

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249309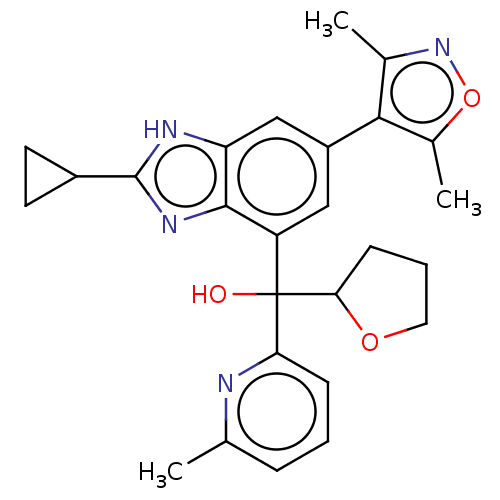 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249314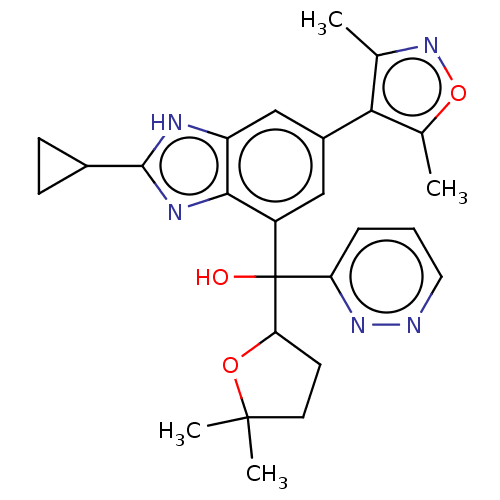 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277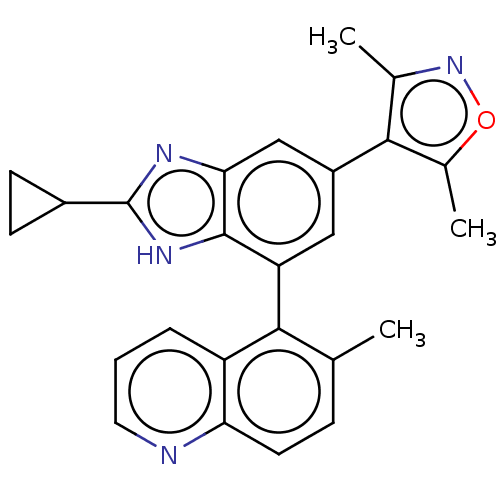 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249309 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249314 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249310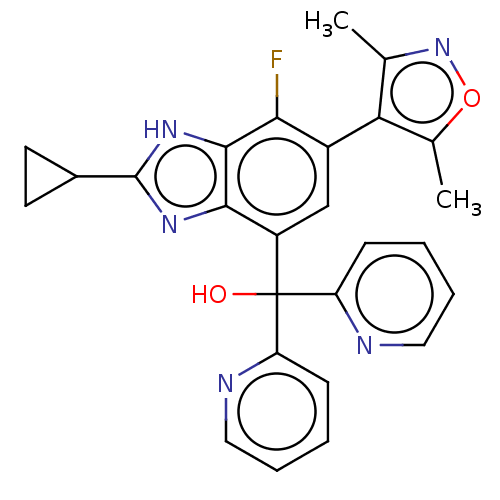 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249310 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573212 (US11453681, Compound C57) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50563730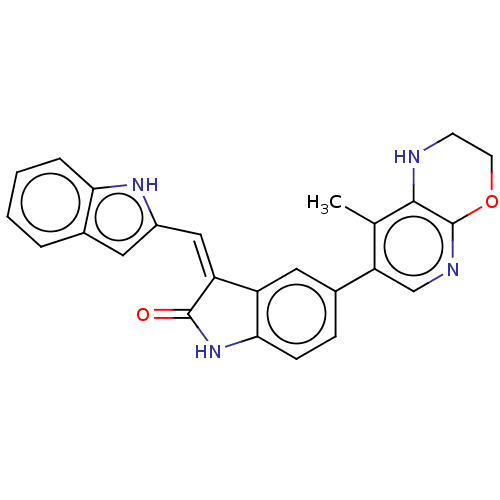 (CHEMBL4750047 | US11453681, Compound C62) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573156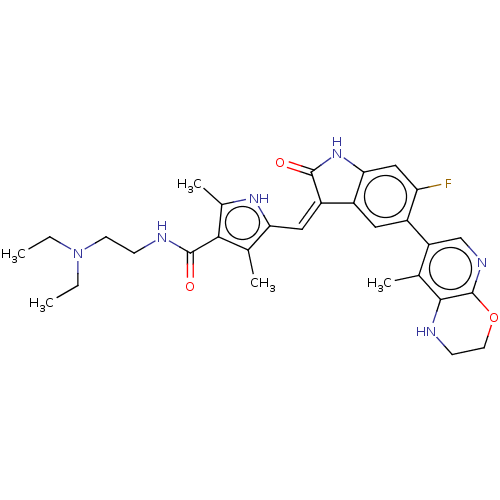 (US11453681, Compound C1) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573171 (US11453681, Compound C16) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50563735 (CHEMBL4786511 | US11453681, Compound C17) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573180 (US11453681, Compound C25) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573181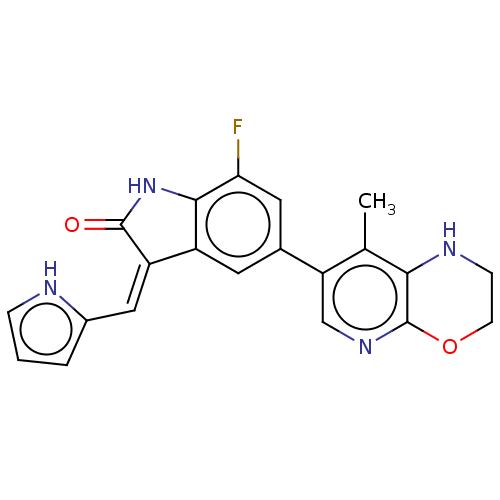 (US11453681, Compound C26) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573183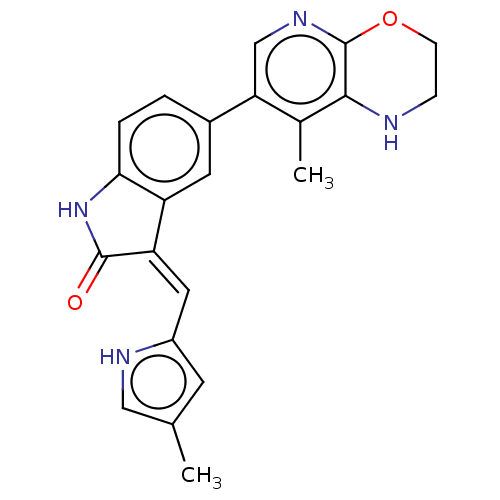 (US11453681, Compound C28) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573184 (US11453681, Compound C29) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573190 (US11453681, Compound C35) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50563731 (CHEMBL4799073 | US11453681, Compound C44) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM573201 (US11453681, Compound C46) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM50563732 (CHEMBL4776545 | US11453681, Compound C60) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activity of human HPK1 (MAP4K1) was monitored in a biochemical assay in the presence or absence of compounds and using a synthetic pept... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452113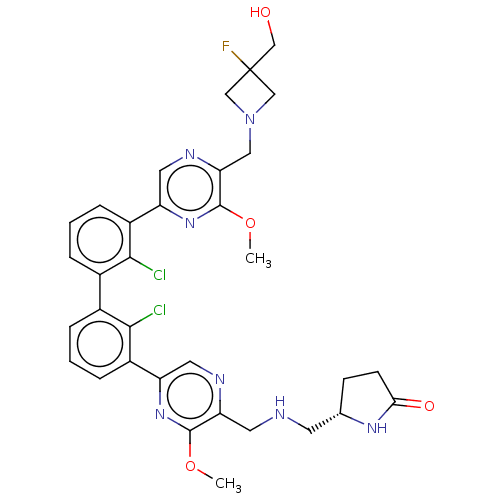 (US10710986, Example 252 | US11555029, No. 252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452147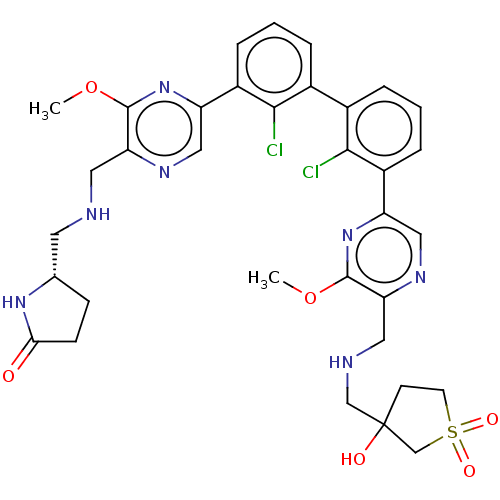 (US10710986, Example 286 | US11555029, No. 286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452150 (US10710986, Example 289 | US11555029, No. 289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452007 (US10710986, Example 147 | US11555029, No. 147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM452008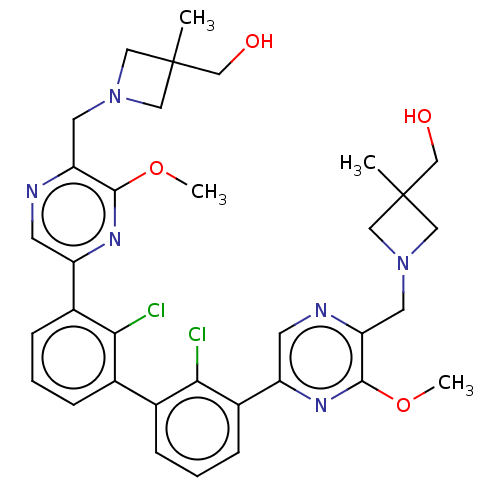 (US10710986, Example 148 | US11555029, No. 148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM451887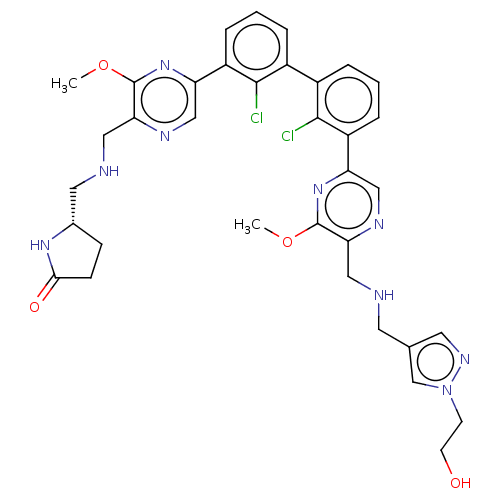 (US10710986, Example 26 | US11555029, No. 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P27329 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452113 (US10710986, Example 252 | US11555029, No. 252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452147 (US10710986, Example 286 | US11555029, No. 286) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand 1 [19-238]/protein 1 [25-167] (Homo sapiens (Human)) | BDBM452150 (US10710986, Example 289 | US11555029, No. 289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Compounds were tested in biochemical protein-protein interaction assays to determine if they can specifically block the interaction between the extra... | US Patent US10710986 (2020) BindingDB Entry DOI: 10.7270/Q28S4SZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2937 total ) | Next | Last >> |