

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50037996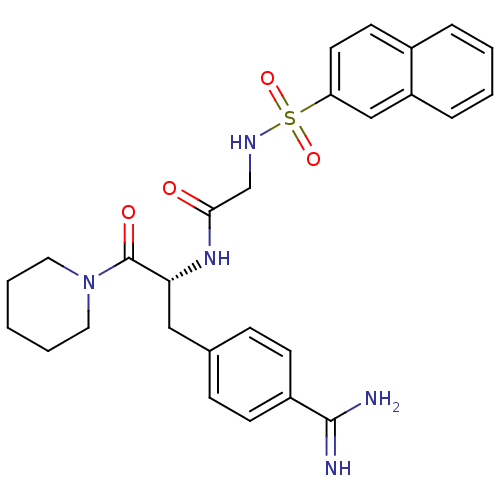 (1-[N-(naphthalen-2-ylsulfonyl)glycyl-4-carbamimido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 42: 3251-64 (1999) Article DOI: 10.1021/jm9806998 BindingDB Entry DOI: 10.7270/Q2CF9QSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079881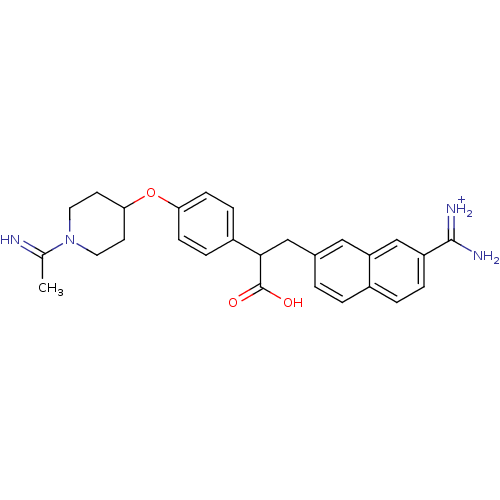 (3-(7-Carbamimidoyl-naphthalen-2-yl)-2-{4-[1-(1-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor Xa | J Med Chem 42: 3251-64 (1999) Article DOI: 10.1021/jm9806998 BindingDB Entry DOI: 10.7270/Q2CF9QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079882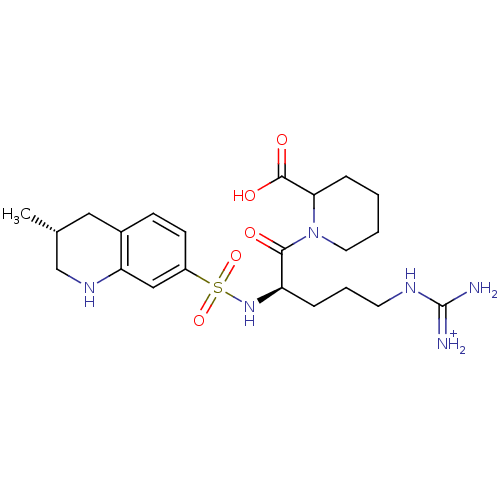 (1-[(R)-5-Guanidino-2-(3-methyl-1,2,3,4-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 42: 3251-64 (1999) Article DOI: 10.1021/jm9806998 BindingDB Entry DOI: 10.7270/Q2CF9QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070632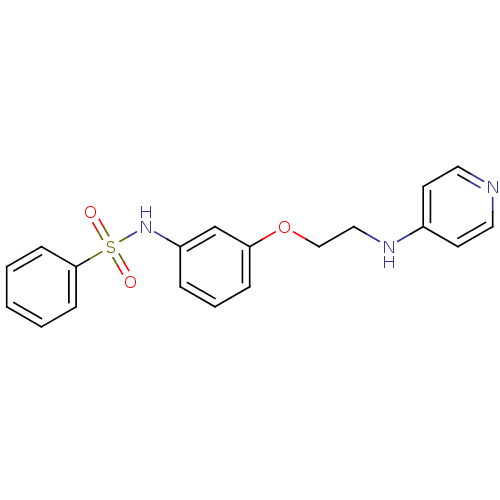 (4-[2-(3-Benzenesulfonylamino-phenoxy)-ethylamino]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 42: 3251-64 (1999) Article DOI: 10.1021/jm9806998 BindingDB Entry DOI: 10.7270/Q2CF9QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50080477 (3-[(S)-3-(Naphthalene-2-sulfonylamino)-2-oxo-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor Xa | J Med Chem 42: 3251-64 (1999) Article DOI: 10.1021/jm9806998 BindingDB Entry DOI: 10.7270/Q2CF9QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86095 (2-Cyanopyrrolidine Derivative, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86096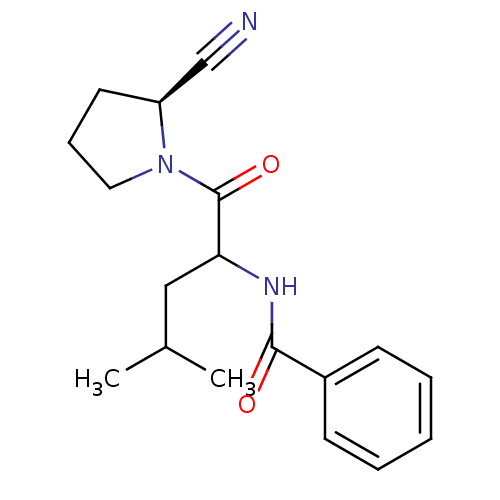 (2-Cyanopyrrolidine Derivative, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | -28.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86094 (2-Cyanopyrrolidine Derivative, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+4 | -23.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86099 (2-Cyanopyrrolidine Derivative, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.50E+4 | -23.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86093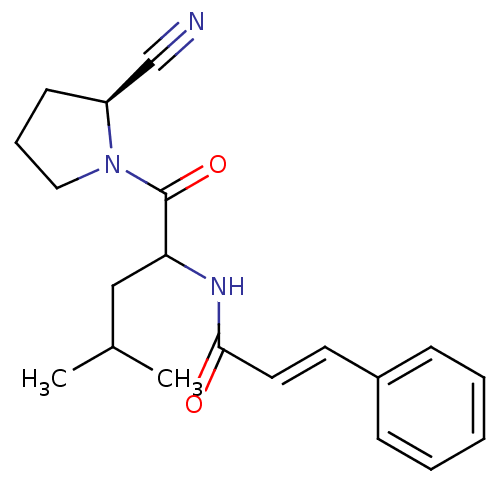 (2-Cyanopyrrolidine Derivative, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.50E+4 | -23.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86094 (2-Cyanopyrrolidine Derivative, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20E+4 | -22.9 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86097 (2-Cyanopyrrolidine Derivative, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86098 (2-Cyanopyrrolidine Derivative, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86100 (2-Cyanopyrrolidine Derivative, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86100 (2-Cyanopyrrolidine Derivative, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86098 (2-Cyanopyrrolidine Derivative, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86097 (2-Cyanopyrrolidine Derivative, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86096 (2-Cyanopyrrolidine Derivative, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86095 (2-Cyanopyrrolidine Derivative, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86093 (2-Cyanopyrrolidine Derivative, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM86099 (2-Cyanopyrrolidine Derivative, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
The M S University of Baroda | Assay Description The fluorescence for the assay was monitored on a Varian Gemini spectrofluorometer with the excitation and emission wavelengths at 380 and 440nm. Th... | J Enzyme Inhib Med Chem 23: 190-7 (2008) Article DOI: 10.1080/14756360701504842 BindingDB Entry DOI: 10.7270/Q2DV1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020833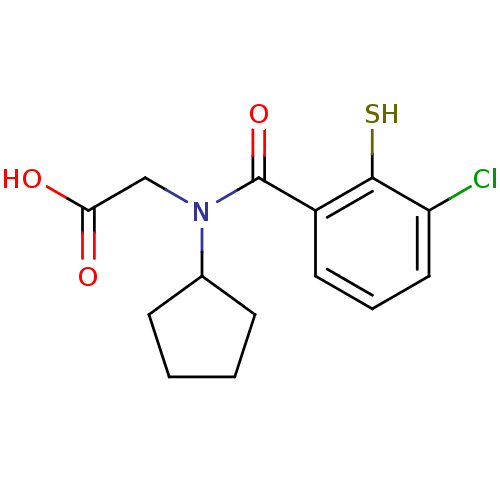 (CHEMBL326137 | [(3-Chloro-2-mercapto-benzoyl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020840 (CHEMBL113276 | [Cyclopentyl-(2-mercapto-3-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020829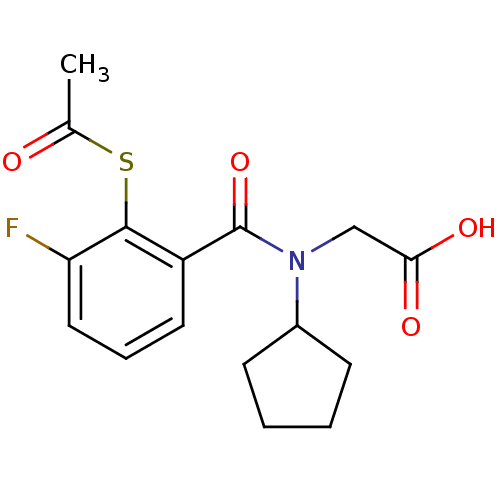 (CHEMBL114242 | [(2-Acetylsulfanyl-3-fluoro-benzoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020830 (CHEMBL263056 | [Cyclopentyl-(2-mercapto-3-trifluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020824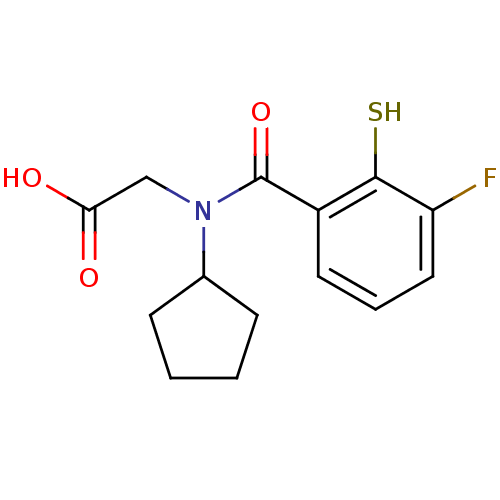 (CHEMBL325659 | [Cyclopentyl-(3-fluoro-2-mercapto-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020831 (CHEMBL113315 | [(2-Acetylsulfanyl-3-methyl-benzoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020842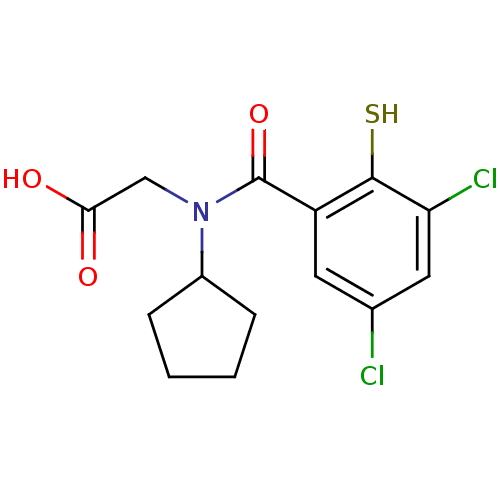 (CHEMBL114294 | [Cyclopentyl-(3,5-dichloro-2-mercap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020841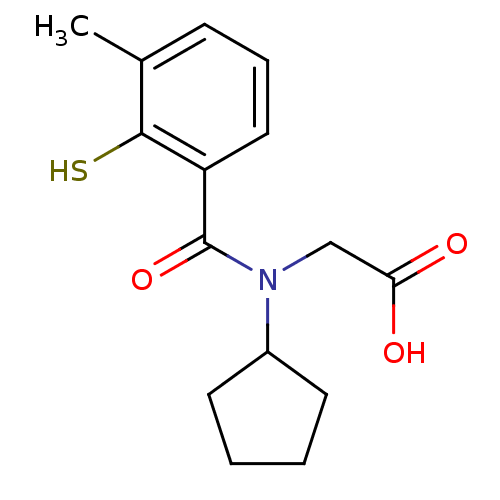 (CHEMBL324242 | [Cyclopentyl-(2-mercapto-3-methyl-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020825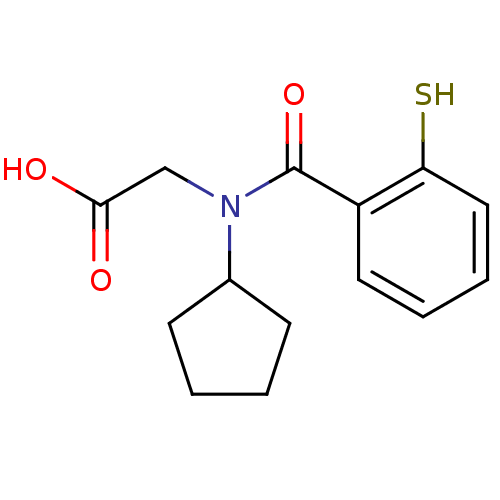 (CHEMBL112168 | [Cyclopentyl-(2-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020823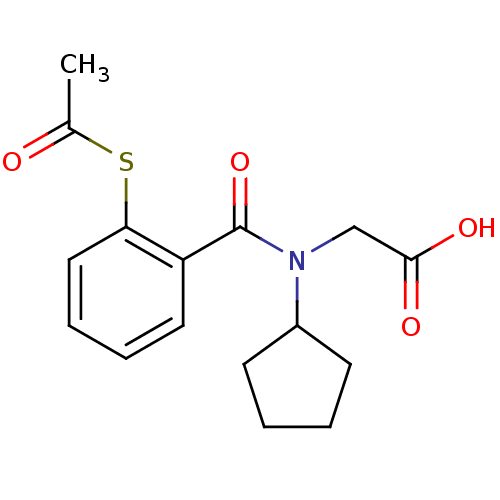 (CHEMBL112477 | [(2-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020821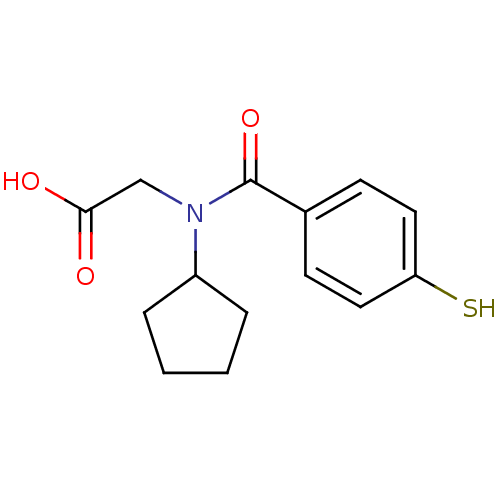 (CHEMBL113612 | [Cyclopentyl-(4-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020832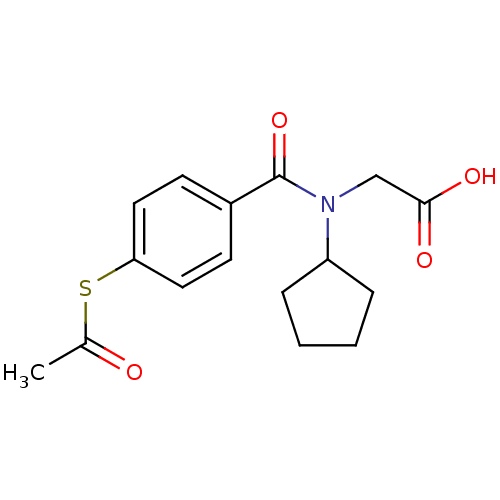 (CHEMBL112589 | [(4-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020827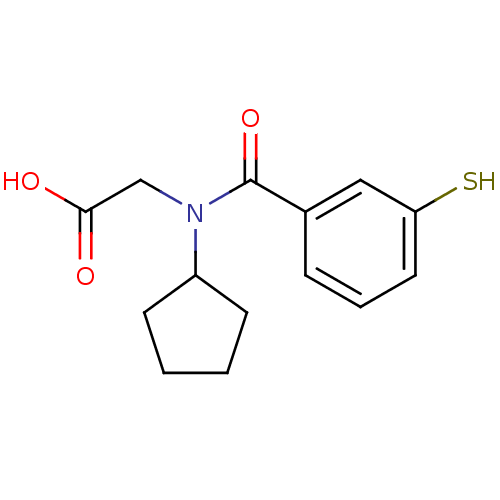 (CHEMBL324898 | [Cyclopentyl-(3-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020838 (CHEMBL323879 | [(3-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020820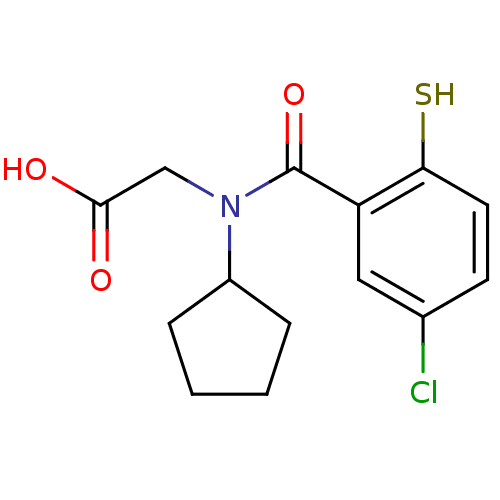 (CHEMBL112922 | [(5-Chloro-2-mercapto-benzoyl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020837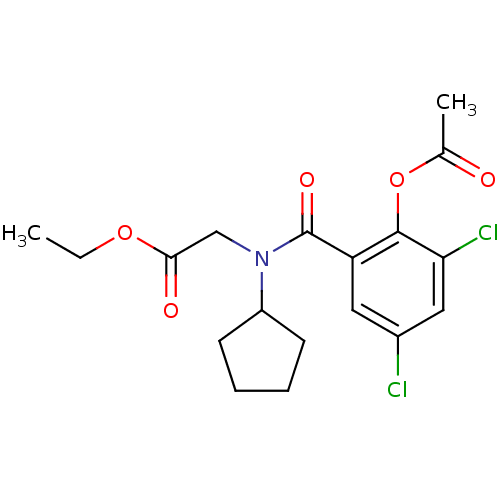 (CHEMBL114018 | [(2-Acetoxy-3,5-dichloro-benzoyl)-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020836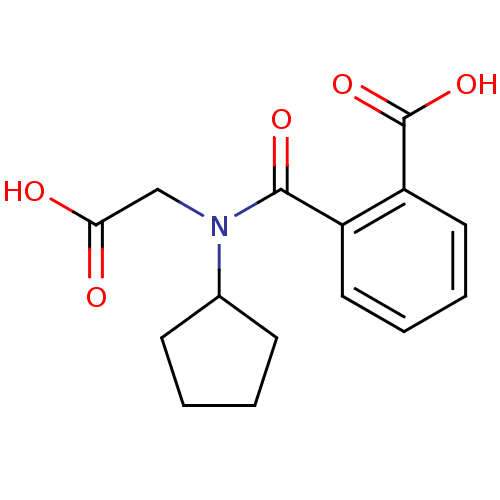 (CHEMBL115153 | N-Carboxymethyl-N-cyclopentyl-phtha...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121904 (CHEMBL30401 | [(S)-1-((3S,4R)-2-Oxo-4-phenoxy-azet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin S | Bioorg Med Chem Lett 13: 139-41 (2002) BindingDB Entry DOI: 10.7270/Q27W6BJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020839 (CHEMBL324703 | [Cyclopentyl-(2-nitro-benzoyl)-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121907 (CHEMBL31788 | [(S)-1-((3R,4S)-2-Oxo-4-phenylsulfan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Cathepsin L | Bioorg Med Chem Lett 13: 139-41 (2002) BindingDB Entry DOI: 10.7270/Q27W6BJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121902 (CHEMBL287630 | [(S)-1-((2S,3R)-2-Benzenesulfonyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human Cathepsin L | Bioorg Med Chem Lett 13: 139-41 (2002) BindingDB Entry DOI: 10.7270/Q27W6BJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020828 (CHEMBL324676 | [Cyclopentyl-(3,5-dichloro-2-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020834 (CHEMBL325305 | [Cyclopentyl-(3-isopropyl-2-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50121041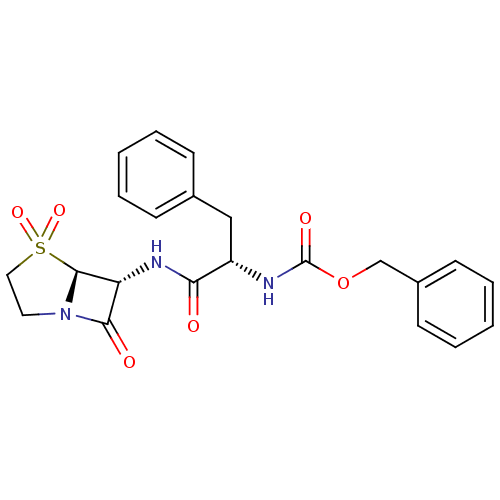 (CHEMBL326093 | [(S)-1-((R)-4,7-Dioxo-4-(S)-oxo-4la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc. Curated by ChEMBL | Assay Description Inhibition of Cbz-Phe-Arg-AMC binding to Cathepsin K cysteine protease | Bioorg Med Chem Lett 12: 3417-9 (2002) BindingDB Entry DOI: 10.7270/Q280520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121039 (CHEMBL114410 | [(S)-2-Cyclohexyl-1-((5S,6R)-4,4,7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc. Curated by ChEMBL | Assay Description In vitro Inhibitory activity of the compound was determined against Cathepsin S cysteine protease by using Cbz-Val-Arg-AMC | Bioorg Med Chem Lett 12: 3417-9 (2002) BindingDB Entry DOI: 10.7270/Q280520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Rattus norvegicus) | BDBM50121039 (CHEMBL114410 | [(S)-2-Cyclohexyl-1-((5S,6R)-4,4,7-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc. Curated by ChEMBL | Assay Description Inhibition of Cbz-Phe-Arg-AMC binding to Cathepsin L cysteine protease | Bioorg Med Chem Lett 12: 3417-9 (2002) BindingDB Entry DOI: 10.7270/Q280520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50367254 (ENALAPRILAT) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in Hog plasma | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L (Rattus norvegicus) | BDBM50121041 (CHEMBL326093 | [(S)-1-((R)-4,7-Dioxo-4-(S)-oxo-4la...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
currently NAEJA Pharmaceutical Inc. Curated by ChEMBL | Assay Description Inhibition of Cbz-Phe-Arg-AMC binding to Cathepsin L cysteine protease | Bioorg Med Chem Lett 12: 3417-9 (2002) BindingDB Entry DOI: 10.7270/Q280520T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 222 total ) | Next | Last >> |