

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572983 (CHEMBL4848846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572968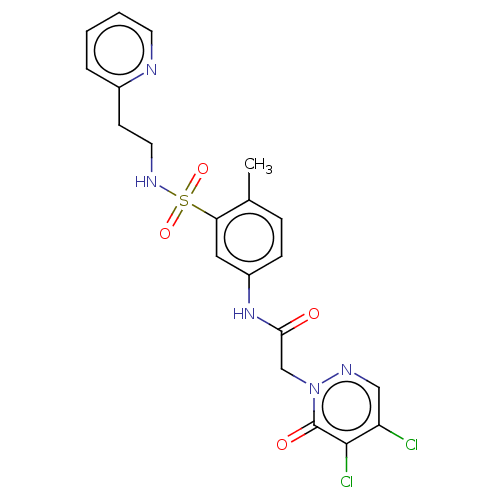 (CHEMBL4862851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572964 (CHEMBL4867592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572989 (CHEMBL4846332) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572991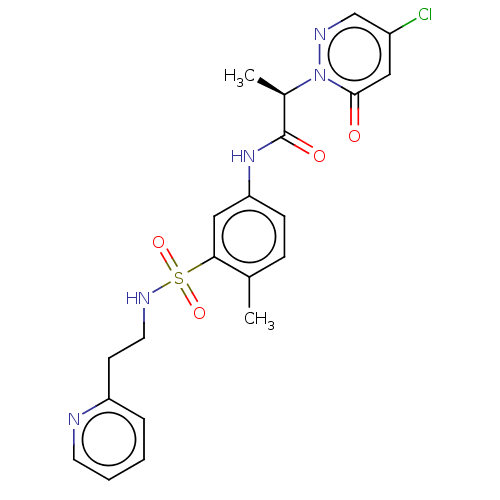 (CHEMBL4858967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572990 (CHEMBL4859105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572988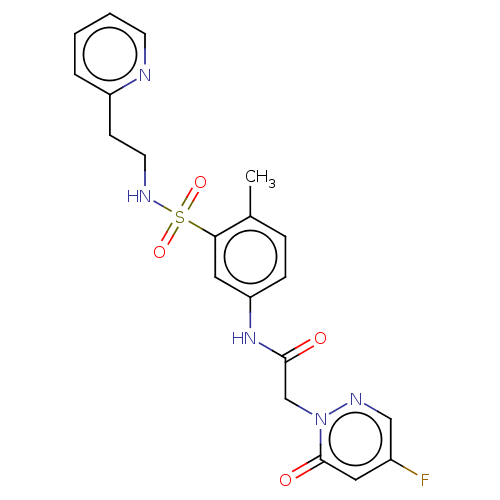 (CHEMBL4855695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50572984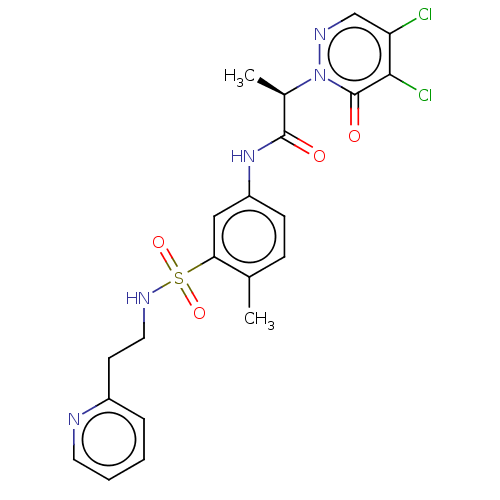 (CHEMBL4874198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00507 BindingDB Entry DOI: 10.7270/Q26977DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652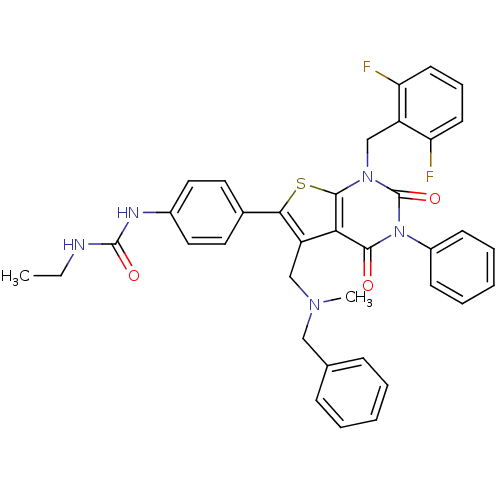 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR Y290L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR Y290L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR N27A mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His5, D-Tyr6]GnRH from GnRHR F272L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His5, D-Tyr6]GnRH from GnRHR F272L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR N27A mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR Q208E mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR Q208E mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR L300A mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR L300A mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173889 (CHEMBL3809400) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173875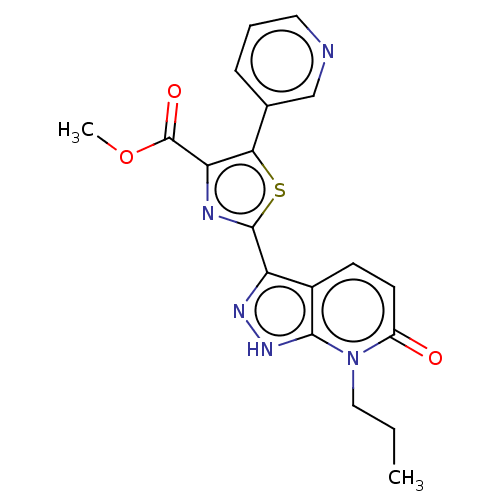 (CHEMBL3808756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR F309L mutant expressed in COS-7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173879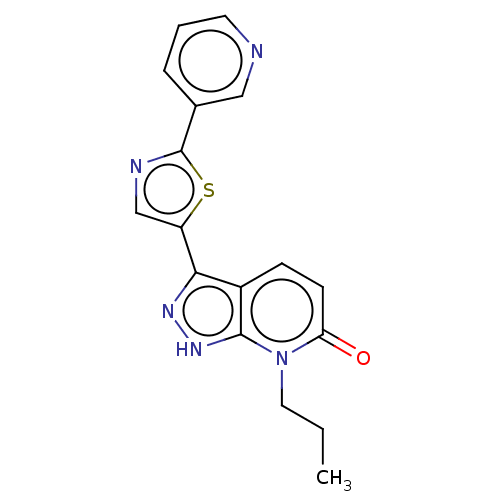 (CHEMBL3810359) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122655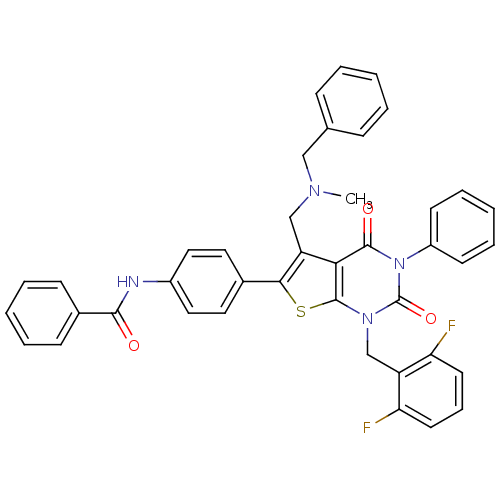 (CHEMBL280212 | N-(4-(1-(2,6-difluorobenzyl)-5-((be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His5, D-Tyr6]GnRH from GnRHR F272L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173720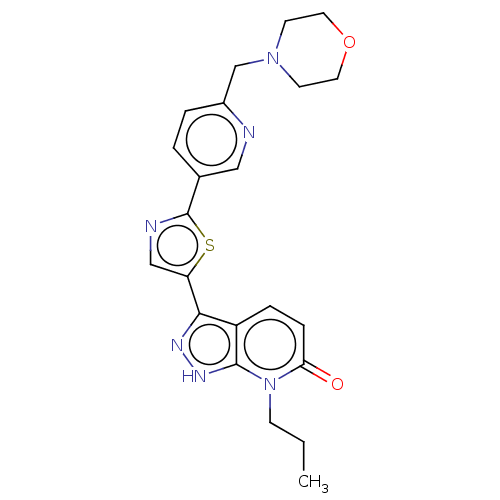 (CHEMBL3809947) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173718 (CHEMBL3809821) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173644 (CHEMBL3810048) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173641 (CHEMBL3810223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173857 (CHEMBL3808789) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173823 (CHEMBL3809278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173874 (CHEMBL3808699) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173723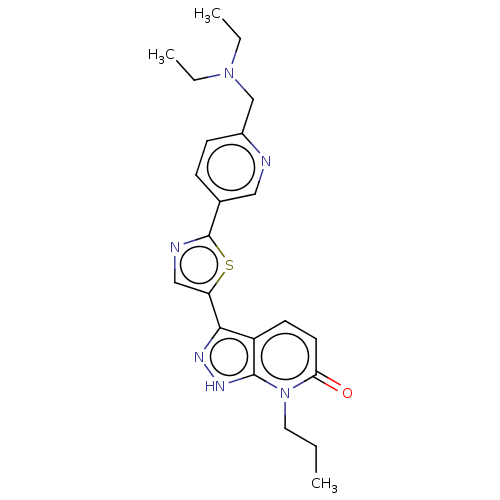 (CHEMBL3809816) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR F309L mutant expressed in COS-7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR F309Q mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173722 (CHEMBL3809648) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR Y284L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122655 (CHEMBL280212 | N-(4-(1-(2,6-difluorobenzyl)-5-((be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR D302N mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173876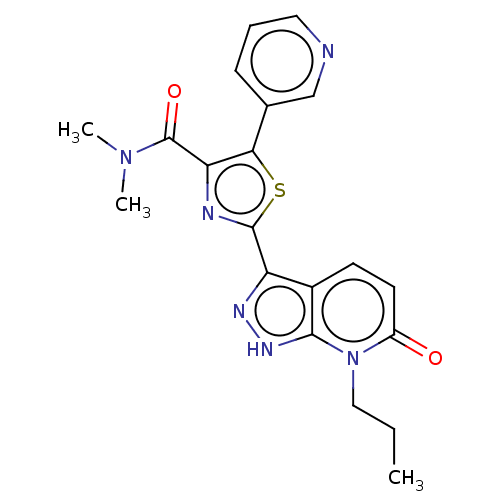 (CHEMBL3808760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173872 (CHEMBL3810056) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122655 (CHEMBL280212 | N-(4-(1-(2,6-difluorobenzyl)-5-((be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR D302A mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173724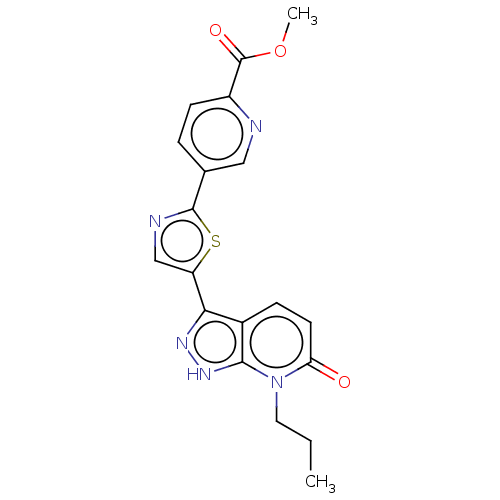 (CHEMBL3809114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173605 (CHEMBL3808603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50196040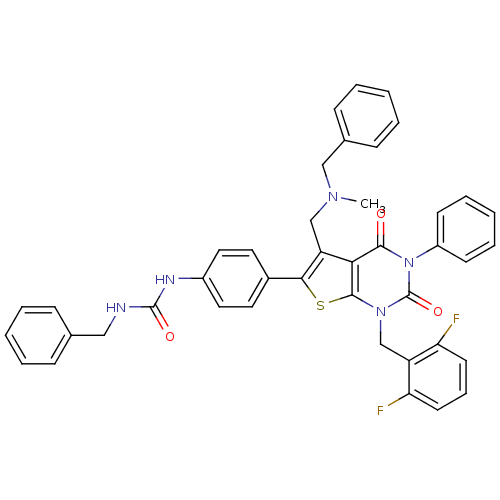 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His5, D-Tyr6]GnRH from GnRHR F272L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173878 (CHEMBL3809316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122655 (CHEMBL280212 | N-(4-(1-(2,6-difluorobenzyl)-5-((be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR Y290L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122655 (CHEMBL280212 | N-(4-(1-(2,6-difluorobenzyl)-5-((be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR L300A mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50173719 (CHEMBL3808784) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyraseB ATPase activity using linear pBR322 DNA substrate incubated for 30 mins by fluorescence polarization ... | ACS Med Chem Lett 7: 374-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00368 BindingDB Entry DOI: 10.7270/Q20R9RB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR F313L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR H306A mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR F313L mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [His5, D-Tyr6]GnRH from GnRHR M24I mutant expressed in COS7 cells | J Med Chem 49: 6170-6 (2006) Article DOI: 10.1021/jm060580w BindingDB Entry DOI: 10.7270/Q20V8CFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |