

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasoactive intestinal polypeptide receptor 1 (Homo sapiens (Human)) | BDBM50435130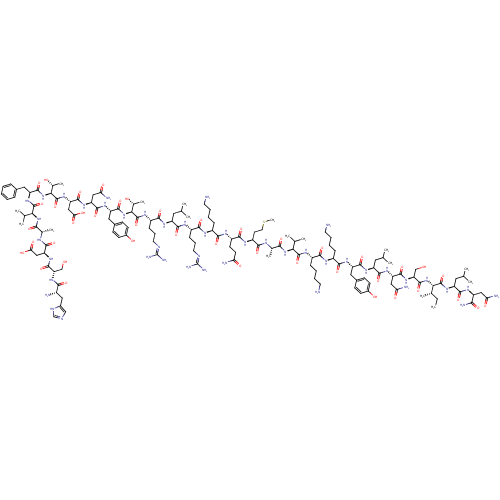 (CHEMBL1893324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human VPAC1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50015490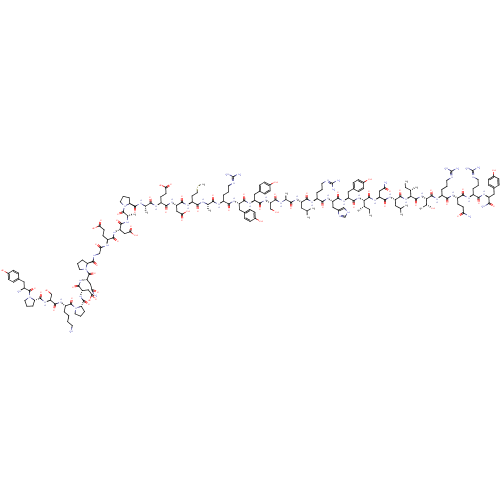 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human neuropeptide Y receptor type 2 by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50166996 (2-(4-Methyl-piperazin-1-yl)-3-propyl-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human neuropeptide Y receptor type 1 by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167002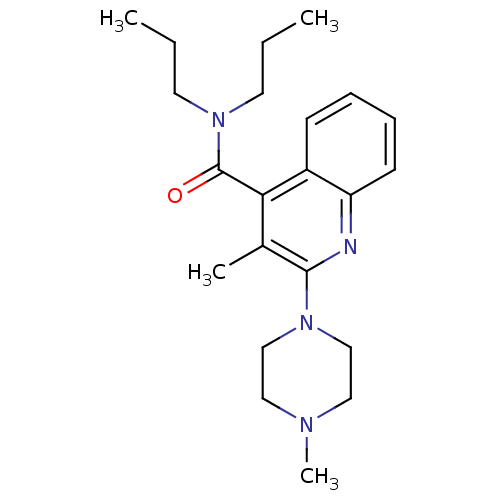 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266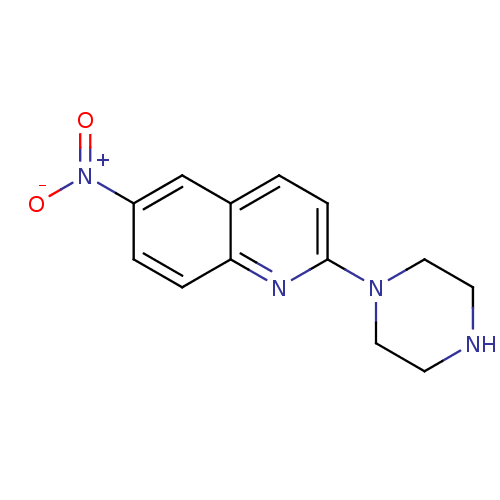 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to displace [3H]paroxetine bound to Serotonin transporter receptor in rat forebrain | J Med Chem 41: 728-41 (1998) Article DOI: 10.1021/jm970645i BindingDB Entry DOI: 10.7270/Q2QV3N6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Binding affinity towards Serotonin transporter was determined in rat forebrain membrane | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50335566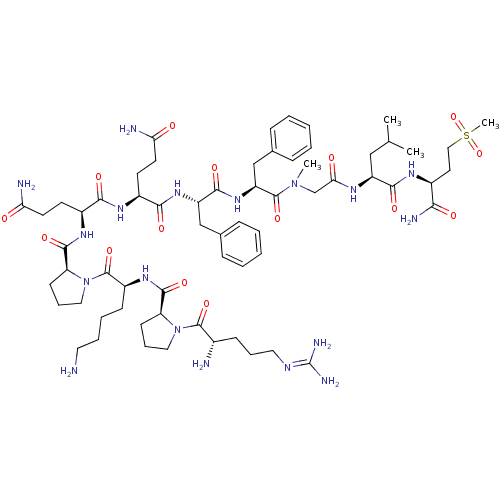 (CHEMBL1651026 | Substance P [Sar9,Met(O2)11]) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human NK1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167013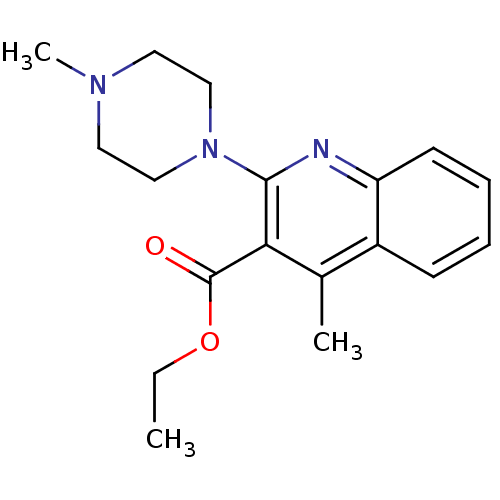 (4-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-3-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167015 (3-Ethyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50063263 (6-(4-Methyl-piperazin-1-yl)-7,8,9,10-tetrahydro-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50063263 (6-(4-Methyl-piperazin-1-yl)-7,8,9,10-tetrahydro-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to displace [3H]granisetron specifically bound to 5-hydroxytryptamine 3 receptor in rat cortical membrane | J Med Chem 41: 728-41 (1998) Article DOI: 10.1021/jm970645i BindingDB Entry DOI: 10.7270/Q2QV3N6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50063263 (6-(4-Methyl-piperazin-1-yl)-7,8,9,10-tetrahydro-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167011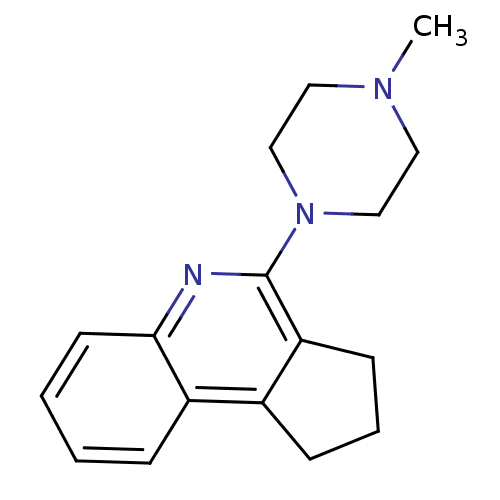 (4-(4-Methyl-piperazin-1-yl)-2,3-dihydro-1H-cyclope...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human NTS1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50360276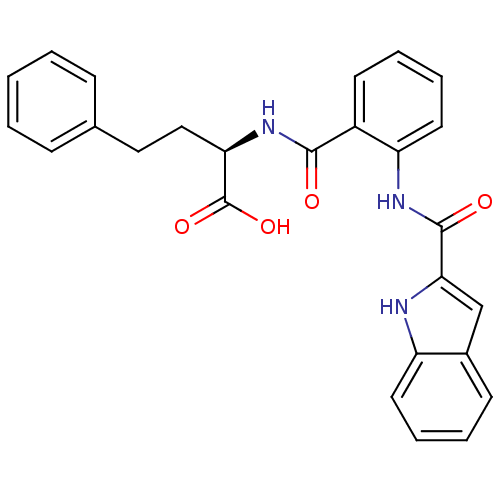 (CHEMBL1933104) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste Curated by ChEMBL | Assay Description Displacement of [125I]-BH-JMV-179 from wild type human CCK1 receptor expressed in COS7 cells after 60 mins by gamma counting | J Med Chem 54: 5769-85 (2011) Article DOI: 10.1021/jm200438b BindingDB Entry DOI: 10.7270/Q2BP036J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50449636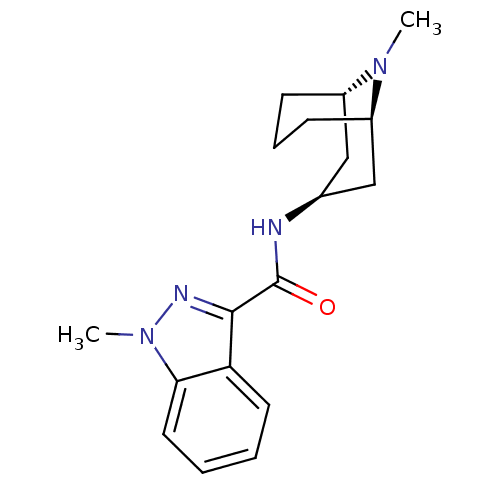 (BRL-43694 | GRANISETRON | Kytril | LY-278584 | San...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to displace [3H]granisetron specifically bound to 5-hydroxytryptamine 3 receptor in rat cortical membrane | J Med Chem 41: 728-41 (1998) Article DOI: 10.1021/jm970645i BindingDB Entry DOI: 10.7270/Q2QV3N6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167014 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076415 (2-[2-(4-Methyl-piperazin-1-yl)-4-phenyl-quinolin-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167004 (2-(4-Methyl-piperazin-1-yl)-quinoline-4-carboxylic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167003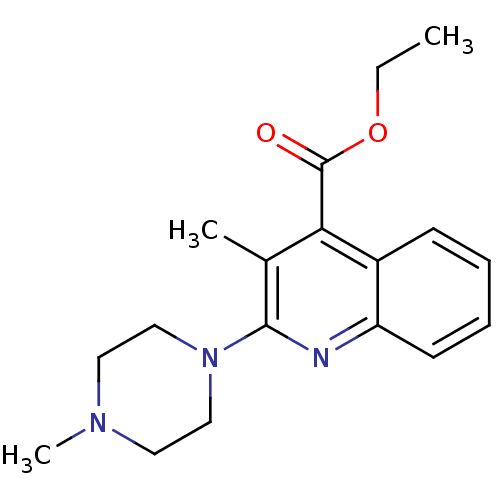 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50166997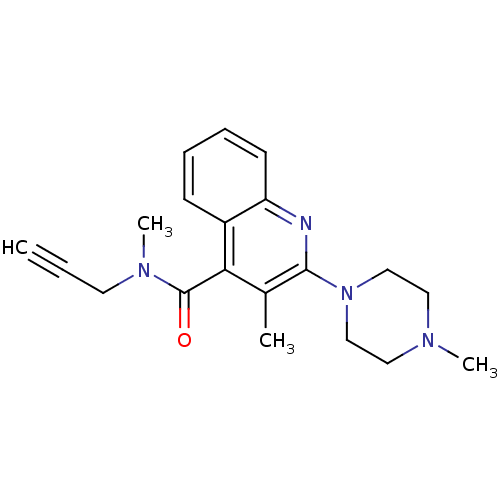 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 1 (Homo sapiens (Human)) | BDBM50378616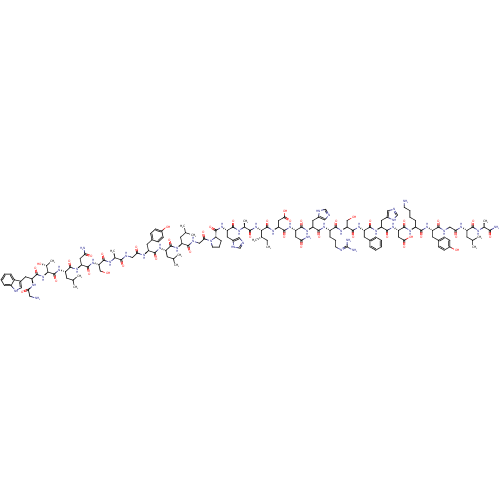 (GALANIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human GAL1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50333104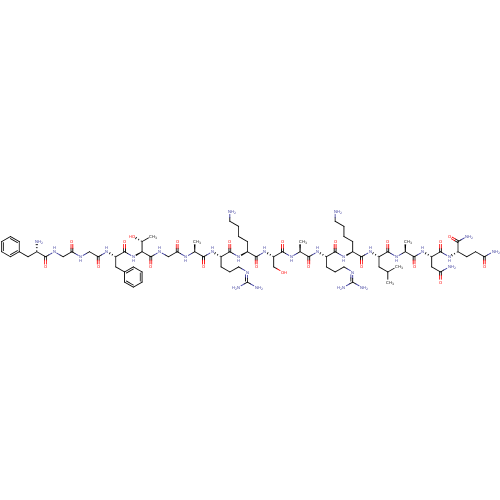 (CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human NOP receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049806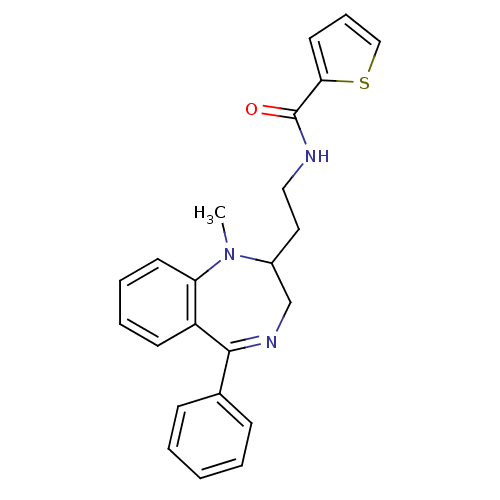 (CHEMBL127969 | Thiophene-2-carboxylic acid [2-(1-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Siena Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 of guinea pig using [3H]-U-65,693 as radioligand | J Med Chem 39: 860-72 (1996) Article DOI: 10.1021/jm950423p BindingDB Entry DOI: 10.7270/Q2222SVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167016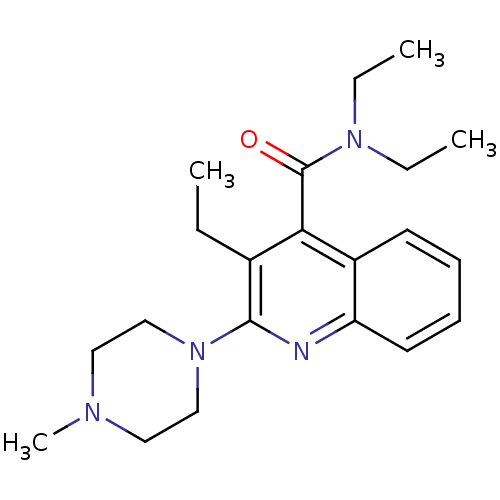 (3-Ethyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167005 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049802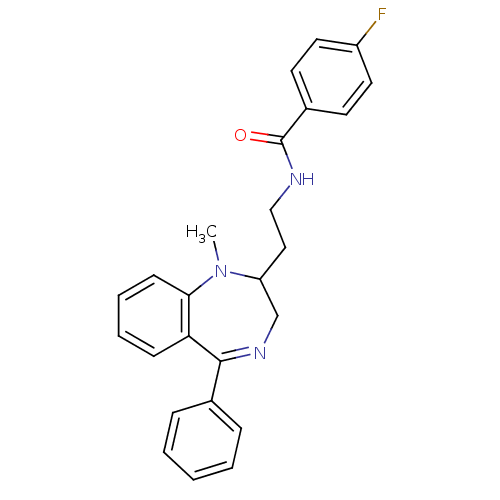 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Siena Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 of guinea pig using [3H]-U-65,693 as radioligand | J Med Chem 39: 860-72 (1996) Article DOI: 10.1021/jm950423p BindingDB Entry DOI: 10.7270/Q2222SVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50166991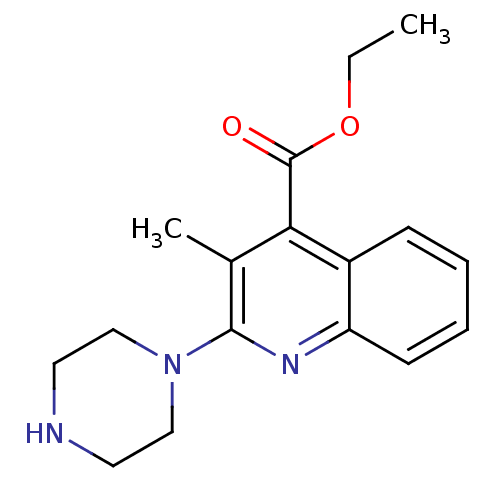 (3-Methyl-2-piperazin-1-yl-quinoline-4-carboxylic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076416 (2-[4-(2-Fluoro-phenyl)-2-(4-methyl-piperazin-1-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076401 (CHEMBL41511 | [2-(4-Methyl-piperazin-1-yl)-4-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167000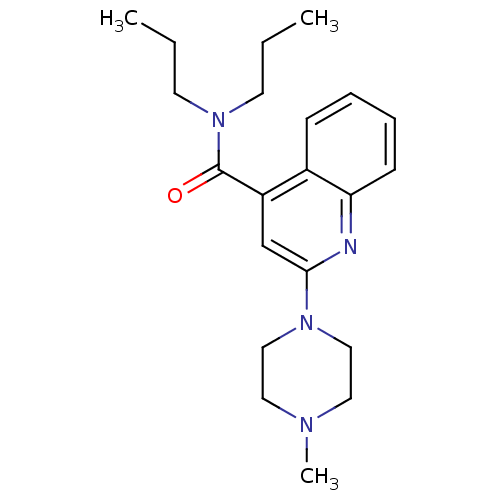 (2-(4-Methyl-piperazin-1-yl)-quinoline-4-carboxylic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076408 (4-(2-Fluoro-phenyl)-2-(4-methyl-piperazin-1-yl)-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50451023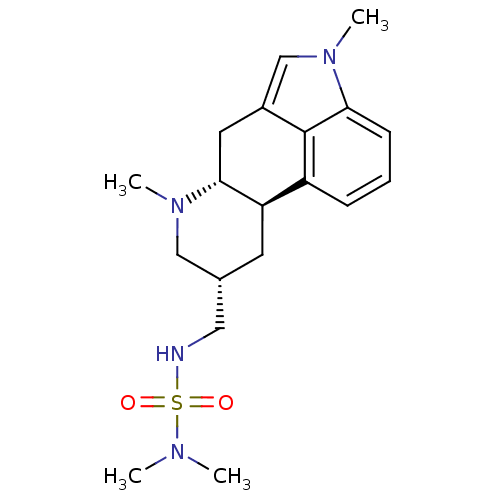 (MESULERGINE) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to displace [3H]mesulergine bound to 5-hydroxytryptamine 2C receptor in rat cortex | J Med Chem 41: 728-41 (1998) Article DOI: 10.1021/jm970645i BindingDB Entry DOI: 10.7270/Q2QV3N6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50366495 ((+)butaclamol | CHEMBL1255588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human dopamine D2S receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049805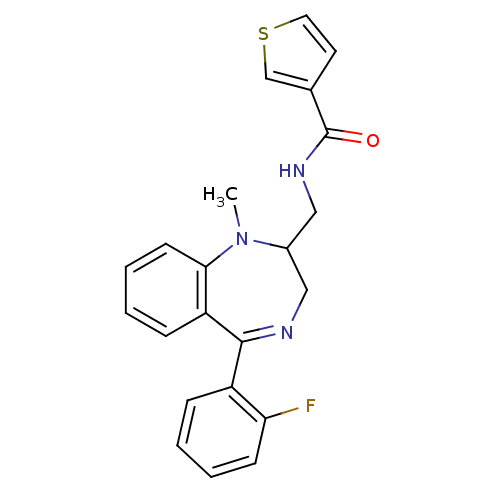 (CHEMBL169703 | Thiophene-3-carboxylic acid [5-(2-f...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Siena Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 of guinea pig using [3H]-U-65,693 as radioligand | J Med Chem 39: 860-72 (1996) Article DOI: 10.1021/jm950423p BindingDB Entry DOI: 10.7270/Q2222SVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50166981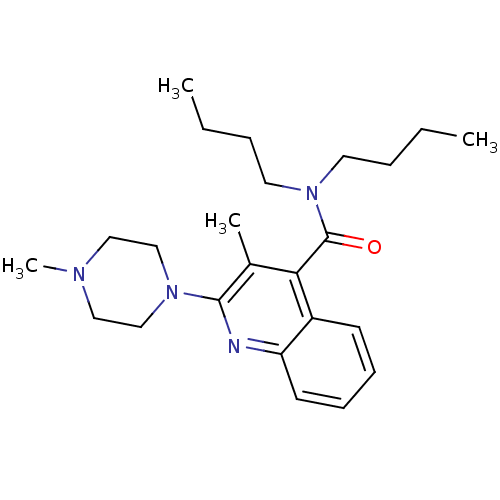 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076427 (2-(4-Methyl-piperazin-1-yl)-4-phenyl-3-[2-(tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50063260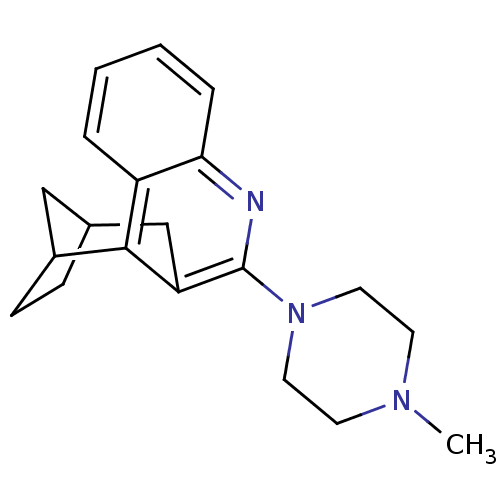 (10-(4-methylhexahydro-1-pyrazinyl)-9-azatetracyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167001 (CHEMBL192794 | [3-Methyl-2-(4-methyl-piperazin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50166993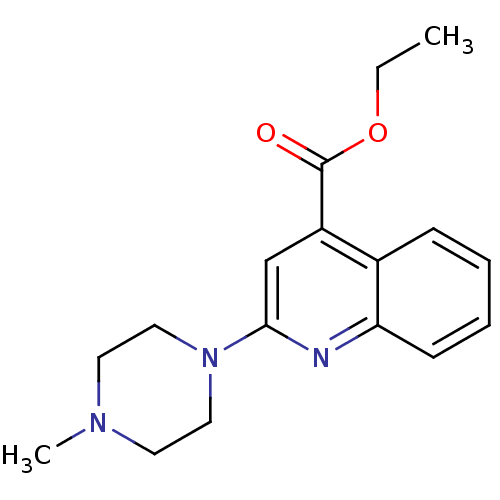 (2-(4-Methyl-piperazin-1-yl)-quinoline-4-carboxylic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076410 (2-[2-(4-Methyl-piperazin-1-yl)-3-vinyl-quinolin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor (5-HT 3 receptor) of rat cortex and hippocampus tissue | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167009 (2-(4-Methyl-piperazin-1-yl)-3-propyl-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human histamine H1 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50076421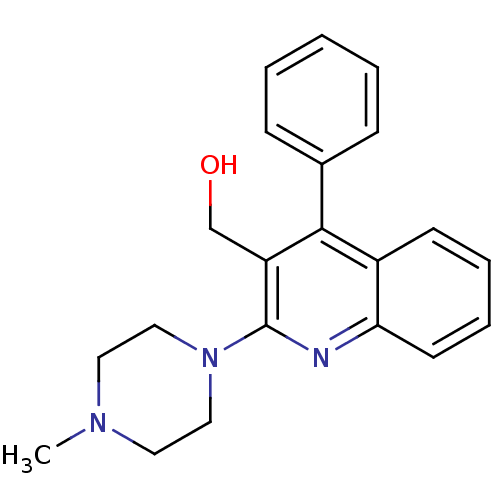 (CHEMBL42912 | [2-(4-Methyl-piperazin-1-yl)-4-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]granisetron binding to 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049807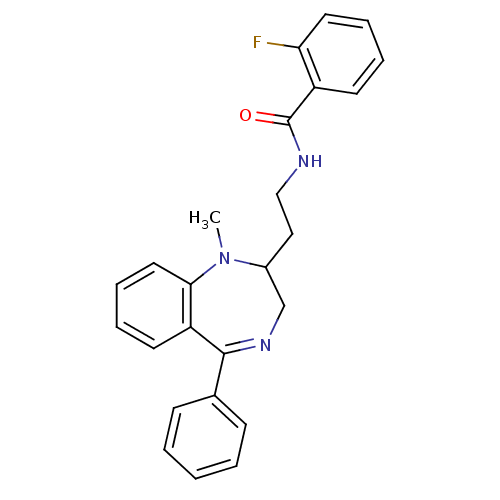 (2-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Siena Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 of guinea pig using [3H]-U-65,693 as radioligand | J Med Chem 39: 860-72 (1996) Article DOI: 10.1021/jm950423p BindingDB Entry DOI: 10.7270/Q2222SVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50166985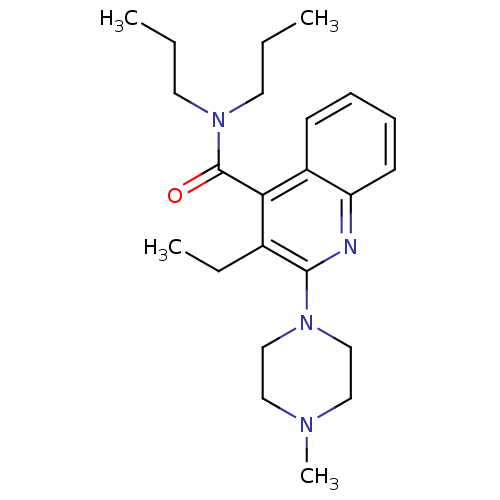 (3-Ethyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to displace [3H]5-HT bound to 5-hydroxytryptamine 1B receptor in rat striatum | J Med Chem 41: 728-41 (1998) Article DOI: 10.1021/jm970645i BindingDB Entry DOI: 10.7270/Q2QV3N6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1B receptor of rat striatum. | J Med Chem 42: 1556-75 (1999) Article DOI: 10.1021/jm981112s BindingDB Entry DOI: 10.7270/Q2D50NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50166989 (2-(4-Methyl-piperazin-1-yl)-3-propyl-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 737 total ) | Next | Last >> |