 Found 32 hits with Last Name = 'mendes' and Initial = 'e'
Found 32 hits with Last Name = 'mendes' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50384793

(CHEMBL2037384)Show InChI InChI=1S/C16H18N6O/c1-8(23)18-15-11-7-10-13(17)9-5-3-4-6-12(9)19-14(10)20-16(11)22(2)21-15/h7H,3-6H2,1-2H3,(H2,17,19,20)(H,18,21,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50384793

(CHEMBL2037384)Show InChI InChI=1S/C16H18N6O/c1-8(23)18-15-11-7-10-13(17)9-5-3-4-6-12(9)19-14(10)20-16(11)22(2)21-15/h7H,3-6H2,1-2H3,(H2,17,19,20)(H,18,21,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50384792

(CHEMBL2037385)Show SMILES CC(=O)Nc1nn(C)c2nc3nc4CCCCc4c(N)c3c(-c3ccccc3)c12 Show InChI InChI=1S/C22H22N6O/c1-12(29)24-21-18-16(13-8-4-3-5-9-13)17-19(23)14-10-6-7-11-15(14)25-20(17)26-22(18)28(2)27-21/h3-5,8-9H,6-7,10-11H2,1-2H3,(H2,23,25,26)(H,24,27,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589258
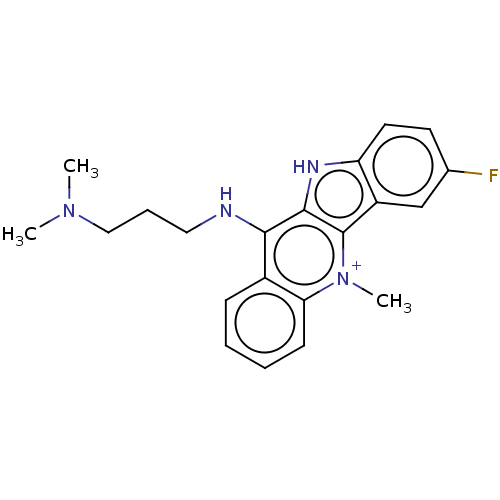
(CHEMBL1187140)Show SMILES CN(C)CCCNc1c2[nH]c3ccc(F)cc3c2[n+](C)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50176906
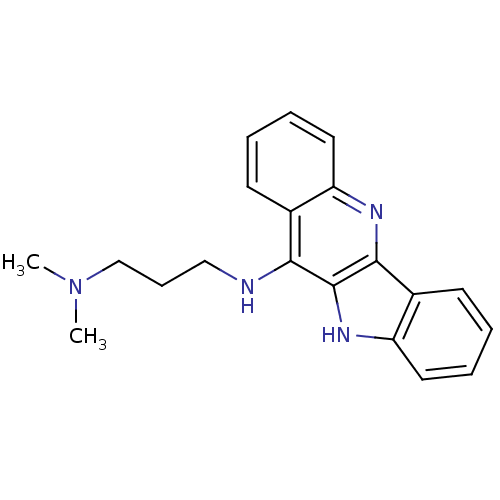
(CHEMBL219728 | N'-(10H-indolo[3,2-b]quinolin-11-yl...)Show InChI InChI=1S/C20H22N4/c1-24(2)13-7-12-21-18-14-8-3-5-10-16(14)22-19-15-9-4-6-11-17(15)23-20(18)19/h3-6,8-11,23H,7,12-13H2,1-2H3,(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50111322

(CHEMBL274335 | N,N-Dimethyl-N'-(11-nitro-13H-5,13-...)Show SMILES CN(C)CCCNc1nc2ccccc2c2[nH]c3c(ccc4ccc(cc34)[N+]([O-])=O)c12 Show InChI InChI=1S/C24H23N5O2/c1-28(2)13-5-12-25-24-21-18-11-9-15-8-10-16(29(30)31)14-19(15)22(18)27-23(21)17-6-3-4-7-20(17)26-24/h3-4,6-11,14,27H,5,12-13H2,1-2H3,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50384794

(CHEMBL2037380)Show InChI InChI=1S/C12H13N5/c1-17-9(6-13)10-11(14)7-4-2-3-5-8(7)15-12(10)16-17/h2-5,14H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50384796

(CHEMBL2037382)Show InChI InChI=1S/C12H15N5O/c1-17-10(11(14)18)8-9(13)6-4-2-3-5-7(6)15-12(8)16-17/h2-5,13H2,1H3,(H2,14,18) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50111320

(CHEMBL274096 | N'-(10-Methoxy-13H-5,13-diaza-diben...)Show SMILES COc1ccc2c3[nH]c4c(c(NCCCN(C)C)nc5ccccc45)c3ccc2c1 Show InChI InChI=1S/C25H26N4O/c1-29(2)14-6-13-26-25-22-20-11-9-16-15-17(30-3)10-12-18(16)23(20)28-24(22)19-7-4-5-8-21(19)27-25/h4-5,7-12,15,28H,6,13-14H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50111323
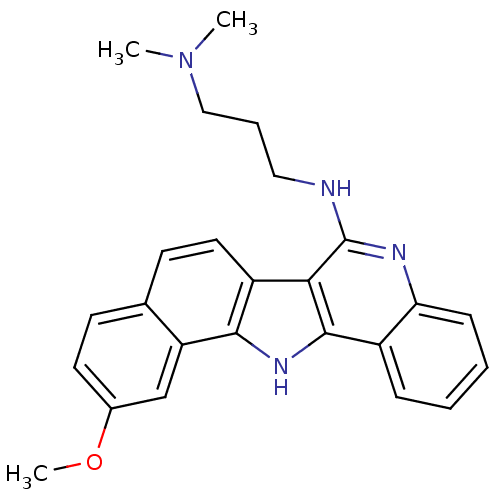
(CHEMBL276202 | N'-(11-Methoxy-13H-5,13-diaza-diben...)Show SMILES COc1ccc2ccc3c([nH]c4c3c(NCCCN(C)C)nc3ccccc43)c2c1 Show InChI InChI=1S/C25H26N4O/c1-29(2)14-6-13-26-25-22-19-12-10-16-9-11-17(30-3)15-20(16)23(19)28-24(22)18-7-4-5-8-21(18)27-25/h4-5,7-12,15,28H,6,13-14H2,1-3H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50384795

(CHEMBL2037381)Show InChI InChI=1S/C12H13N5/c1-17-12-10(9(6-13)16-17)11(14)7-4-2-3-5-8(7)15-12/h2-5H2,1H3,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50384796

(CHEMBL2037382)Show InChI InChI=1S/C12H15N5O/c1-17-10(11(14)18)8-9(13)6-4-2-3-5-7(6)15-12(8)16-17/h2-5,13H2,1H3,(H2,14,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589267

(CHEMBL5198560)Show SMILES C[n+]1c2c3ccccc3oc2c(NCC(=O)[C@@H](N)Cc2ccccc2)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589266
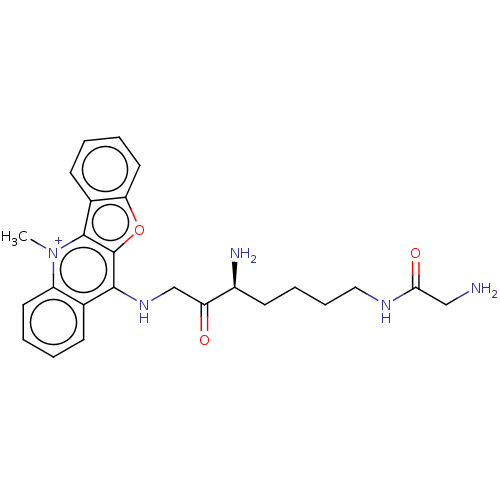
(CHEMBL5208334)Show SMILES C[n+]1c2c3ccccc3oc2c(NCC(=O)[C@@H](N)CCCCNC(=O)CN)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589265

(CHEMBL5180652)Show SMILES C[n+]1c2c3ccccc3oc2c(NCC(=O)CNC(=O)[C@@H](N)CCCCN)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589264

(CHEMBL5175786)Show SMILES C[n+]1c2c3ccccc3oc2c(NCC(=O)CNC(=O)[C@@H](N)Cc2cnc[nH]2)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589263

(CHEMBL5188921)Show SMILES C[n+]1c2c3ccccc3oc2c(NCC(=O)CNC(=O)[C@@H](N)Cc2ccccc2)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589262

(CHEMBL5178646)Show SMILES C[n+]1c2c(oc3ccccc23)c(NCC(=O)CNC(=O)[C@@H](N)CCCNC(N)=N)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589261

(CHEMBL5195420)Show SMILES C[n+]1c2c(oc3ccccc23)c(NCC(=O)CNC(=O)CN)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589260

(CHEMBL5171310) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589268

(CHEMBL5173036)Show SMILES C[n+]1c2c3ccccc3oc2c(NCC(=O)[C@@H](N)Cc2cnc[nH]2)c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50384795

(CHEMBL2037381)Show InChI InChI=1S/C12H13N5/c1-17-12-10(9(6-13)16-17)11(14)7-4-2-3-5-8(7)15-12/h2-5H2,1H3,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50384793

(CHEMBL2037384)Show InChI InChI=1S/C16H18N6O/c1-8(23)18-15-11-7-10-13(17)9-5-3-4-6-12(9)19-14(10)20-16(11)22(2)21-15/h7H,3-6H2,1-2H3,(H2,17,19,20)(H,18,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589257

(CHEMBL5182039)Show SMILES C(CN1CCCC1)Cc1ccc2[nH]c3cc4cc(CCCN5CCCC5)ccc4nc3c2c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50384792

(CHEMBL2037385)Show SMILES CC(=O)Nc1nn(C)c2nc3nc4CCCCc4c(N)c3c(-c3ccccc3)c12 Show InChI InChI=1S/C22H22N6O/c1-12(29)24-21-18-16(13-8-4-3-5-9-13)17-19(23)14-10-6-7-11-15(14)25-20(17)26-22(18)28(2)27-21/h3-5,8-9H,6-7,10-11H2,1-2H3,(H2,23,25,26)(H,24,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50384797

(CHEMBL2037383)Show SMILES Cn1nc(C(N)=O)c2c(NCCCCCCOc3ccccc3)c3CCCCc3nc12 Show InChI InChI=1S/C24H31N5O2/c1-29-24-20(22(28-29)23(25)30)21(18-13-7-8-14-19(18)27-24)26-15-9-2-3-10-16-31-17-11-5-4-6-12-17/h4-6,11-12H,2-3,7-10,13-16H2,1H3,(H2,25,30)(H,26,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50589259
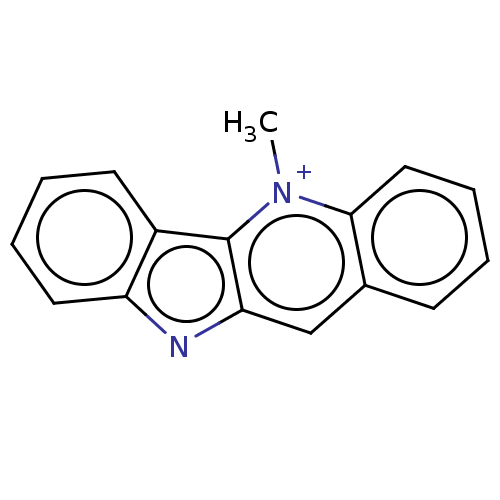
(CHEBI:3930 | CRYPTOLEPINE | Cryptolepine) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50091956

(CHEMBL292124 | {2-[2-(2-Dimethylamino-ethyl)-indol...)Show SMILES CN(C)CCc1ccc2nc3c(cc2c1)n(CCN(C)C)c1ccccc31 Show InChI InChI=1S/C23H28N4/c1-25(2)12-11-17-9-10-20-18(15-17)16-22-23(24-20)19-7-5-6-8-21(19)27(22)14-13-26(3)4/h5-10,15-16H,11-14H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128862
BindingDB Entry DOI: 10.7270/Q2KH0S85 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50384794

(CHEMBL2037380)Show InChI InChI=1S/C12H13N5/c1-17-9(6-13)10-11(14)7-4-2-3-5-8(7)15-12(10)16-17/h2-5,14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50384797

(CHEMBL2037383)Show SMILES Cn1nc(C(N)=O)c2c(NCCCCCCOc3ccccc3)c3CCCCc3nc12 Show InChI InChI=1S/C24H31N5O2/c1-29-24-20(22(28-29)23(25)30)21(18-13-7-8-14-19(18)27-24)26-15-9-2-3-10-16-31-17-11-5-4-6-12-17/h4-6,11-12H,2-3,7-10,13-16H2,1H3,(H2,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method |
Eur J Med Chem 46: 4676-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.068
BindingDB Entry DOI: 10.7270/Q24T6KDT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data