

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human membrane bound carbonic anhydrase 4 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116638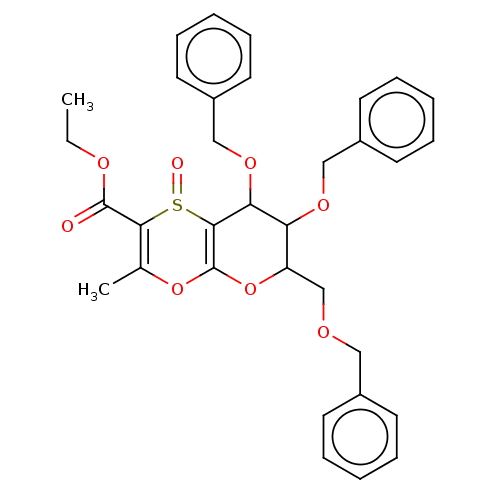 (7,8-Bis-benzyloxy-6-benzyloxymethyl-3-methyl-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 1 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116647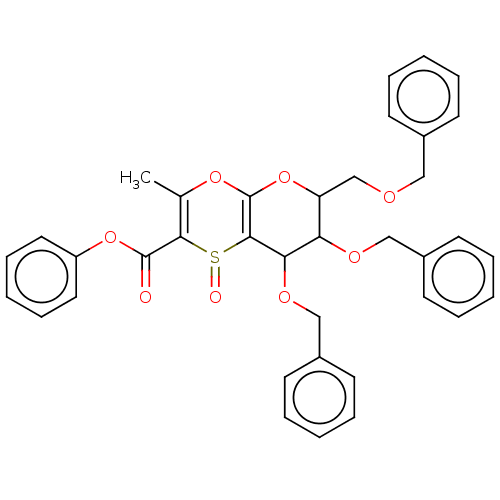 (7,8-Bis-benzyloxy-6-benzyloxymethyl-3-methyl-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248421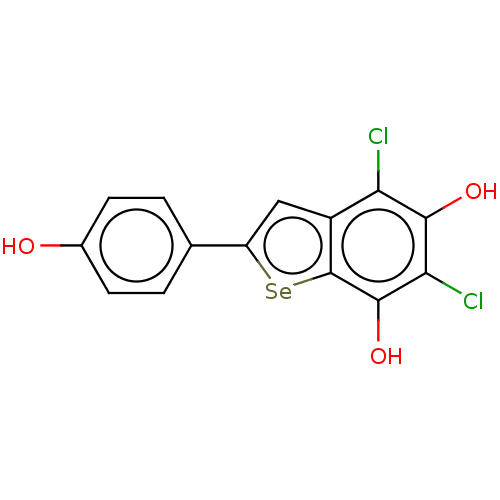 (CHEMBL4101066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116645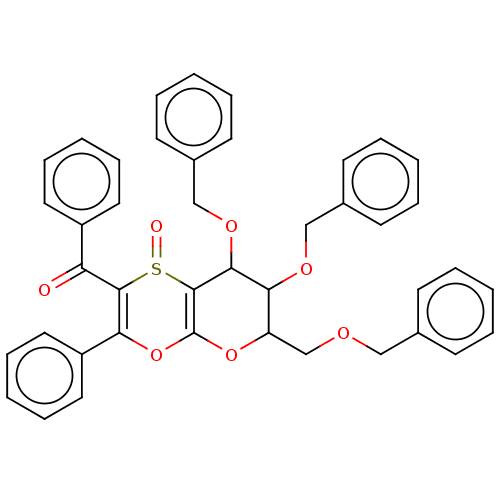 ((7,8-Bis-benzyloxy-6-benzyloxymethyl-1-oxo-3-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116649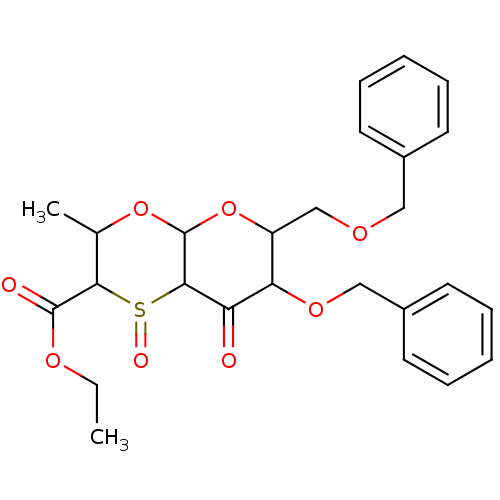 (7-Benzyloxy-6-benzyloxymethyl-8-hydroxy-3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248421 (CHEMBL4101066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50248564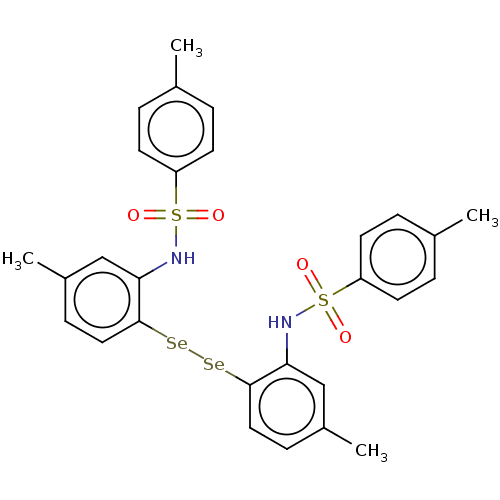 (CHEMBL4069637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248422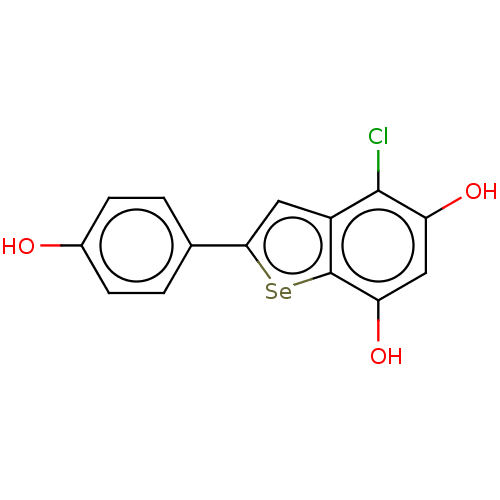 (CHEMBL4086505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248512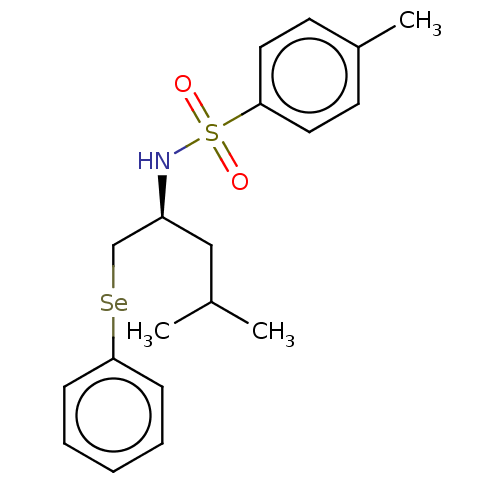 (CHEMBL4067403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116643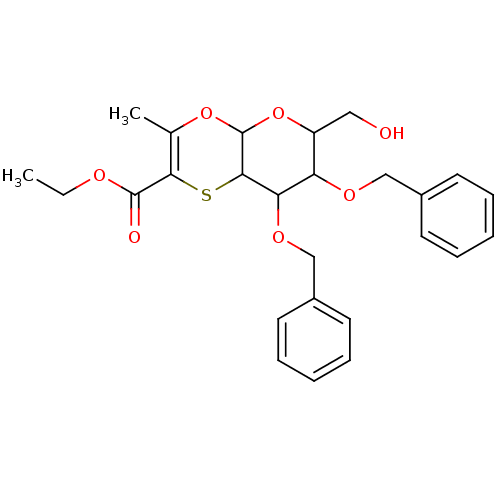 (7,8-Bis-benzyloxy-6-hydroxymethyl-3-methyl-6,7,8,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116644 (7,8-Bis-benzyloxy-6-hydroxymethyl-3-methyl-1-oxo-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248515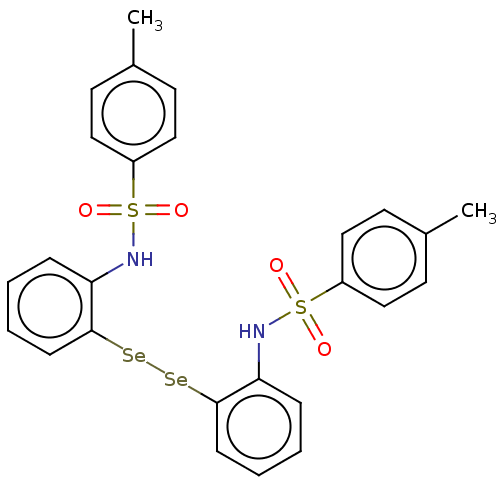 (CHEMBL4082845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116651 ((7,8-Bis-benzyloxy-6-benzyloxymethyl-1,1-dioxo-3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248513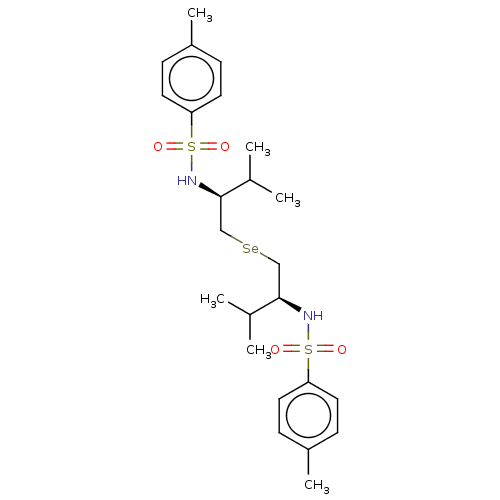 (CHEMBL4088203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248564 (CHEMBL4069637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248517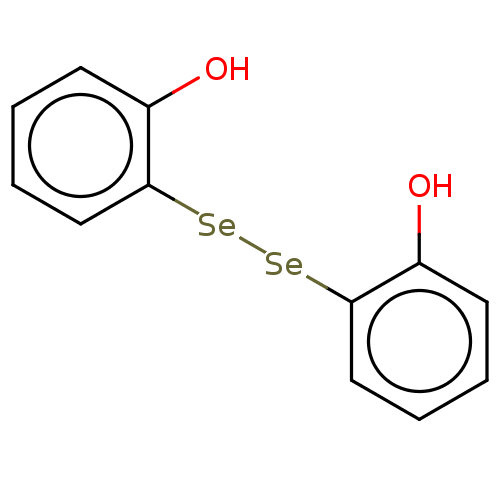 (CHEMBL3314696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50248515 (CHEMBL4082845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human membrane bound carbonic anhydrase 4 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248423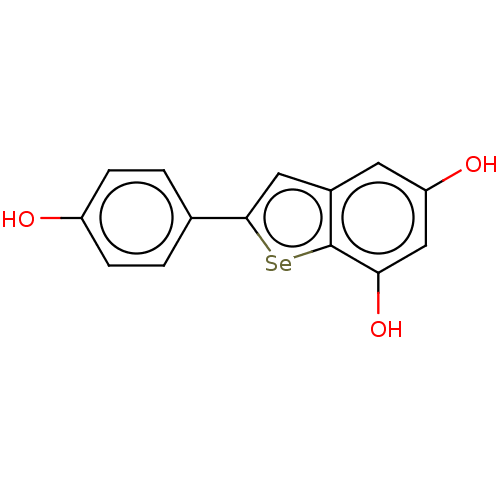 (CHEMBL4104399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248562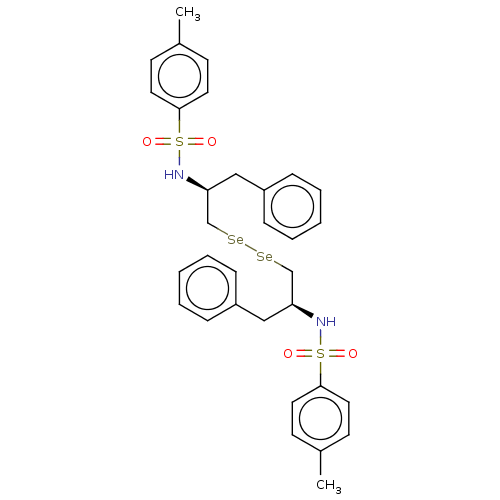 (CHEMBL4073396) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50248515 (CHEMBL4082845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50248512 (CHEMBL4067403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50248421 (CHEMBL4101066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50248422 (CHEMBL4086505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human membrane bound carbonic anhydrase 4 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116650 (7-Benzyloxy-8-hydroxy-6-hydroxymethyl-3-methyl-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116646 (7,8-Bis-benzyloxy-6-benzyloxymethyl-3-methyl-6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116642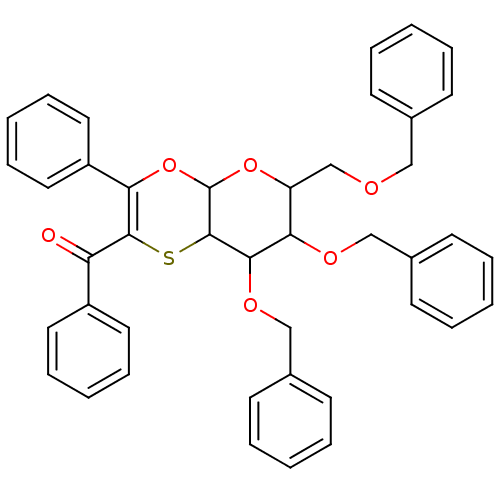 ((7,8-Bis-benzyloxy-6-benzyloxymethyl-3-phenyl-6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116641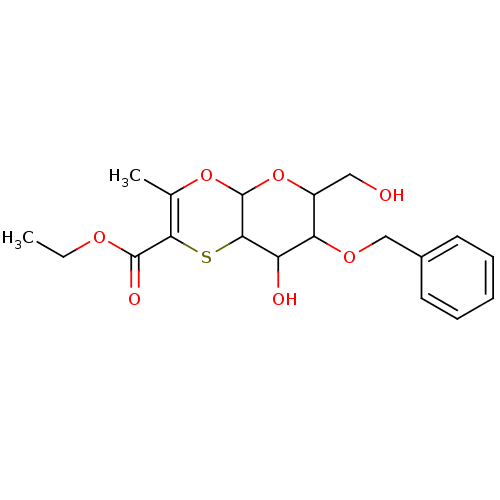 (7-Benzyloxy-8-hydroxy-6-hydroxymethyl-3-methyl-6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116640 (8-Benzyloxy-6-benzyloxymethyl-7-hydroxy-3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116648 (7-Benzyloxy-6-benzyloxymethyl-8-hydroxy-3-methyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50116639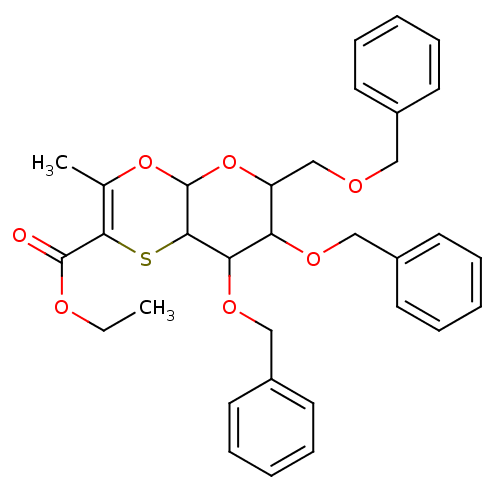 (7,8-Bis-benzyloxy-6-benzyloxymethyl-3-methyl-6,7,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' di Firenze Curated by ChEMBL | Assay Description Displacement of [125I]-NKA from human Tachykinin receptor 2 expressed in CHO cells | Bioorg Med Chem Lett 12: 2263-6 (2002) BindingDB Entry DOI: 10.7270/Q20864NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248468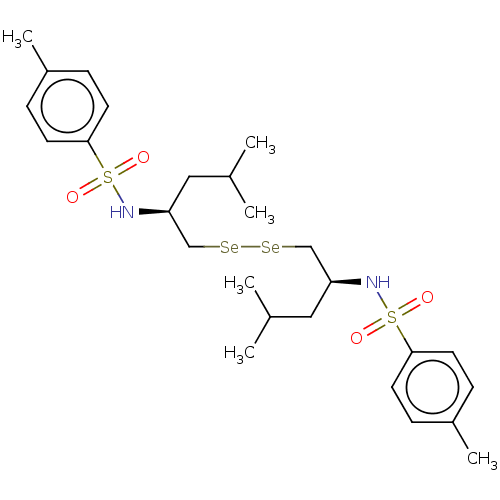 (CHEMBL4095150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50248421 (CHEMBL4101066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 1 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50248561 (CHEMBL4083092) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 1 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248422 (CHEMBL4086505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248423 (CHEMBL4104399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50248422 (CHEMBL4086505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50248468 (CHEMBL4095150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 1 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50248468 (CHEMBL4095150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human membrane bound carbonic anhydrase 4 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248517 (CHEMBL3314696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50248517 (CHEMBL3314696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human membrane bound carbonic anhydrase 4 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50248422 (CHEMBL4086505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 1 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50248468 (CHEMBL4095150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50248517 (CHEMBL3314696) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 1 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50248564 (CHEMBL4069637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50248423 (CHEMBL4104399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ... | Bioorg Med Chem 25: 2518-2523 (2017) Article DOI: 10.1016/j.bmc.2017.03.013 BindingDB Entry DOI: 10.7270/Q25B04XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 134 total ) | Next | Last >> |