

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897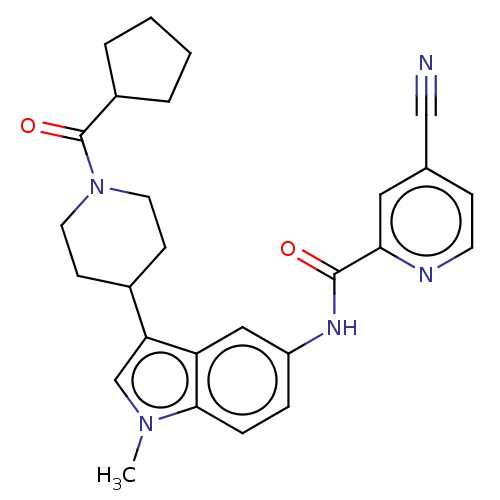 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891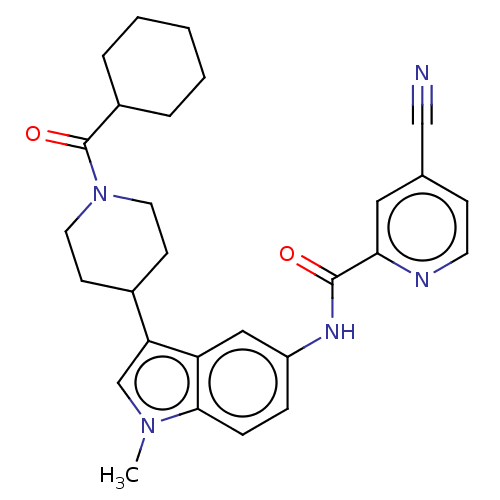 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893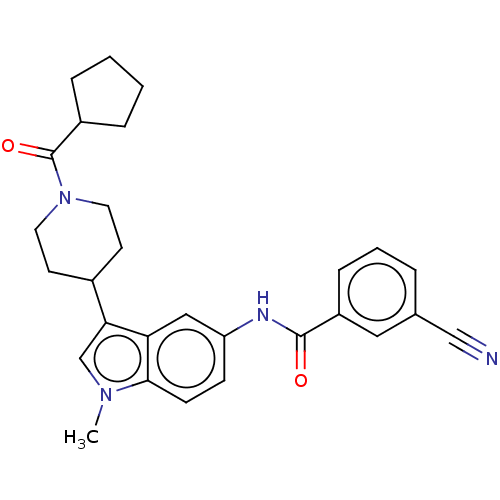 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892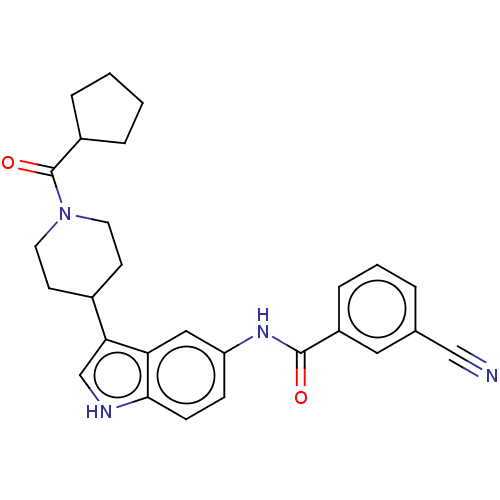 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50297122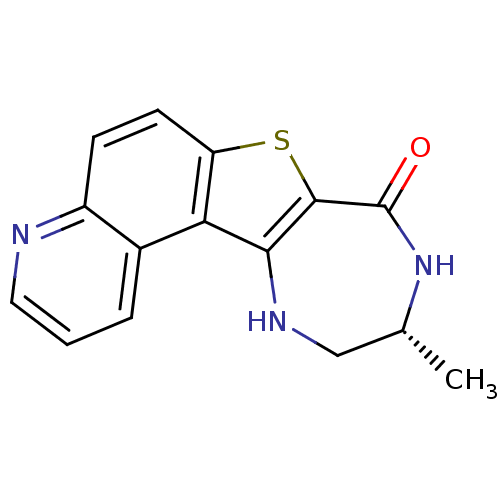 ((R)-10-Methyl-9,10,11,12-tetrahydro-7-thia-4,9,12-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 19: 4882-4 (2009) Article DOI: 10.1016/j.bmcl.2009.02.017 BindingDB Entry DOI: 10.7270/Q2J38SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50297120 ((R)-9-Methyl-8,9,10,11-tetrahydro-3-oxa-6-thia-8,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 19: 4882-4 (2009) Article DOI: 10.1016/j.bmcl.2009.02.017 BindingDB Entry DOI: 10.7270/Q2J38SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50297122 ((R)-10-Methyl-9,10,11,12-tetrahydro-7-thia-4,9,12-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 19: 4882-4 (2009) Article DOI: 10.1016/j.bmcl.2009.02.017 BindingDB Entry DOI: 10.7270/Q2J38SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013412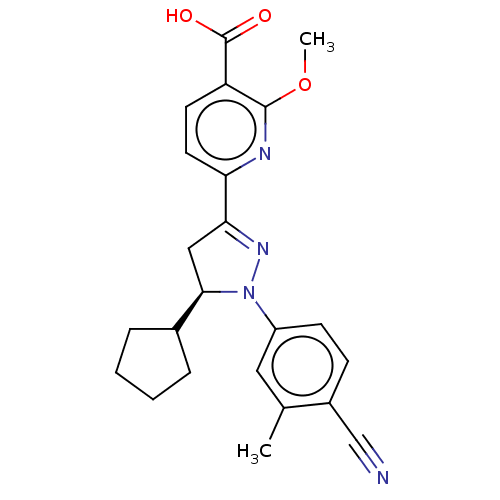 (CHEMBL3263752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to mineralocorticoid receptor (unknown origin) | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234547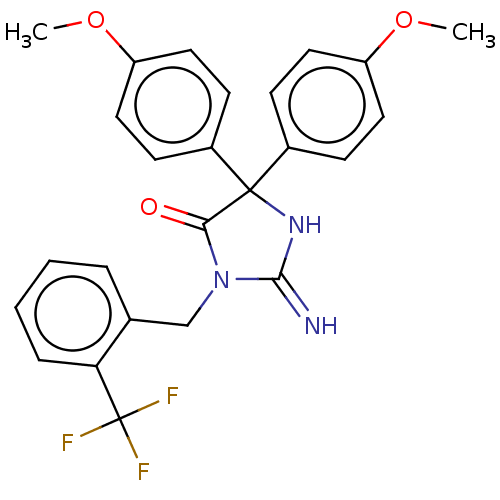 (US9353089, 304) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.88 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234542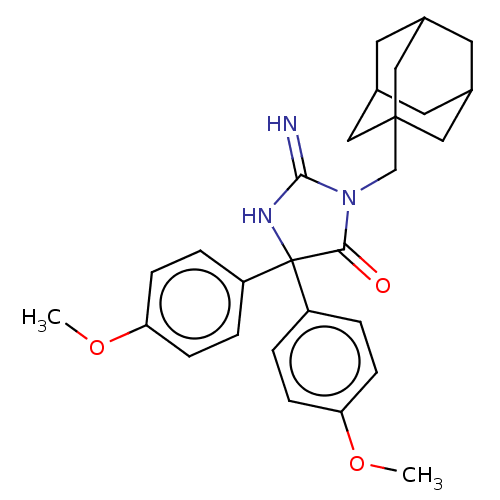 (US9353089, 276) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234565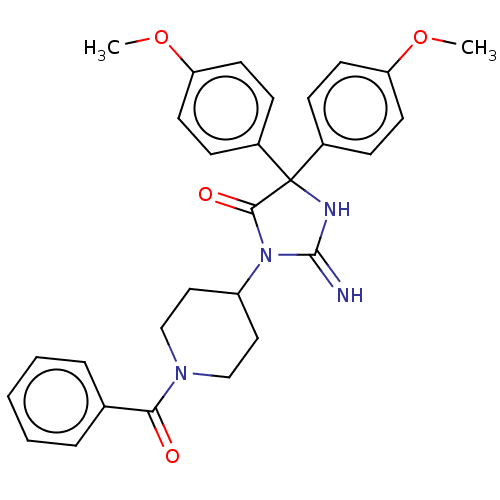 (US9353089, 311) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50297124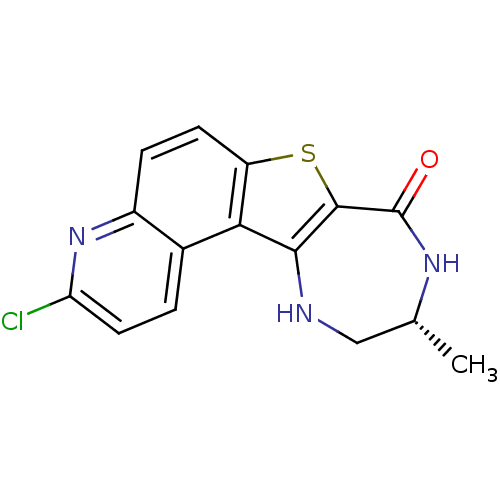 ((R)-3-Chloro-10-methyl-9,10,11,12-tetrahydro-7-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 19: 4882-4 (2009) Article DOI: 10.1016/j.bmcl.2009.02.017 BindingDB Entry DOI: 10.7270/Q2J38SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234527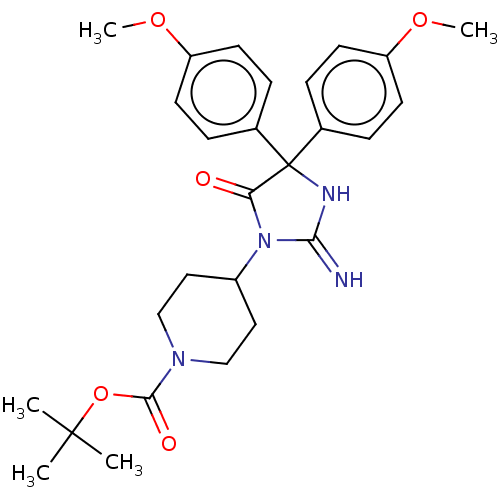 (US9353089, 206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324200 (4-[(5R)-1-(3-Chloro-4-cyanophenyl)-5-cyclopentyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at Gal4-tagged mineralocorticoid receptor expressed in human Huh7 cells by luciferase reporter gene assay | J Med Chem 53: 5979-6002 (2010) Article DOI: 10.1021/jm100505n BindingDB Entry DOI: 10.7270/Q2T43V2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013430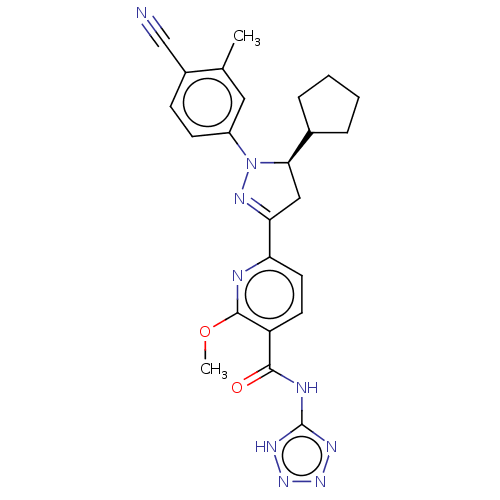 (CHEMBL3263768) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234537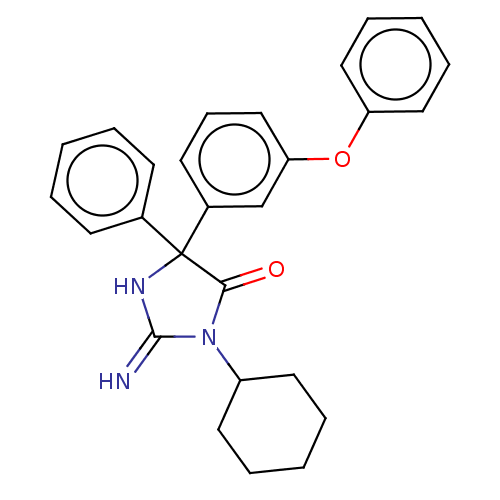 (US9353089, 238) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234534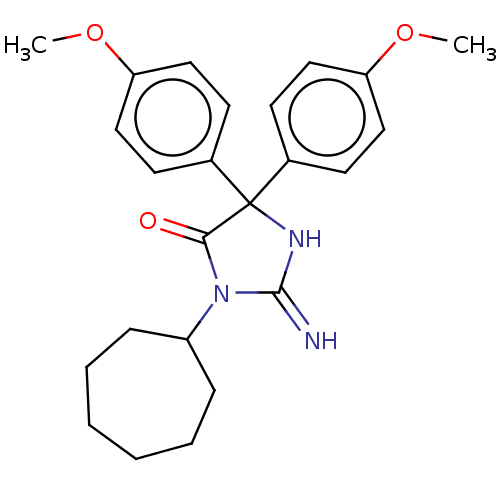 (US9353089, 220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.37 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234535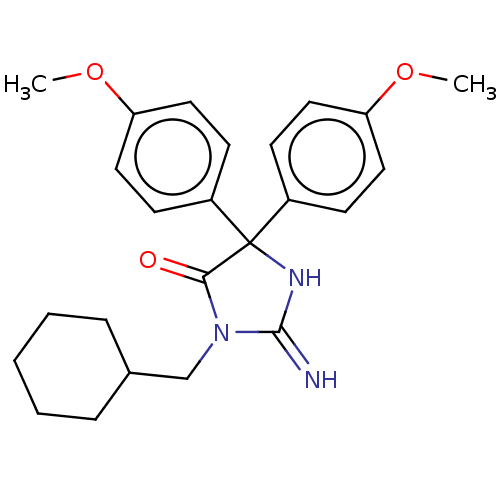 (US9353089, 221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.39 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013422 (CHEMBL3263760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234527 (US9353089, 206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234544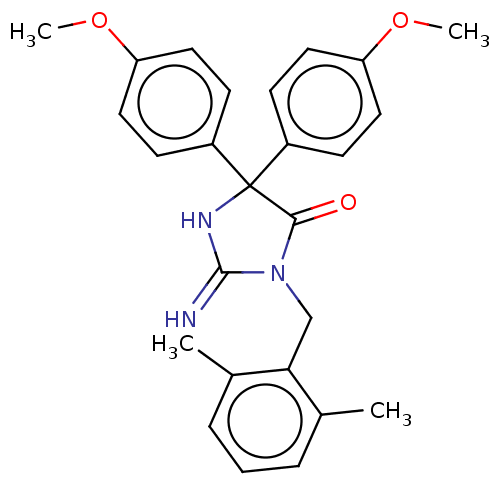 (US9353089, 278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013428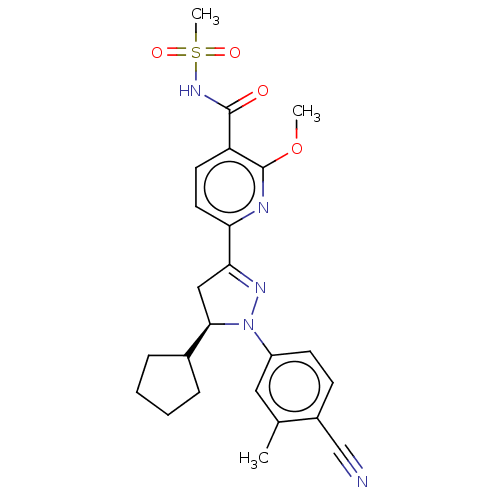 (CHEMBL3263766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234536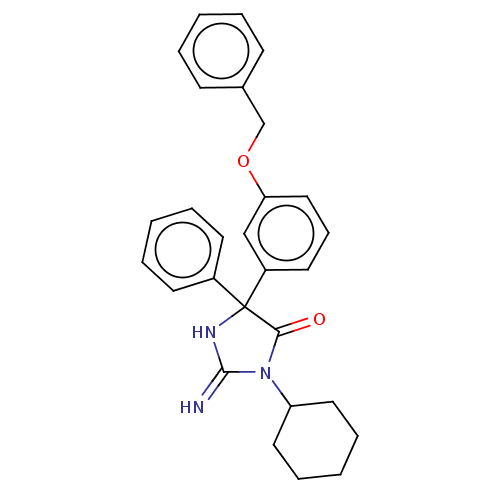 (US9353089, 228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.89 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234546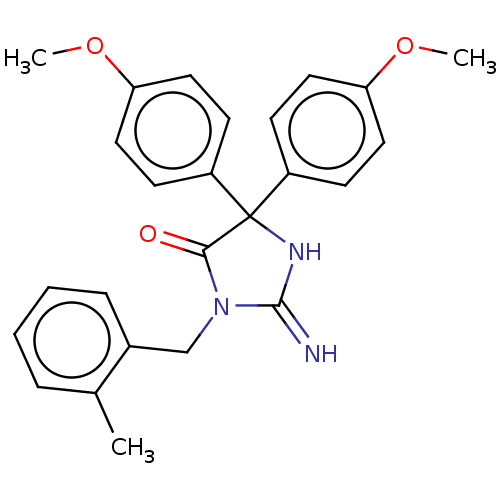 (US9353089, 303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890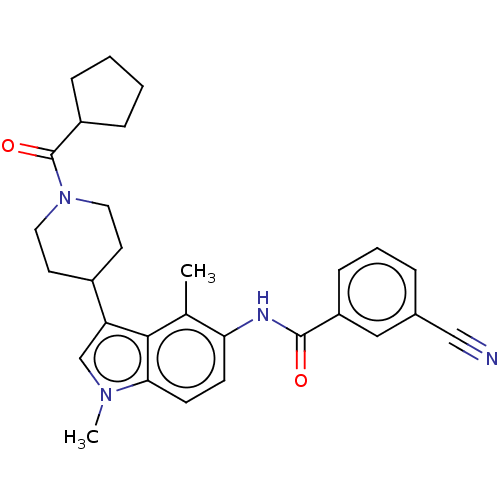 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013426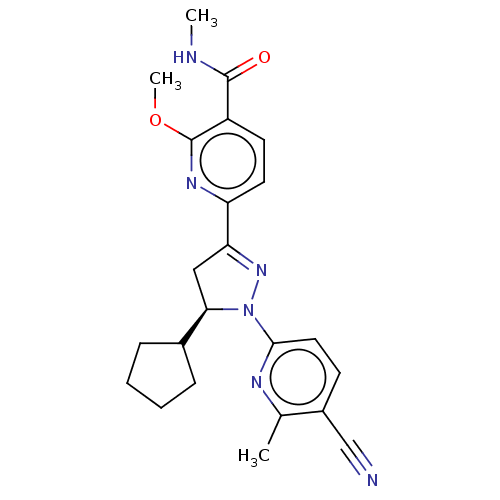 (CHEMBL3263764) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013424 (CHEMBL3263762) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234497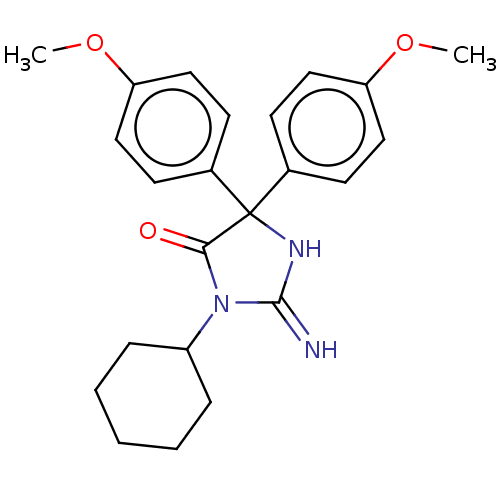 (US9353089, 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.96 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324215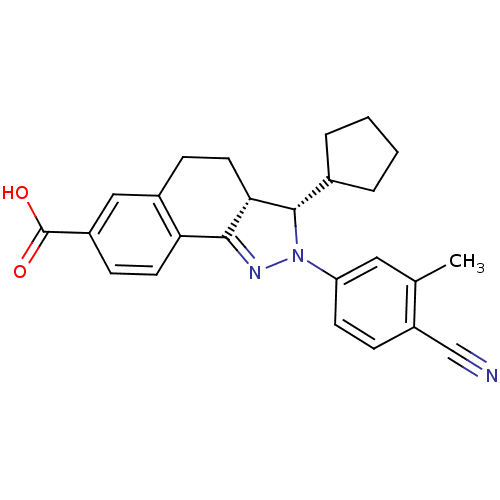 ((+/-)-(3SR,3aRS)-2-(4-Cyano-3-methylphenyl)-3-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at Gal4-tagged mineralocorticoid receptor expressed in human Huh7 cells by luciferase reporter gene assay | J Med Chem 53: 5979-6002 (2010) Article DOI: 10.1021/jm100505n BindingDB Entry DOI: 10.7270/Q2T43V2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234497 (US9353089, 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189877 (US10227346, Example 5 | US10426135, Example 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013429 (CHEMBL3263767) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013425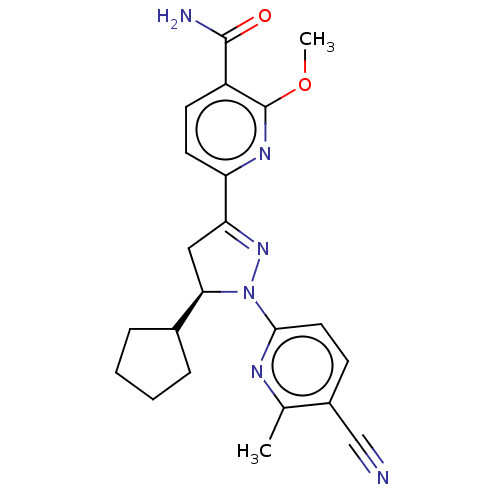 (CHEMBL3263763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234543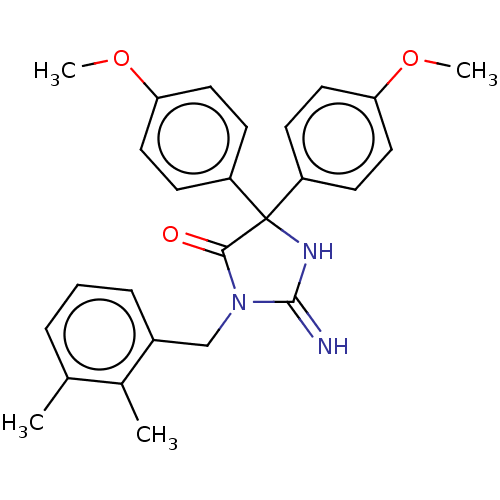 (US9353089, 277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.39 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013419 (CHEMBL3263757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50013412 (CHEMBL3263752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor (unknown origin) by Gal4-based cellular assay | J Med Chem 57: 4273-88 (2014) Article DOI: 10.1021/jm500206r BindingDB Entry DOI: 10.7270/Q2D50PGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466912 (CHEMBL4283051) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50297121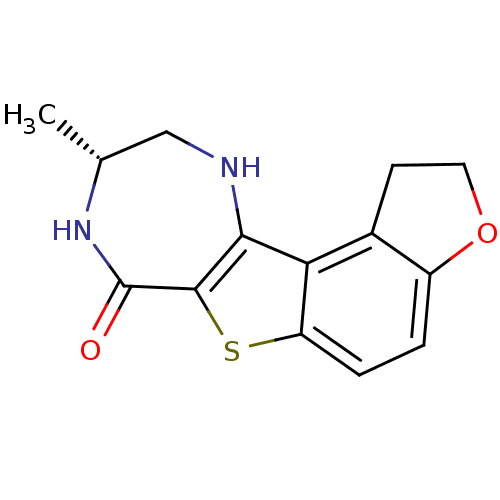 ((R)-9-Methyl-1,2,8,9,10,11-hexahydro-3-oxa-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 19: 4882-4 (2009) Article DOI: 10.1016/j.bmcl.2009.02.017 BindingDB Entry DOI: 10.7270/Q2J38SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50297113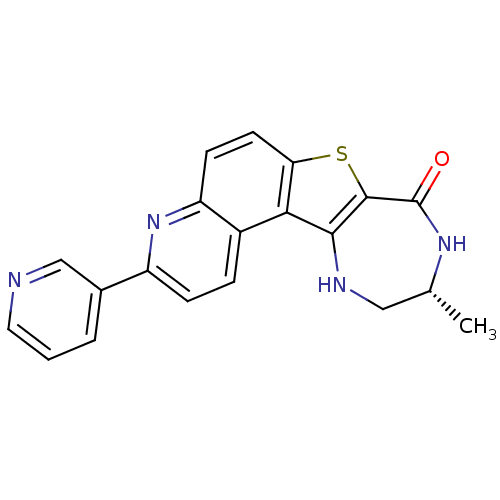 ((R)-10-Methyl-3-pyridin-3-yl-9,10,11,12-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 19: 4882-4 (2009) Article DOI: 10.1016/j.bmcl.2009.02.017 BindingDB Entry DOI: 10.7270/Q2J38SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50297151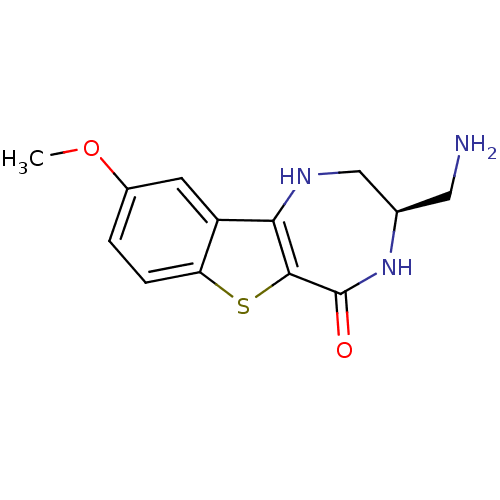 ((3R)-3-(aminomethyl)-9-methoxy-1,2,3,4-tetrahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 19: 4878-81 (2009) Article DOI: 10.1016/j.bmcl.2009.02.015 BindingDB Entry DOI: 10.7270/Q28K795K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50297119 ((R)-10-Methyl-3-(4-methyl-pyridin-3-yl)-9,10,11,12...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 19: 4882-4 (2009) Article DOI: 10.1016/j.bmcl.2009.02.017 BindingDB Entry DOI: 10.7270/Q2J38SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234539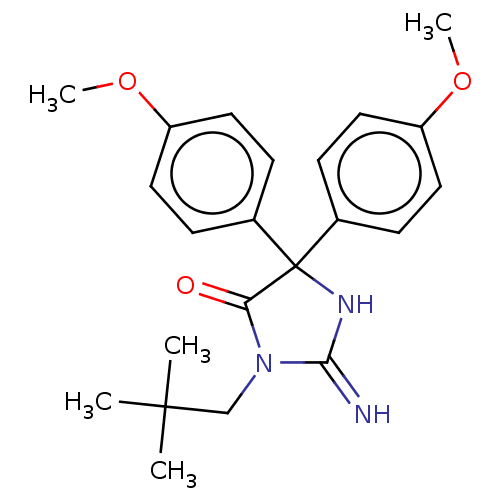 (US9353089, 252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.28 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234529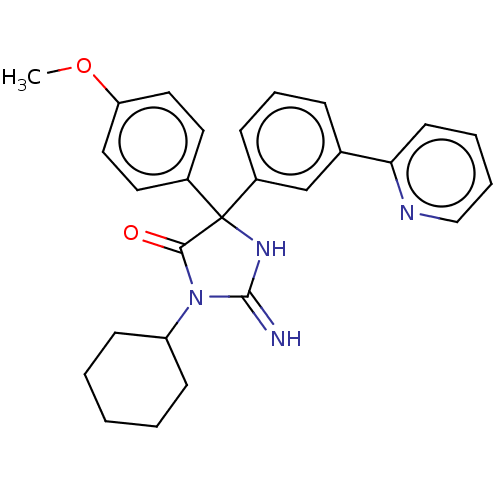 (US9353089, 210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.47 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234564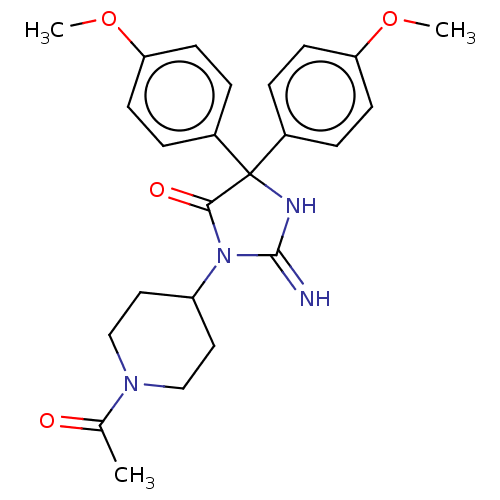 (US9353089, 310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.84 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234508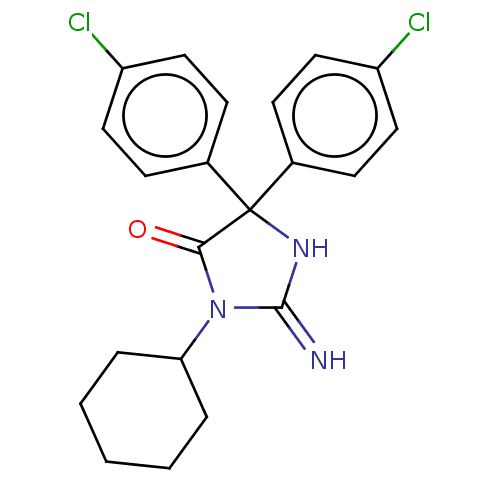 (US9353089, 155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 791 total ) | Next | Last >> |