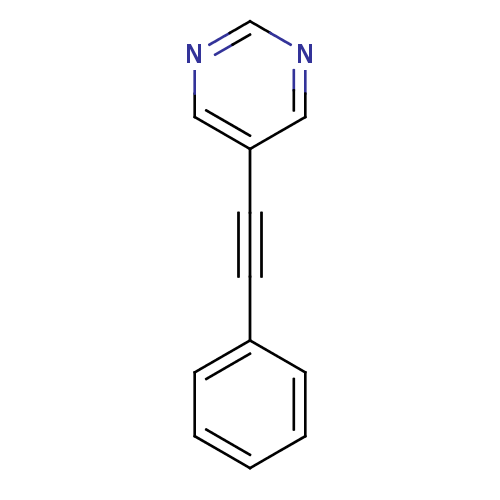 (5-(phenylethynyl)pyrimidine | CHEMBL486244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 486 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulation activity at mGlu5 receptor | J Med Chem 55: 1445-64 (2012) Article DOI: 10.1021/jm201139r BindingDB Entry DOI: 10.7270/Q25D8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387768 (CHEMBL2057507) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387768 (CHEMBL2057507) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387769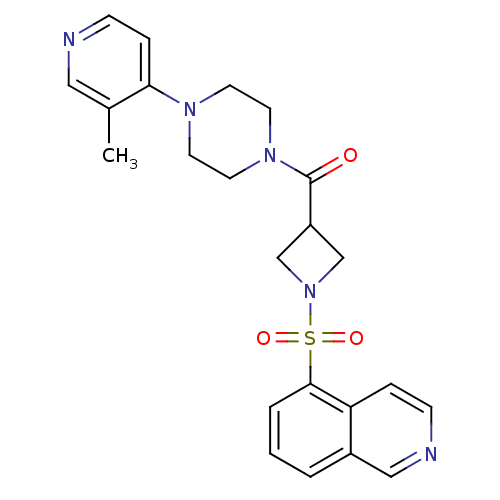 (CHEMBL2057774) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50387769 (CHEMBL2057774) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by fl... | Bioorg Med Chem Lett 22: 5035-40 (2012) Article DOI: 10.1016/j.bmcl.2012.06.018 BindingDB Entry DOI: 10.7270/Q2KW5H39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50207516 (CHEMBL3942511) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]-Paroxetine from recombinant human SERT expressed in HEK293 cells | Bioorg Med Chem Lett 27: 171-175 (2017) Article DOI: 10.1016/j.bmcl.2016.11.086 BindingDB Entry DOI: 10.7270/Q2WM1GC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1028 total ) | Next | Last >> |