

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055260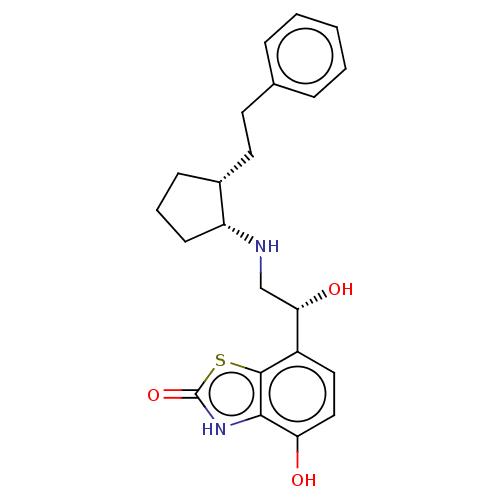 (CHEMBL3323658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055263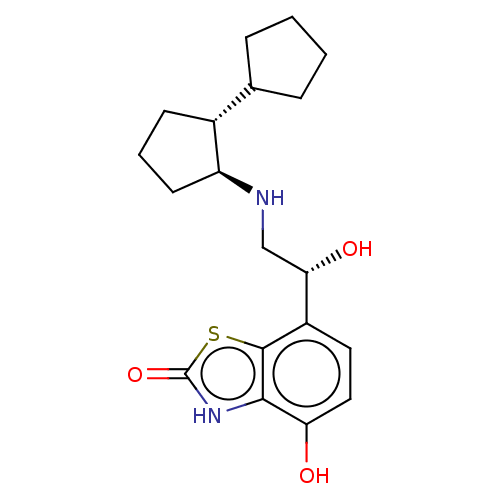 (CHEMBL3323654) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055260 (CHEMBL3323658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324856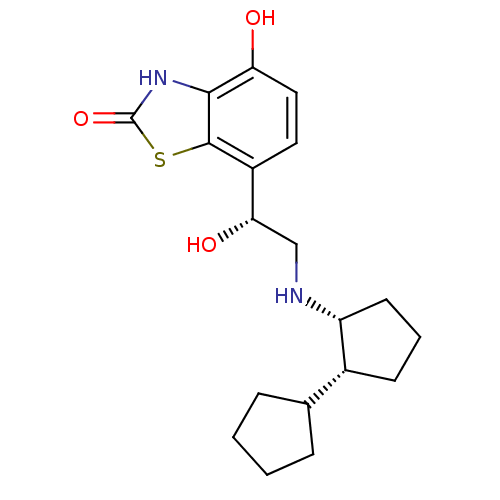 (7-((R)-2-((cis)-bi(cyclopentan)-2-ylamino)-1-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055245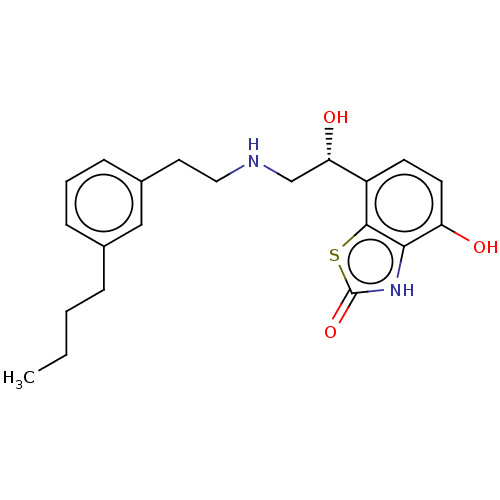 (CHEMBL3323668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055249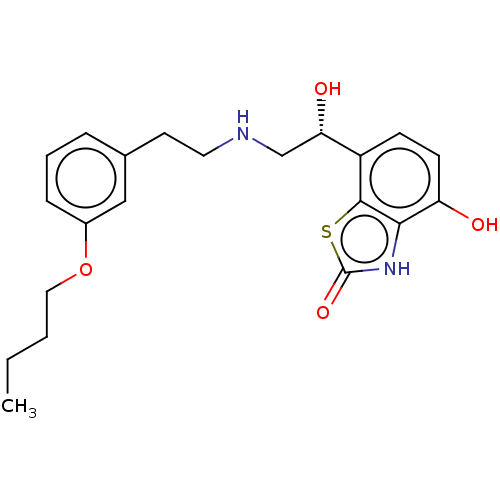 (CHEMBL3323670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055251 (CHEMBL3323655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324855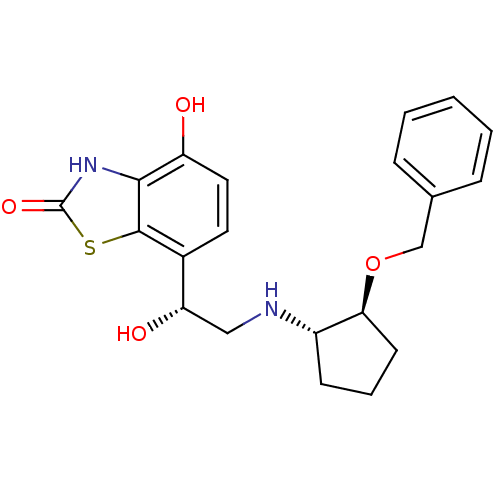 (7-((R)-2-((1S,2S)-2-(benzyloxy)cyclopentylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM86453 (CAS_73573-87-2 | Formoterol | NSC_3083544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25771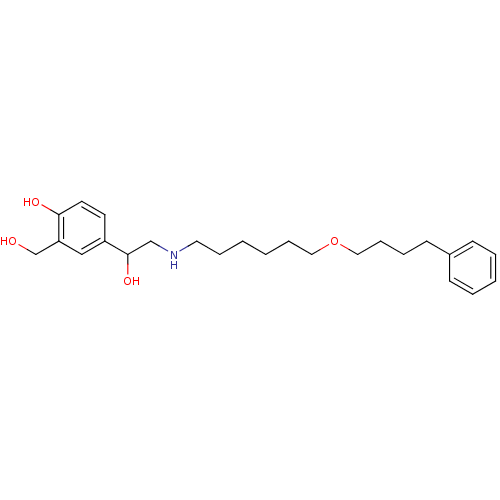 (1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055254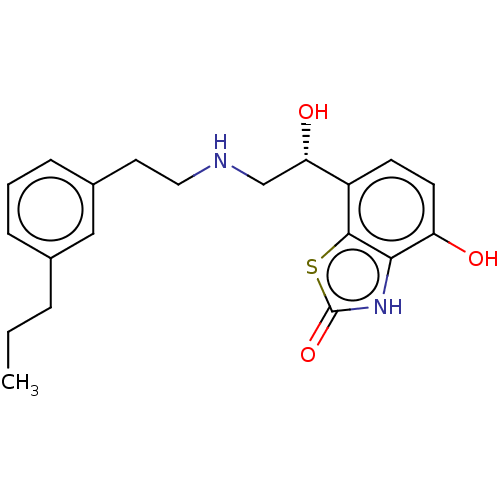 (CHEMBL3323666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055246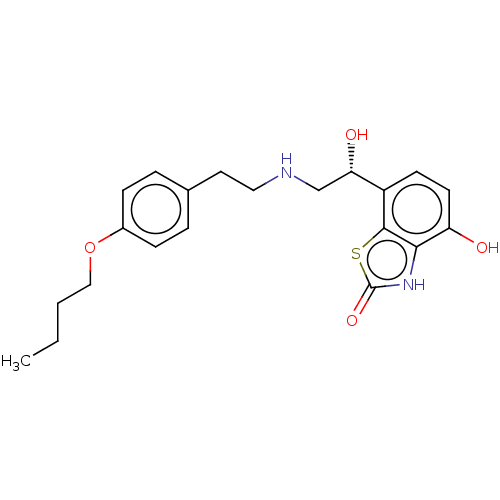 (CHEMBL3323669) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055252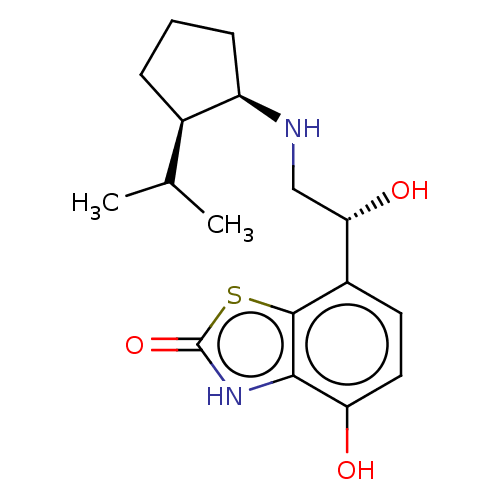 (CHEMBL3323657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055249 (CHEMBL3323670) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055254 (CHEMBL3323666) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055253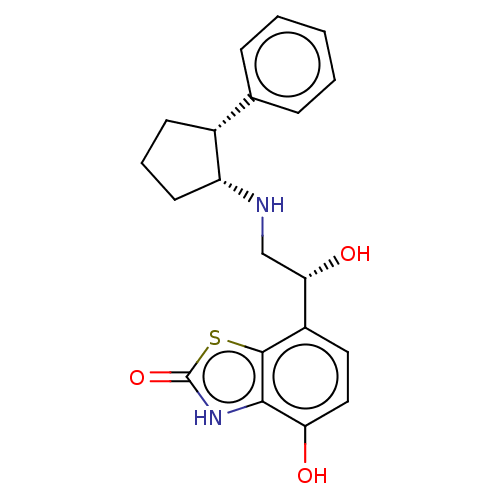 (CHEMBL3323659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055243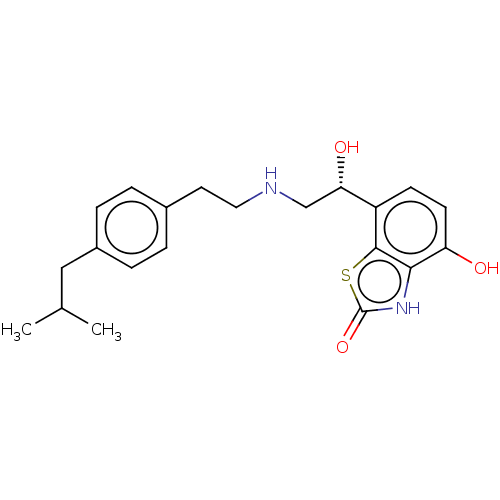 (CHEMBL3323667) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055250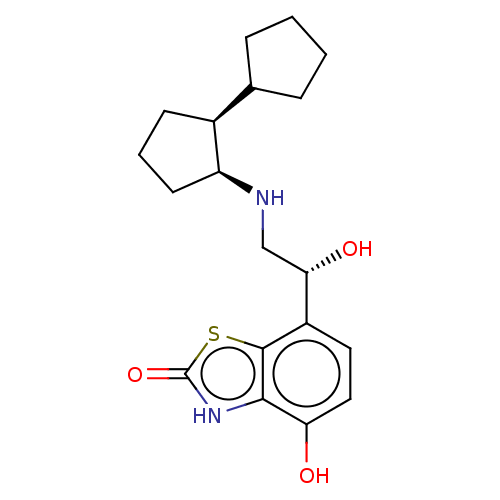 (CHEMBL3323653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055250 (CHEMBL3323653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055243 (CHEMBL3323667) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055259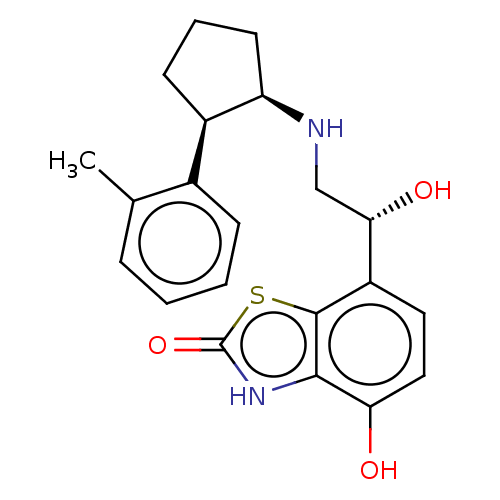 (CHEMBL3323660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055245 (CHEMBL3323668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166735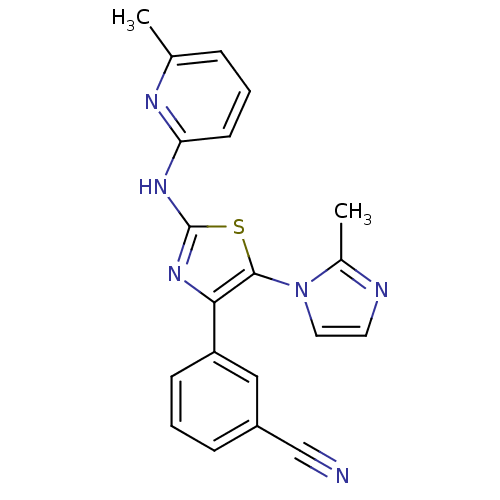 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073296 (4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055256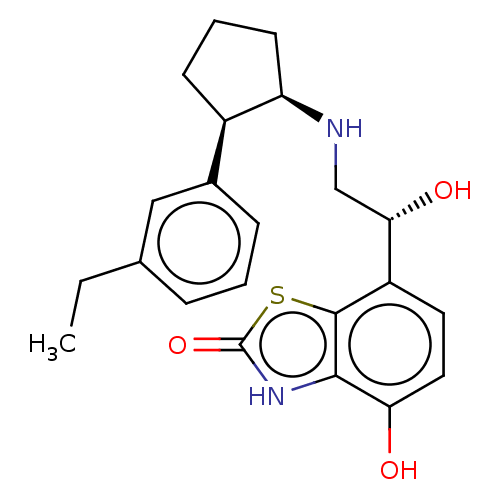 (CHEMBL3323663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076828 (4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 9: 1103-8 (1999) BindingDB Entry DOI: 10.7270/Q2SB44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055246 (CHEMBL3323669) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076819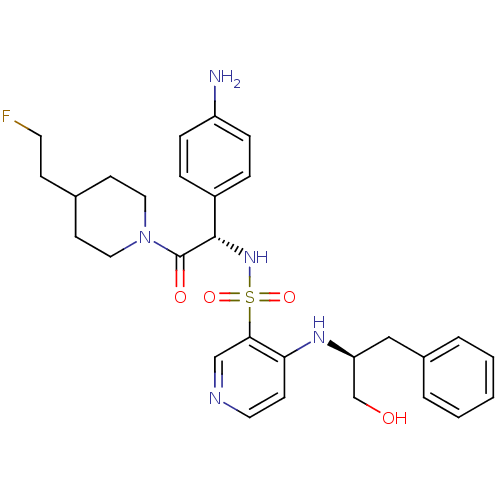 (4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 9: 1103-8 (1999) BindingDB Entry DOI: 10.7270/Q2SB44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055258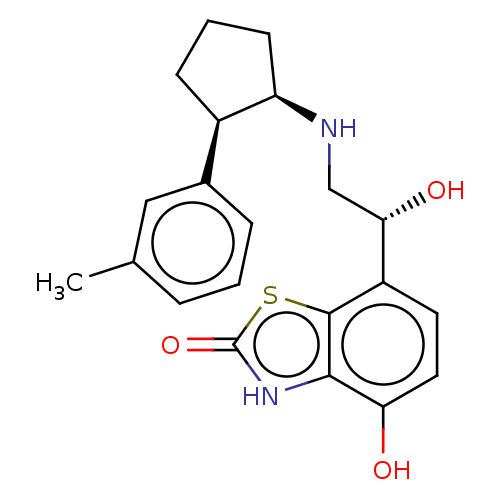 (CHEMBL3323661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073308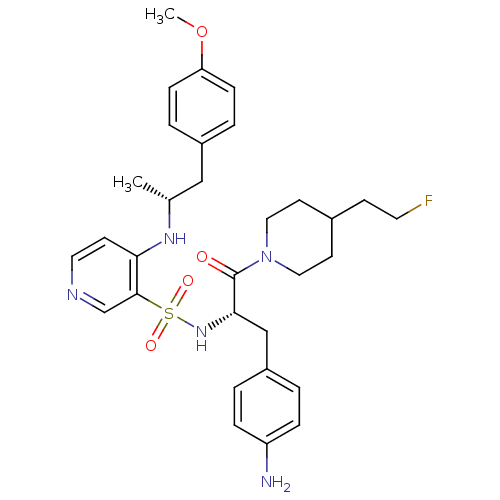 (4-[(R)-2-(4-Methoxy-phenyl)-1-methyl-ethylamino]-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076823 (4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 9: 1103-8 (1999) BindingDB Entry DOI: 10.7270/Q2SB44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50324856 (7-((R)-2-((cis)-bi(cyclopentan)-2-ylamino)-1-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055263 (CHEMBL3323654) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073307 (4-((R)-1-Methyl-2-phenyl-ethylamino)-pyridine-3-su...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073302 (4-((S)-2-Cyclohexyl-1-hydroxymethyl-ethylamino)-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073283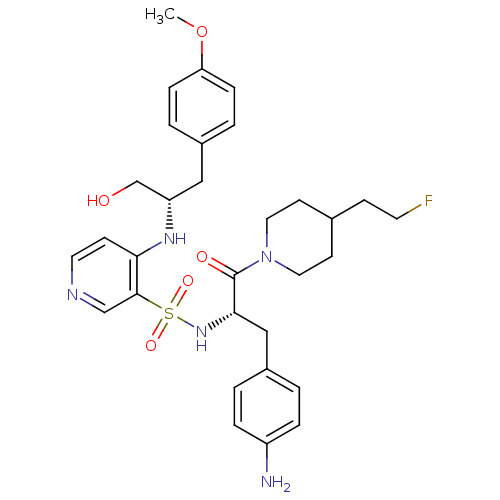 (4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073283 (4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073283 (4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055255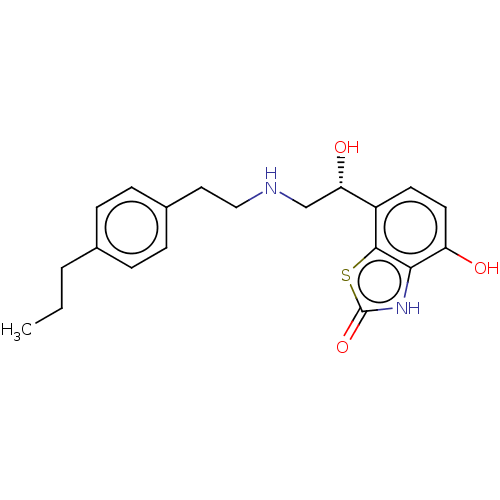 (CHEMBL3323665) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073297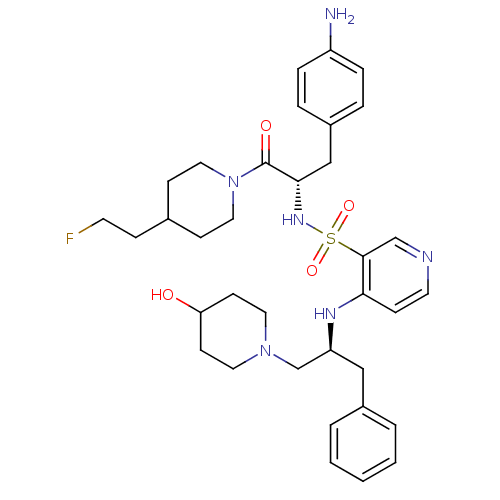 (4-[(S)-1-(4-Hydroxy-piperidin-1-ylmethyl)-2-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073297 (4-[(S)-1-(4-Hydroxy-piperidin-1-ylmethyl)-2-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073290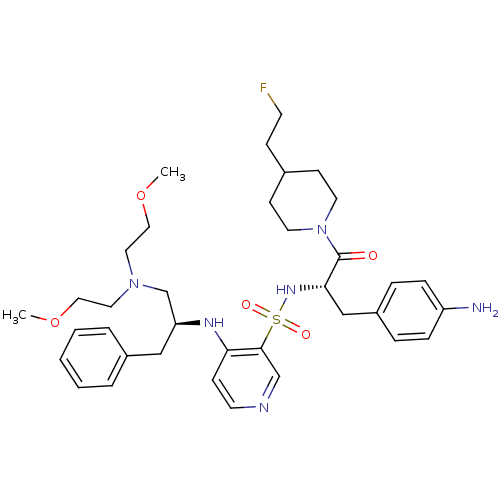 (4-{(S)-1-Benzyl-2-[bis-(2-methoxy-ethyl)-amino]-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166742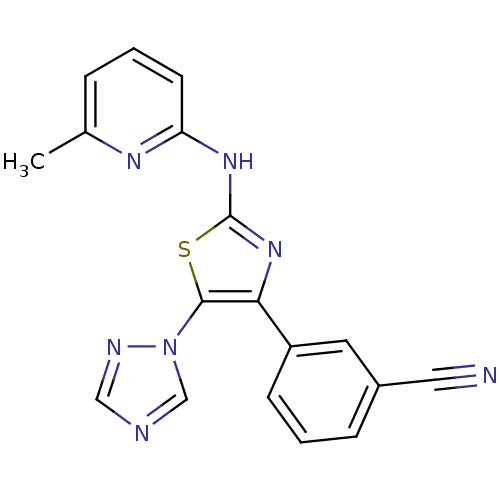 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055264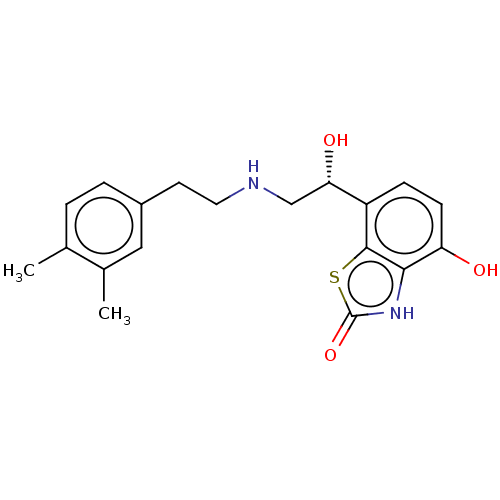 (CHEMBL3323671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159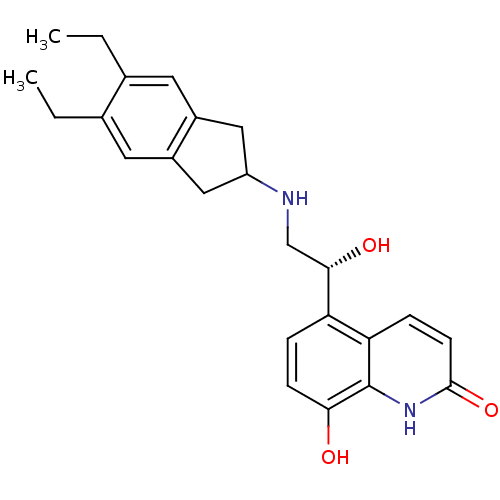 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073298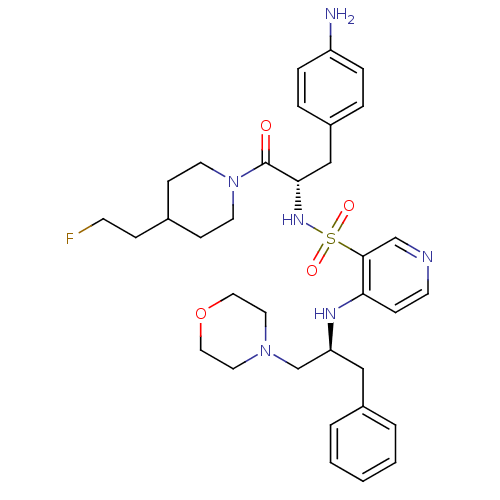 (4-((S)-1-Benzyl-2-morpholin-4-yl-ethylamino)-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073298 (4-((S)-1-Benzyl-2-morpholin-4-yl-ethylamino)-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro binding affinity for human thrombin. | Bioorg Med Chem Lett 8: 3583-8 (1999) BindingDB Entry DOI: 10.7270/Q24B30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073298 (4-((S)-1-Benzyl-2-morpholin-4-yl-ethylamino)-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166739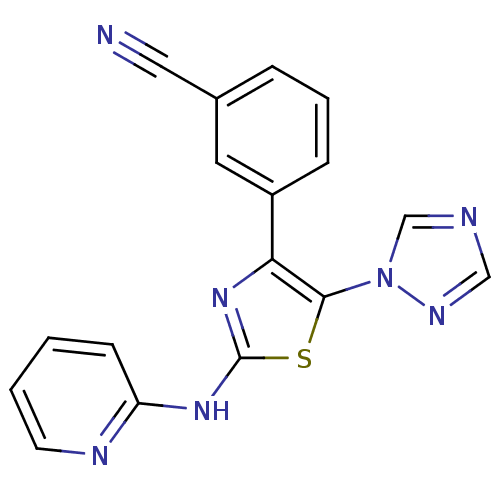 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073294 (4-((S)-1-Benzyl-2-dimethylamino-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 246 total ) | Next | Last >> |