 Found 299 hits with Last Name = 'mesic' and Initial = 'm'
Found 299 hits with Last Name = 'mesic' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM214798
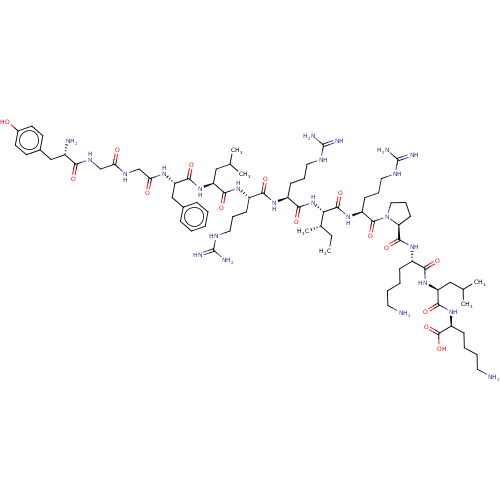
(Dynorphin A (1-17) | YGGFLRRIRPKLK)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50040123
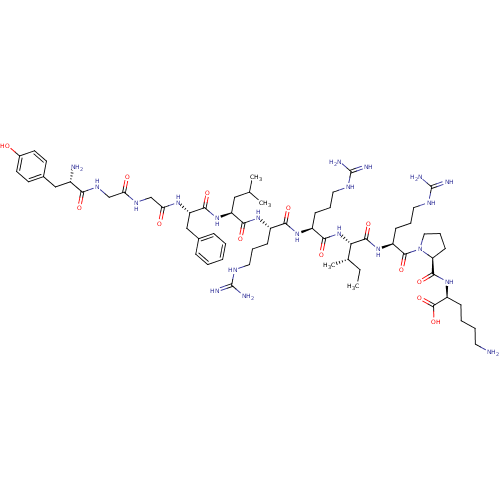
(CHEMBL438223 | HTry-Gly-Gly-Phe-Leu-Arg-Arg-lle-Ar...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C63H103N21O13/c1-5-37(4)51(58(94)80-44(20-13-29-74-63(70)71)59(95)84-30-14-21-48(84)57(93)81-45(60(96)97)17-9-10-26-64)83-54(90)43(19-12-28-73-62(68)69)78-53(89)42(18-11-27-72-61(66)67)79-55(91)46(31-36(2)3)82-56(92)47(33-38-15-7-6-8-16-38)77-50(87)35-75-49(86)34-76-52(88)41(65)32-39-22-24-40(85)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,85H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H,75,86)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,94)(H,81,93)(H,82,92)(H,83,90)(H,96,97)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50002087
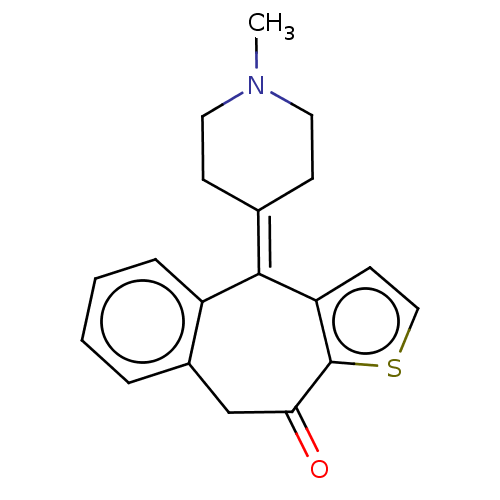
(4-(1-Methyl-piperidin-4-ylidene)-4,9-dihydro-1-thi...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccsc2-[#6](=O)-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H19NOS/c1-20-9-6-13(7-10-20)18-15-5-3-2-4-14(15)12-17(21)19-16(18)8-11-22-19/h2-5,8,11H,6-7,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107
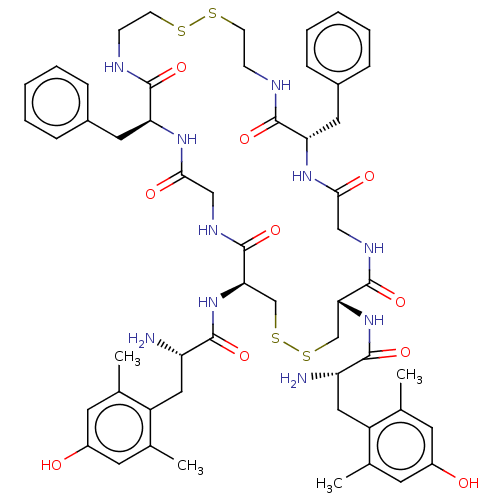
(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558711
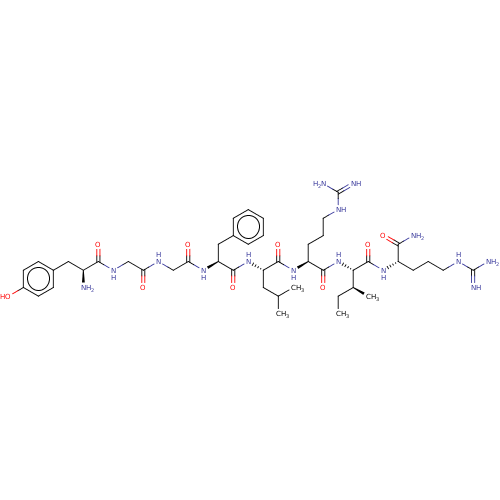
(CHEMBL4754961)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558723
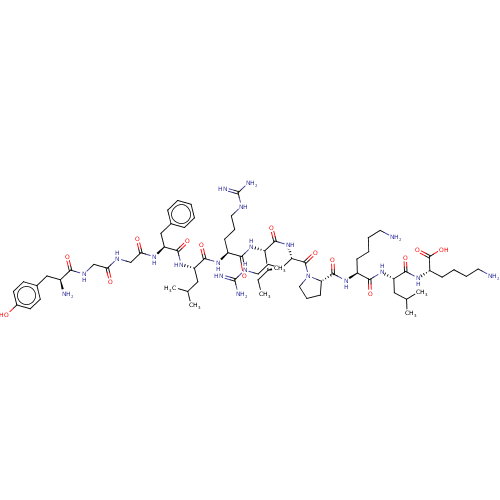
(CHEMBL4760958)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558722
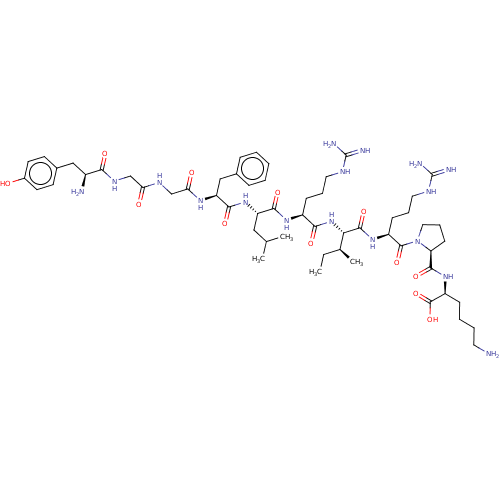
(CHEMBL4757601)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in mouse NG108-15 cell membranes incubated for 2 hrs by liquid scintillation counting base... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111
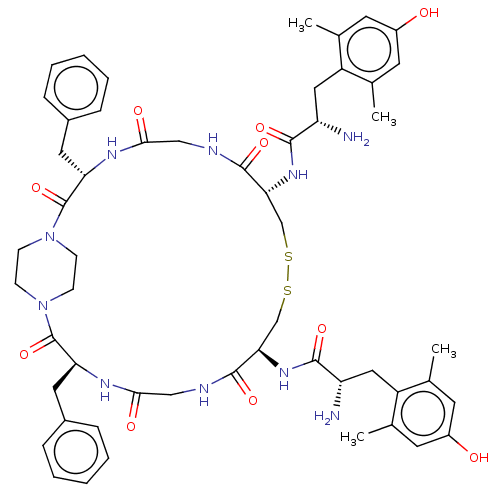
(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275108

(CHEMBL4129668)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C50H62N10O10S4/c51-37(23-33-11-15-35(61)16-12-33)45(65)59-41-29-73-74-30-42(60-46(66)38(52)24-34-13-17-36(62)18-14-34)50(70)56-28-44(64)58-40(26-32-9-5-2-6-10-32)48(68)54-20-22-72-71-21-19-53-47(67)39(25-31-7-3-1-4-8-31)57-43(63)27-55-49(41)69/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,69)(H,56,70)(H,57,63)(H,58,64)(H,59,65)(H,60,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275108

(CHEMBL4129668)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C50H62N10O10S4/c51-37(23-33-11-15-35(61)16-12-33)45(65)59-41-29-73-74-30-42(60-46(66)38(52)24-34-13-17-36(62)18-14-34)50(70)56-28-44(64)58-40(26-32-9-5-2-6-10-32)48(68)54-20-22-72-71-21-19-53-47(67)39(25-31-7-3-1-4-8-31)57-43(63)27-55-49(41)69/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,69)(H,56,70)(H,57,63)(H,58,64)(H,59,65)(H,60,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]deltorphin-II from human DOR expressed in CHO cell membranes incubated for 2 hrs by liquid scintillation counting based radioliga... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]deltorphin-II from human DOR expressed in CHO cell membranes incubated for 2 hrs by liquid scintillation counting based radioliga... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50417376
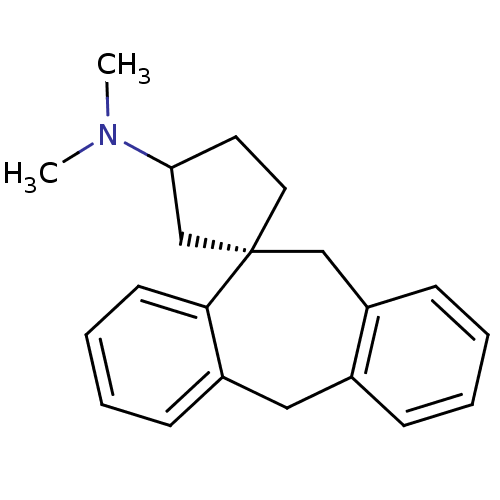
(CHEMBL1278116)Show InChI InChI=1S/C21H25N/c1-22(2)19-11-12-21(15-19)14-18-9-4-3-7-16(18)13-17-8-5-6-10-20(17)21/h3-10,19H,11-15H2,1-2H3/t19?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275108

(CHEMBL4129668)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C50H62N10O10S4/c51-37(23-33-11-15-35(61)16-12-33)45(65)59-41-29-73-74-30-42(60-46(66)38(52)24-34-13-17-36(62)18-14-34)50(70)56-28-44(64)58-40(26-32-9-5-2-6-10-32)48(68)54-20-22-72-71-21-19-53-47(67)39(25-31-7-3-1-4-8-31)57-43(63)27-55-49(41)69/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,69)(H,56,70)(H,57,63)(H,58,64)(H,59,65)(H,60,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50558723

(CHEMBL4760958)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in mouse NG108-15 cell membranes incubated for 2 hrs by liquid scintillation counting base... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275108

(CHEMBL4129668)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C50H62N10O10S4/c51-37(23-33-11-15-35(61)16-12-33)45(65)59-41-29-73-74-30-42(60-46(66)38(52)24-34-13-17-36(62)18-14-34)50(70)56-28-44(64)58-40(26-32-9-5-2-6-10-32)48(68)54-20-22-72-71-21-19-53-47(67)39(25-31-7-3-1-4-8-31)57-43(63)27-55-49(41)69/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,69)(H,56,70)(H,57,63)(H,58,64)(H,59,65)(H,60,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50558723

(CHEMBL4760958)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]deltorphin-II from human DOR expressed in CHO cell membranes incubated for 2 hrs by liquid scintillation counting based radioliga... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21014
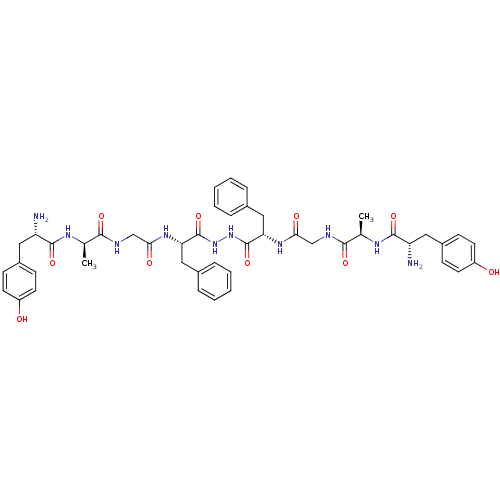
((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C46H56N10O10/c1-27(51-43(63)35(47)21-31-13-17-33(57)18-14-31)41(61)49-25-39(59)53-37(23-29-9-5-3-6-10-29)45(65)55-56-46(66)38(24-30-11-7-4-8-12-30)54-40(60)26-50-42(62)28(2)52-44(64)36(48)22-32-15-19-34(58)20-16-32/h3-20,27-28,35-38,57-58H,21-26,47-48H2,1-2H3,(H,49,61)(H,50,62)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,65)(H,56,66)/t27-,28-,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity to DOR (unknown origin) |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50330747
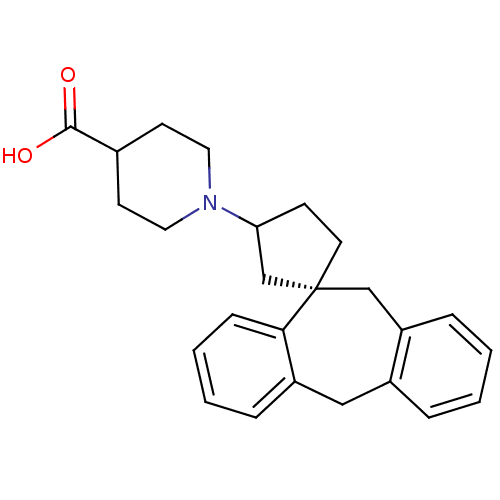
((-)-1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'...)Show SMILES OC(=O)C1CCN(CC1)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r| Show InChI InChI=1S/C25H29NO2/c27-24(28)18-10-13-26(14-11-18)22-9-12-25(17-22)16-21-7-2-1-5-19(21)15-20-6-3-4-8-23(20)25/h1-8,18,22H,9-17H2,(H,27,28)/t22?,25-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50330752
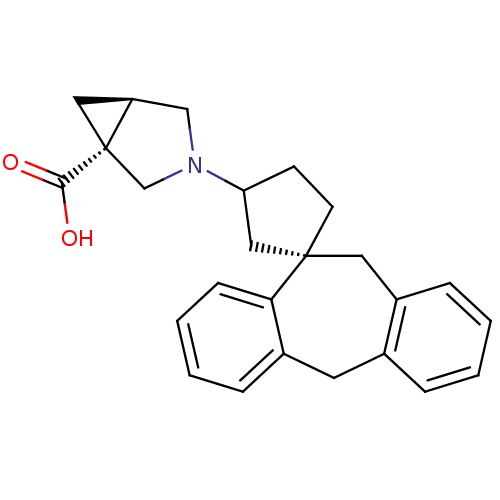
(3-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...)Show SMILES OC(=O)[C@]12C[C@H]1CN(C2)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r| Show InChI InChI=1S/C25H27NO2/c27-23(28)25-13-20(25)15-26(16-25)21-9-10-24(14-21)12-19-7-2-1-5-17(19)11-18-6-3-4-8-22(18)24/h1-8,20-21H,9-16H2,(H,27,28)/t20-,21?,24-,25-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in mouse NG108-15 cell membranes incubated for 2 hrs by liquid scintillation counting base... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50330751
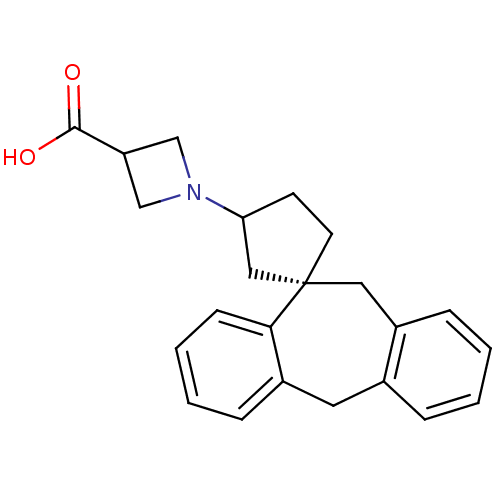
(1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...)Show SMILES OC(=O)C1CN(C1)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r| Show InChI InChI=1S/C23H25NO2/c25-22(26)19-14-24(15-19)20-9-10-23(13-20)12-18-7-2-1-5-16(18)11-17-6-3-4-8-21(17)23/h1-8,19-20H,9-15H2,(H,25,26)/t20?,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50558711

(CHEMBL4754961)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]deltorphin-II from human DOR expressed in CHO cell membranes incubated for 2 hrs by liquid scintillation counting based radioliga... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50558722

(CHEMBL4757601)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]deltorphin-II from human DOR expressed in CHO cell membranes incubated for 2 hrs by liquid scintillation counting based radioliga... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50558722

(CHEMBL4757601)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in mouse NG108-15 cell membranes incubated for 2 hrs by liquid scintillation counting base... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50330751

(1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...)Show SMILES OC(=O)C1CN(C1)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r| Show InChI InChI=1S/C23H25NO2/c25-22(26)19-14-24(15-19)20-9-10-23(13-20)12-18-7-2-1-5-16(18)11-17-6-3-4-8-21(17)23/h1-8,19-20H,9-15H2,(H,25,26)/t20?,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM214798

(Dynorphin A (1-17) | YGGFLRRIRPKLK)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in mouse NG108-15 cell membranes incubated for 2 hrs by liquid scintillation counting base... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM214798

(Dynorphin A (1-17) | YGGFLRRIRPKLK)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]deltorphin-II from human DOR expressed in CHO cell membranes incubated for 2 hrs by liquid scintillation counting based radioliga... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50330748
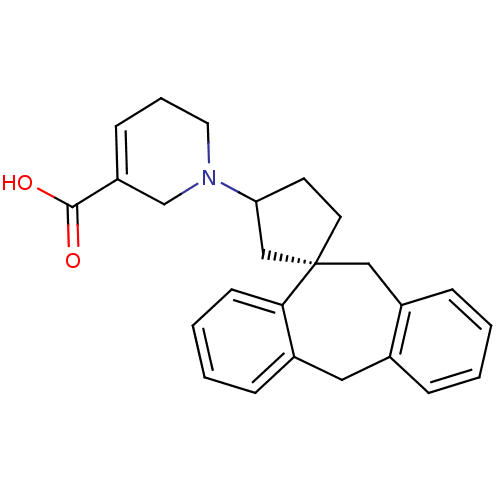
(1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...)Show SMILES OC(=O)C1=CCCN(C1)C1CC[C@@]2(C1)Cc1ccccc1Cc1ccccc21 |r,t:3| Show InChI InChI=1S/C25H27NO2/c27-24(28)21-9-5-13-26(17-21)22-11-12-25(16-22)15-20-8-2-1-6-18(20)14-19-7-3-4-10-23(19)25/h1-4,6-10,22H,5,11-17H2,(H,27,28)/t22?,25-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting |
J Med Chem 53: 7778-95 (2010)
Article DOI: 10.1021/jm100856p
BindingDB Entry DOI: 10.7270/Q2FQ9WWQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275110

(CHEMBL4130069)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:1.1,13.13,57.61,wD:18.19,22.24,39.41,(4.38,-11.48,;5.71,-12.25,;7.04,-11.48,;7.04,-9.94,;5.71,-9.17,;5.71,-7.63,;7.04,-6.86,;7.04,-5.32,;8.38,-7.63,;8.38,-9.17,;5.71,-13.79,;4.38,-14.56,;7.04,-14.56,;7.04,-16.11,;5.71,-16.88,;5.71,-18.42,;8.38,-21.5,;8.38,-23.04,;9.72,-23.81,;9.72,-25.35,;8.38,-26.12,;7.04,-25.35,;8.38,-27.66,;7.04,-28.44,;9.72,-28.44,;9.72,-29.98,;11.05,-30.75,;11.05,-32.29,;9.72,-33.06,;9.72,-34.6,;8.38,-32.29,;8.38,-30.75,;11.05,-23.04,;11.05,-21.5,;12.38,-23.81,;13.72,-23.04,;15.05,-23.81,;15.05,-25.35,;16.38,-23.04,;16.38,-21.5,;17.72,-20.73,;19.06,-21.5,;20.39,-20.73,;21.72,-21.5,;21.72,-23.04,;20.39,-23.81,;19.06,-23.04,;15.05,-20.73,;13.72,-21.5,;15.05,-19.19,;13.72,-18.42,;13.72,-16.88,;15.05,-16.11,;16.38,-16.88,;16.38,-18.42,;15.05,-14.56,;16.38,-13.79,;13.72,-13.79,;13.72,-12.25,;15.05,-11.48,;16.38,-12.25,;17.72,-11.48,;17.72,-9.94,;16.38,-9.17,;15.05,-9.94,;12.38,-14.56,;11.05,-13.79,;11.05,-12.25,;9.71,-14.56,;9.71,-16.11,;8.38,-16.88,;8.38,-18.42,)| Show InChI InChI=1S/C50H60N10O10S2/c51-37(23-33-11-15-35(61)16-12-33)45(65)57-41-29-71-72-30-42(58-46(66)38(52)24-34-13-17-36(62)18-14-34)48(68)54-28-44(64)56-40(26-32-9-5-2-6-10-32)50(70)60-21-19-59(20-22-60)49(69)39(25-31-7-3-1-4-8-31)55-43(63)27-53-47(41)67/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,63)(H,56,64)(H,57,65)(H,58,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275110

(CHEMBL4130069)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:1.1,13.13,57.61,wD:18.19,22.24,39.41,(4.38,-11.48,;5.71,-12.25,;7.04,-11.48,;7.04,-9.94,;5.71,-9.17,;5.71,-7.63,;7.04,-6.86,;7.04,-5.32,;8.38,-7.63,;8.38,-9.17,;5.71,-13.79,;4.38,-14.56,;7.04,-14.56,;7.04,-16.11,;5.71,-16.88,;5.71,-18.42,;8.38,-21.5,;8.38,-23.04,;9.72,-23.81,;9.72,-25.35,;8.38,-26.12,;7.04,-25.35,;8.38,-27.66,;7.04,-28.44,;9.72,-28.44,;9.72,-29.98,;11.05,-30.75,;11.05,-32.29,;9.72,-33.06,;9.72,-34.6,;8.38,-32.29,;8.38,-30.75,;11.05,-23.04,;11.05,-21.5,;12.38,-23.81,;13.72,-23.04,;15.05,-23.81,;15.05,-25.35,;16.38,-23.04,;16.38,-21.5,;17.72,-20.73,;19.06,-21.5,;20.39,-20.73,;21.72,-21.5,;21.72,-23.04,;20.39,-23.81,;19.06,-23.04,;15.05,-20.73,;13.72,-21.5,;15.05,-19.19,;13.72,-18.42,;13.72,-16.88,;15.05,-16.11,;16.38,-16.88,;16.38,-18.42,;15.05,-14.56,;16.38,-13.79,;13.72,-13.79,;13.72,-12.25,;15.05,-11.48,;16.38,-12.25,;17.72,-11.48,;17.72,-9.94,;16.38,-9.17,;15.05,-9.94,;12.38,-14.56,;11.05,-13.79,;11.05,-12.25,;9.71,-14.56,;9.71,-16.11,;8.38,-16.88,;8.38,-18.42,)| Show InChI InChI=1S/C50H60N10O10S2/c51-37(23-33-11-15-35(61)16-12-33)45(65)57-41-29-71-72-30-42(58-46(66)38(52)24-34-13-17-36(62)18-14-34)48(68)54-28-44(64)56-40(26-32-9-5-2-6-10-32)50(70)60-21-19-59(20-22-60)49(69)39(25-31-7-3-1-4-8-31)55-43(63)27-53-47(41)67/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,63)(H,56,64)(H,57,65)(H,58,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data