 Found 1675 hits with Last Name = 'middleton' and Initial = 'sa'
Found 1675 hits with Last Name = 'middleton' and Initial = 'sa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50211341

(5-bromo-6-chloro-N-((1s,4s)-4-(4-(2-isopropoxyphen...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@@H]1CC[C@@H](CC1)NS(=O)(=O)c1cnc(Cl)c(Br)c1 |wU:16.17,19.24,(-7.75,-8.21,;-8.54,-9.53,;-10.08,-9.51,;-7.79,-10.88,;-8.57,-12.2,;-10.11,-12.18,;-10.9,-13.51,;-10.14,-14.86,;-8.6,-14.87,;-7.82,-13.55,;-6.29,-13.56,;-5.53,-14.9,;-3.99,-14.92,;-3.21,-13.6,;-3.95,-12.26,;-5.49,-12.24,;-1.67,-13.62,;-.92,-14.97,;.61,-14.99,;1.41,-13.67,;.65,-12.32,;-.89,-12.3,;2.96,-13.7,;3.74,-12.37,;2.41,-11.59,;5.06,-13.14,;4.5,-11.03,;6.05,-11.02,;6.8,-9.68,;6.02,-8.35,;6.78,-7.01,;4.47,-8.37,;3.69,-7.05,;3.72,-9.71,)| Show InChI InChI=1S/C24H32BrClN4O3S/c1-17(2)33-23-6-4-3-5-22(23)30-13-11-29(12-14-30)19-9-7-18(8-10-19)28-34(31,32)20-15-21(25)24(26)27-16-20/h3-6,15-19,28H,7-14H2,1-2H3/t18-,19+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1D receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM86846
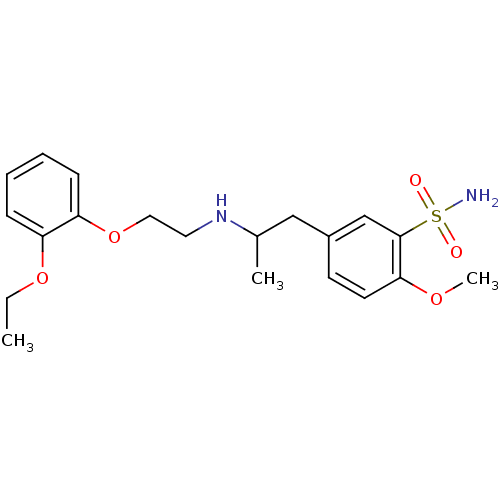
(CAS_106133-20-4 | NSC_60147 | Tamsulosin)Show SMILES CCOc1ccccc1OCCNC(C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964
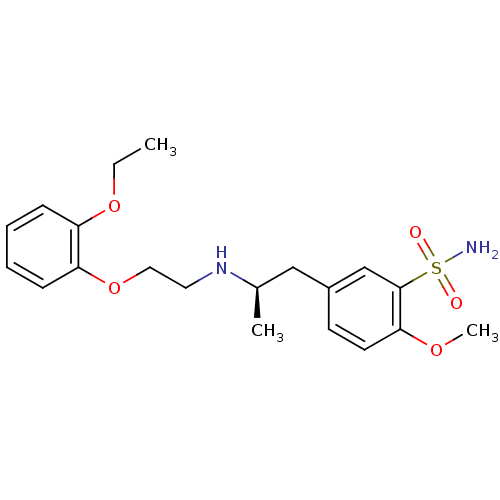
((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964

((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1a receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964

((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1d receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50203481
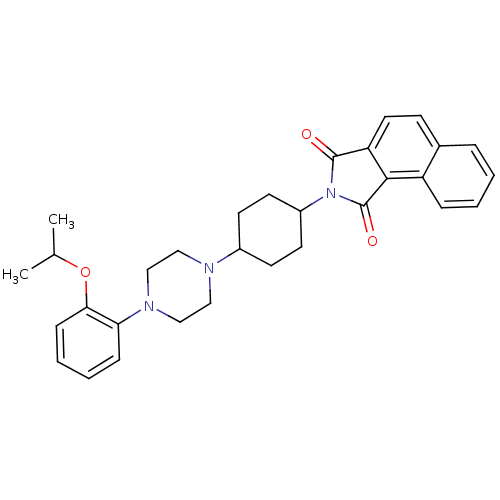
(2-(4-(4-(2-isopropoxyphenyl)piperazin-1-yl)cyclohe...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2ccc3ccccc3c2C1=O |(15.23,-21.71,;14.46,-20.38,;12.92,-20.38,;15.23,-19.04,;14.46,-17.71,;12.92,-17.71,;12.15,-16.39,;12.91,-15.06,;14.46,-15.05,;15.23,-16.38,;16.76,-16.38,;17.53,-17.72,;19.07,-17.73,;19.84,-16.39,;19.08,-15.06,;17.53,-15.05,;21.38,-16.4,;22.15,-17.74,;23.69,-17.74,;24.46,-16.41,;23.7,-15.08,;22.16,-15.07,;26,-16.42,;26.91,-15.17,;26.44,-13.71,;28.37,-15.65,;29.7,-14.9,;31.03,-15.67,;31.02,-17.22,;32.35,-17.99,;32.34,-19.52,;31,-20.29,;29.67,-19.51,;29.68,-17.97,;28.36,-17.2,;26.9,-17.67,;26.42,-19.13,)| Show InChI InChI=1S/C31H35N3O3/c1-21(2)37-28-10-6-5-9-27(28)33-19-17-32(18-20-33)23-12-14-24(15-13-23)34-30(35)26-16-11-22-7-3-4-8-25(22)29(26)31(34)36/h3-11,16,21,23-24H,12-15,17-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1d receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM86846

(CAS_106133-20-4 | NSC_60147 | Tamsulosin)Show SMILES CCOc1ccccc1OCCNC(C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50060964

((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1D receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM82517
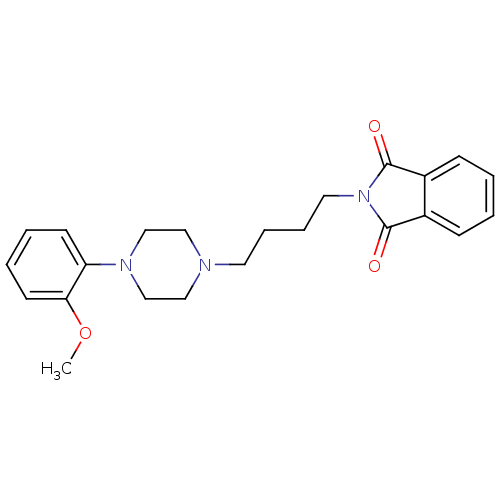
(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1a receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50203485

(5,6-dichloro-2-(4-(4-(2-isopropoxyphenyl)piperazin...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2cc(Cl)c(Cl)cc2C1=O |(-6.31,-12.94,;-7.08,-11.61,;-8.62,-11.61,;-6.3,-10.28,;-7.07,-8.94,;-8.61,-8.94,;-9.38,-7.62,;-8.62,-6.29,;-7.07,-6.28,;-6.31,-7.61,;-4.77,-7.62,;-4,-8.95,;-2.47,-8.96,;-1.69,-7.63,;-2.46,-6.29,;-4,-6.28,;-.15,-7.63,;.62,-8.97,;2.16,-8.98,;2.93,-7.64,;2.17,-6.31,;.63,-6.3,;4.47,-7.65,;5.37,-6.4,;4.9,-4.94,;6.84,-6.89,;8.17,-6.13,;9.5,-6.9,;10.83,-6.13,;9.49,-8.45,;10.82,-9.22,;8.15,-9.21,;6.82,-8.43,;5.36,-8.9,;4.88,-10.36,)| Show InChI InChI=1S/C27H31Cl2N3O3/c1-17(2)35-25-6-4-3-5-24(25)31-13-11-30(12-14-31)18-7-9-19(10-8-18)32-26(33)20-15-22(28)23(29)16-21(20)27(32)34/h3-6,15-19H,7-14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1d receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM82517

(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1d receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50223565
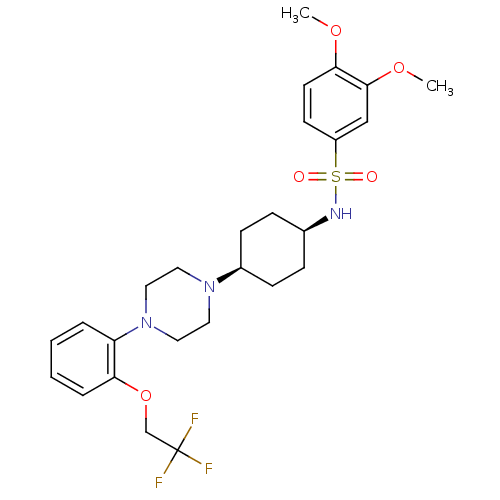
(3,4-dimethoxy-N-((1s,4s)-4-(4-(2-(2,2,2-trifluoroe...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCN(CC1)c1ccccc1OCC(F)(F)F |wU:17.21,14.14,(32.39,2.57,;30.9,2.95,;29.83,1.85,;28.32,2.24,;27.24,1.13,;27.66,-.35,;29.16,-.74,;30.24,.36,;31.73,-.03,;32.14,-1.51,;26.57,-1.45,;25.37,-.49,;27.77,-2.42,;25.74,-2.75,;24.21,-2.68,;23.38,-3.98,;21.85,-3.91,;21.13,-2.55,;21.95,-1.25,;23.49,-1.32,;19.6,-2.48,;18.78,-3.78,;17.25,-3.72,;16.54,-2.36,;17.34,-1.06,;18.89,-1.12,;15,-2.3,;14.19,-3.61,;12.64,-3.55,;11.92,-2.18,;12.74,-.88,;14.29,-.95,;15.11,.35,;14.39,1.72,;15.21,3.02,;15.97,4.36,;16.53,2.23,;13.89,3.81,)| Show InChI InChI=1S/C26H34F3N3O5S/c1-35-24-12-11-21(17-25(24)36-2)38(33,34)30-19-7-9-20(10-8-19)31-13-15-32(16-14-31)22-5-3-4-6-23(22)37-18-26(27,28)29/h3-6,11-12,17,19-20,30H,7-10,13-16,18H2,1-2H3/t19-,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1d receptor |
Bioorg Med Chem Lett 17: 6123-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.051
BindingDB Entry DOI: 10.7270/Q2C82925 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50203486

(2-(4-(4-(2-isopropoxyphenyl)piperazin-1-yl)cyclohe...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2ccncc2C1=O |(16.6,-44.54,;15.83,-43.21,;14.29,-43.21,;16.6,-41.88,;15.83,-40.54,;14.3,-40.54,;13.53,-39.22,;14.29,-37.89,;15.83,-37.88,;16.6,-39.21,;18.14,-39.22,;18.91,-40.55,;20.44,-40.56,;21.22,-39.23,;20.45,-37.89,;18.9,-37.88,;22.76,-39.23,;23.52,-40.57,;25.06,-40.58,;25.84,-39.25,;25.07,-37.91,;23.53,-37.9,;27.38,-39.25,;28.28,-38.01,;27.81,-36.54,;29.74,-38.49,;31.07,-37.73,;32.4,-38.5,;32.4,-40.05,;31.06,-40.81,;29.73,-40.03,;28.27,-40.5,;27.79,-41.96,)| Show InChI InChI=1S/C26H32N4O3/c1-18(2)33-24-6-4-3-5-23(24)29-15-13-28(14-16-29)19-7-9-20(10-8-19)30-25(31)21-11-12-27-17-22(21)26(30)32/h3-6,11-12,17-20H,7-10,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1a receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86848
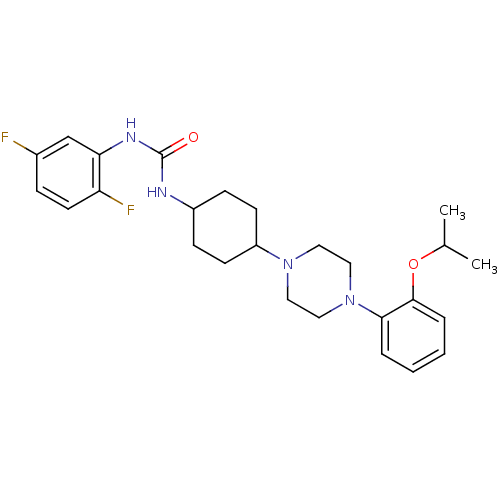
(1-(2,5-difluorophenyl)-3-(4-(4-(2-isopropoxyphenyl...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)NC(=O)Nc1cc(F)ccc1F |(-10.67,-3.08,;-10.67,-4.62,;-9.34,-5.39,;-12,-5.39,;-12,-6.93,;-10.67,-7.7,;-10.67,-9.24,;-12,-10.01,;-13.34,-9.24,;-13.34,-7.7,;-14.67,-6.93,;-16,-7.7,;-17.34,-6.93,;-17.34,-5.39,;-16,-4.62,;-14.67,-5.39,;-18.67,-4.62,;-20.01,-5.39,;-21.34,-4.62,;-21.34,-3.08,;-20.01,-2.31,;-18.67,-3.08,;-22.67,-2.31,;-22.67,-.77,;-21.34,,;-24.01,,;-25.34,-.77,;-26.67,,;-28.01,-.77,;-29.34,,;-28.01,-2.31,;-26.67,-3.08,;-25.34,-2.31,;-24.01,-3.08,)| Show InChI InChI=1S/C26H34F2N4O2/c1-18(2)34-25-6-4-3-5-24(25)32-15-13-31(14-16-32)21-10-8-20(9-11-21)29-26(33)30-23-17-19(27)7-12-22(23)28/h3-7,12,17-18,20-21H,8-11,13-16H2,1-2H3,(H2,29,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50203480
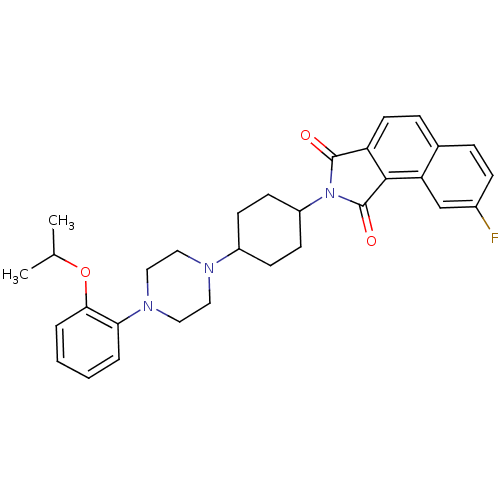
(8-fluoro-2-(4-(4-(2-isopropoxyphenyl)piperazin-1-y...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2ccc3ccc(F)cc3c2C1=O |(-7.03,-31.98,;-7.8,-30.64,;-9.34,-30.64,;-7.03,-29.31,;-7.8,-27.98,;-9.33,-27.97,;-10.1,-26.65,;-9.34,-25.32,;-7.8,-25.32,;-7.03,-26.65,;-5.49,-26.65,;-4.72,-27.99,;-3.19,-27.99,;-2.41,-26.66,;-3.18,-25.32,;-4.73,-25.32,;-.87,-26.67,;-.11,-28,;1.43,-28.01,;2.21,-26.68,;1.44,-25.34,;-.1,-25.34,;3.75,-26.68,;4.65,-25.44,;4.18,-23.97,;6.11,-25.92,;7.45,-25.16,;8.77,-25.93,;8.77,-27.48,;10.09,-28.25,;10.08,-29.79,;8.74,-30.55,;8.73,-32.09,;7.41,-29.77,;7.43,-28.24,;6.1,-27.46,;4.64,-27.93,;4.16,-29.39,)| Show InChI InChI=1S/C31H34FN3O3/c1-20(2)38-28-6-4-3-5-27(28)34-17-15-33(16-18-34)23-10-12-24(13-11-23)35-30(36)25-14-8-21-7-9-22(32)19-26(21)29(25)31(35)37/h3-9,14,19-20,23-24H,10-13,15-18H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1d receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50203480

(8-fluoro-2-(4-(4-(2-isopropoxyphenyl)piperazin-1-y...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2ccc3ccc(F)cc3c2C1=O |(-7.03,-31.98,;-7.8,-30.64,;-9.34,-30.64,;-7.03,-29.31,;-7.8,-27.98,;-9.33,-27.97,;-10.1,-26.65,;-9.34,-25.32,;-7.8,-25.32,;-7.03,-26.65,;-5.49,-26.65,;-4.72,-27.99,;-3.19,-27.99,;-2.41,-26.66,;-3.18,-25.32,;-4.73,-25.32,;-.87,-26.67,;-.11,-28,;1.43,-28.01,;2.21,-26.68,;1.44,-25.34,;-.1,-25.34,;3.75,-26.68,;4.65,-25.44,;4.18,-23.97,;6.11,-25.92,;7.45,-25.16,;8.77,-25.93,;8.77,-27.48,;10.09,-28.25,;10.08,-29.79,;8.74,-30.55,;8.73,-32.09,;7.41,-29.77,;7.43,-28.24,;6.1,-27.46,;4.64,-27.93,;4.16,-29.39,)| Show InChI InChI=1S/C31H34FN3O3/c1-20(2)38-28-6-4-3-5-27(28)34-17-15-33(16-18-34)23-10-12-24(13-11-23)35-30(36)25-14-8-21-7-9-22(32)19-26(21)29(25)31(35)37/h3-9,14,19-20,23-24H,10-13,15-18H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1d receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211318
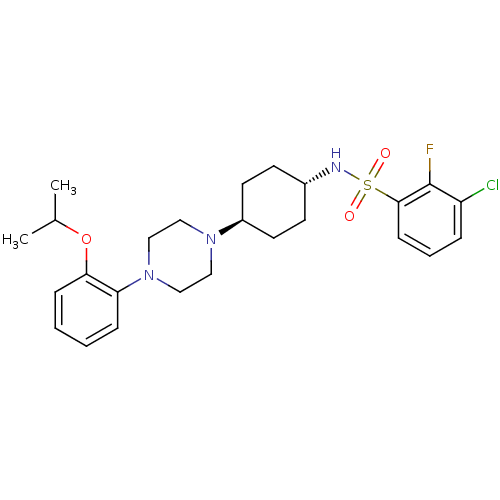
(3-chloro-2-fluoro-N-((1r,4r)-4-(4-(2-isopropoxyphe...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1cccc(Cl)c1F |wU:16.17,wD:19.24,(16.9,6.91,;16.13,5.58,;14.59,5.58,;16.91,4.24,;16.14,2.92,;14.6,2.9,;13.84,1.56,;14.63,.22,;16.16,.24,;16.92,1.58,;18.46,1.58,;19.24,.27,;20.78,.28,;21.54,1.63,;20.77,2.94,;19.23,2.93,;23.08,1.63,;23.84,2.97,;25.38,2.97,;26.16,1.64,;25.39,.31,;23.86,.3,;27.7,1.64,;28.47,2.98,;27.13,3.75,;29.8,2.21,;29.24,4.31,;28.44,5.68,;29.23,7,;30.78,6.98,;31.53,5.64,;33.07,5.64,;30.75,4.31,;31.52,2.98,)| Show InChI InChI=1S/C25H33ClFN3O3S/c1-18(2)33-23-8-4-3-7-22(23)30-16-14-29(15-17-30)20-12-10-19(11-13-20)28-34(31,32)24-9-5-6-21(26)25(24)27/h3-9,18-20,28H,10-17H2,1-2H3/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50211342

(2,4-dichloro-N-((1s,4s)-4-(4-(2-isopropoxyphenyl)p...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(Cl)cc1Cl |wU:16.17,19.24,(-7.91,-40.14,;-8.67,-41.47,;-10.21,-41.47,;-7.91,-42.8,;-8.67,-44.13,;-10.21,-44.13,;-10.98,-45.46,;-10.21,-46.8,;-8.67,-46.8,;-7.91,-45.46,;-6.37,-45.46,;-5.6,-46.8,;-4.06,-46.8,;-3.29,-45.46,;-4.06,-44.13,;-5.6,-44.13,;-1.75,-45.46,;-.99,-46.8,;.55,-46.8,;1.32,-45.46,;.55,-44.13,;-.99,-44.13,;2.86,-45.46,;3.63,-44.13,;2.3,-43.37,;4.95,-44.91,;4.39,-42.8,;3.63,-41.47,;4.39,-40.14,;5.93,-40.14,;6.7,-38.81,;6.7,-41.47,;5.93,-42.8,;6.7,-44.13,)| Show InChI InChI=1S/C25H33Cl2N3O3S/c1-18(2)33-24-6-4-3-5-23(24)30-15-13-29(14-16-30)21-10-8-20(9-11-21)28-34(31,32)25-12-7-19(26)17-22(25)27/h3-7,12,17-18,20-21,28H,8-11,13-16H2,1-2H3/t20-,21+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1D receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50211322

(CHEMBL391530 | N-((1s,4s)-4-(4-(2-isopropoxyphenyl...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCN(CC1)c1ccccc1OC(C)C |wU:17.21,14.14,(9.45,6.98,;7.93,6.98,;7.17,5.66,;5.62,5.66,;4.85,4.32,;5.62,3,;7.17,3,;7.93,4.32,;9.48,4.32,;10.24,3,;4.85,1.66,;3.52,2.44,;6.19,.9,;4.08,.34,;2.54,.34,;1.78,-.99,;.24,-.99,;-.52,.34,;.24,1.67,;1.77,1.67,;-2.05,.34,;-2.82,-.99,;-4.36,-.99,;-5.12,.35,;-4.36,1.67,;-2.82,1.67,;-6.67,.35,;-7.43,-1.02,;-8.98,-1.02,;-9.75,.33,;-8.98,1.67,;-7.43,1.67,;-6.67,3,;-7.44,4.33,;-6.67,5.67,;-8.98,4.33,)| Show InChI InChI=1S/C27H39N3O5S/c1-20(2)35-25-8-6-5-7-24(25)30-17-15-29(16-18-30)22-11-9-21(10-12-22)28-36(31,32)23-13-14-26(33-3)27(19-23)34-4/h5-8,13-14,19-22,28H,9-12,15-18H2,1-4H3/t21-,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1d receptor |
Bioorg Med Chem Lett 17: 6123-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.051
BindingDB Entry DOI: 10.7270/Q2C82925 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86850

(1-(2,4-difluorophenyl)-3-(4-(4-(2-isopropoxyphenyl...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)NC(=O)Nc1ccc(F)cc1F |(-10.67,-3.08,;-10.67,-4.62,;-9.34,-5.39,;-12,-5.39,;-12,-6.93,;-10.67,-7.7,;-10.67,-9.24,;-12,-10.01,;-13.34,-9.24,;-13.34,-7.7,;-14.67,-6.93,;-16,-7.7,;-17.34,-6.93,;-17.34,-5.39,;-16,-4.62,;-14.67,-5.39,;-18.67,-4.62,;-20.01,-5.39,;-21.34,-4.62,;-21.34,-3.08,;-20.01,-2.31,;-18.67,-3.08,;-22.67,-2.31,;-22.67,-.77,;-21.34,,;-24.01,,;-25.34,-.77,;-26.67,,;-28.01,-.77,;-28.01,-2.31,;-29.34,-3.08,;-26.67,-3.08,;-25.34,-2.31,;-24.01,-3.08,)| Show InChI InChI=1S/C26H34F2N4O2/c1-18(2)34-25-6-4-3-5-24(25)32-15-13-31(14-16-32)21-10-8-20(9-11-21)29-26(33)30-23-12-7-19(27)17-22(23)28/h3-7,12,17-18,20-21H,8-11,13-16H2,1-2H3,(H2,29,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211328
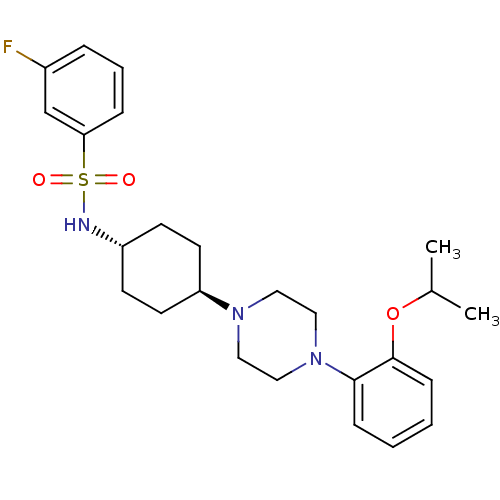
(3-fluoro-N-((1r,4r)-4-(4-(2-isopropoxyphenyl)piper...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1cccc(F)c1 |wU:16.17,wD:19.24,(21.3,5.8,;20.52,4.47,;18.98,4.49,;21.27,3.13,;20.48,1.81,;18.95,1.82,;18.16,.5,;18.92,-.85,;20.46,-.86,;21.24,.46,;22.77,.44,;23.53,-.89,;25.07,-.91,;25.85,.41,;25.1,1.75,;23.56,1.76,;27.38,.39,;28.17,1.71,;29.71,1.69,;30.46,.34,;29.67,-.98,;28.14,-.96,;32.02,.31,;32.79,1.64,;31.46,2.41,;34.12,.87,;33.55,2.98,;32.78,4.3,;33.53,5.64,;35.08,5.65,;35.86,4.33,;37.4,4.34,;35.1,2.99,)| Show InChI InChI=1S/C25H34FN3O3S/c1-19(2)32-25-9-4-3-8-24(25)29-16-14-28(15-17-29)22-12-10-21(11-13-22)27-33(30,31)23-7-5-6-20(26)18-23/h3-9,18-19,21-22,27H,10-17H2,1-2H3/t21-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50213521

(CHEMBL390129 | N-((1s,4s)-4-(4-(2-isopropoxyphenyl...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCC(CC1)c1ccccc1OC(C)C |wU:17.21,14.14,(9.42,6.24,;7.88,6.23,;7.12,4.89,;5.57,4.87,;4.82,3.53,;5.6,2.21,;7.14,2.22,;7.9,3.56,;9.44,3.57,;10.22,2.24,;4.84,.87,;3.49,1.6,;6.19,.12,;4.12,-.5,;2.58,-.55,;1.86,-1.91,;.33,-1.97,;-.49,-.67,;.22,.69,;1.76,.75,;-2.03,-.73,;-2.75,-2.09,;-4.28,-2.16,;-5.11,-.86,;-4.4,.51,;-2.86,.57,;-6.64,-.92,;-7.36,-2.29,;-8.9,-2.36,;-9.72,-1.05,;-9.02,.31,;-7.48,.38,;-6.78,1.75,;-7.61,3.05,;-6.9,4.41,;-9.15,2.97,)| Show InChI InChI=1S/C28H40N2O5S/c1-20(2)35-26-8-6-5-7-25(26)21-15-17-30(18-16-21)23-11-9-22(10-12-23)29-36(31,32)24-13-14-27(33-3)28(19-24)34-4/h5-8,13-14,19-23,29H,9-12,15-18H2,1-4H3/t22-,23+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1d receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211320
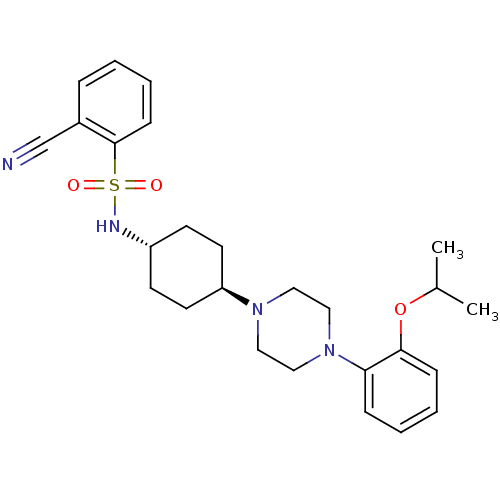
(2-cyano-N-((1r,4r)-4-(4-(2-isopropoxyphenyl)pipera...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccccc1C#N |wU:16.17,wD:19.24,(-5.68,-16.28,;-6.46,-17.61,;-8,-17.59,;-5.71,-18.95,;-6.5,-20.27,;-8.03,-20.26,;-8.82,-21.58,;-8.06,-22.93,;-6.52,-22.94,;-5.74,-21.62,;-4.21,-21.64,;-3.45,-22.98,;-1.91,-22.99,;-1.13,-21.67,;-1.88,-20.34,;-3.42,-20.32,;.41,-21.69,;1.19,-20.37,;2.73,-20.39,;3.48,-21.74,;2.69,-23.06,;1.16,-23.04,;5.04,-21.77,;5.81,-20.44,;4.48,-19.67,;7.14,-21.21,;6.57,-19.1,;5.8,-17.78,;6.55,-16.44,;8.1,-16.43,;8.88,-17.76,;8.12,-19.09,;8.9,-20.42,;9.68,-21.75,)| Show InChI InChI=1S/C26H34N4O3S/c1-20(2)33-25-9-5-4-8-24(25)30-17-15-29(16-18-30)23-13-11-22(12-14-23)28-34(31,32)26-10-6-3-7-21(26)19-27/h3-10,20,22-23,28H,11-18H2,1-2H3/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50203484
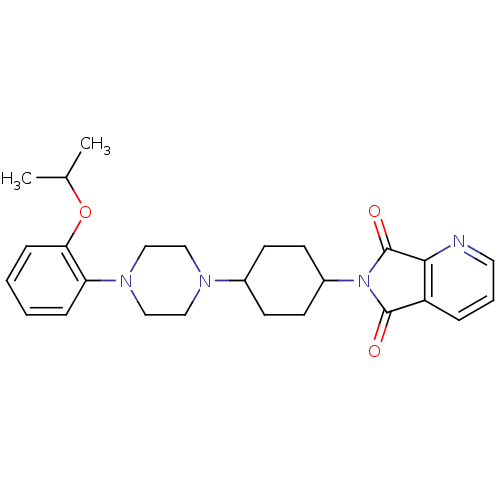
(6-(4-(4-(2-isopropoxyphenyl)piperazin-1-yl)cyclohe...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2cccnc2C1=O |(-5.9,-44.57,;-6.67,-43.23,;-8.21,-43.23,;-5.9,-41.9,;-6.67,-40.57,;-8.21,-40.56,;-8.97,-39.24,;-8.21,-37.91,;-6.67,-37.91,;-5.9,-39.24,;-4.36,-39.24,;-3.6,-40.58,;-2.06,-40.58,;-1.29,-39.25,;-2.05,-37.91,;-3.6,-37.91,;.25,-39.26,;1.02,-40.59,;2.56,-40.6,;3.33,-39.27,;2.57,-37.93,;1.03,-37.93,;4.87,-39.28,;5.78,-38.03,;5.31,-36.56,;7.24,-38.51,;8.57,-37.75,;9.9,-38.52,;9.89,-40.07,;8.55,-40.83,;7.23,-40.05,;5.77,-40.52,;5.29,-41.98,)| Show InChI InChI=1S/C26H32N4O3/c1-18(2)33-23-8-4-3-7-22(23)29-16-14-28(15-17-29)19-9-11-20(12-10-19)30-25(31)21-6-5-13-27-24(21)26(30)32/h3-8,13,18-20H,9-12,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1a receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203781
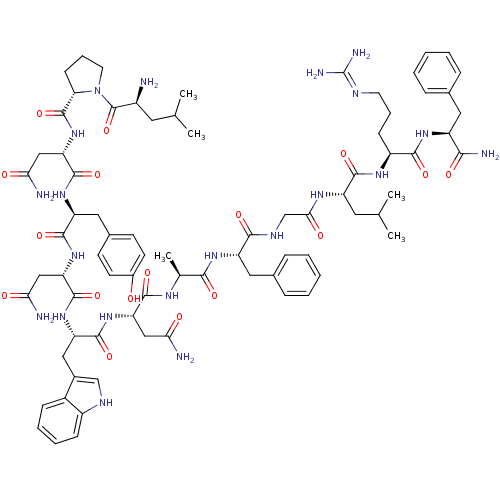
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,71.74,86.90,36.37,12.13,105.109,wD:66.70,24.24,94.98,16.16,44.45,(-10.05,-20.48,;-10.03,-22.02,;-11.35,-22.81,;-8.68,-22.76,;-8.68,-24.32,;-10.01,-25.09,;-7.34,-25.09,;-7.34,-26.63,;-6.01,-24.33,;-5.53,-22.87,;-3.99,-22.87,;-3.52,-24.33,;-4.77,-25.24,;-4.77,-26.78,;-6.11,-27.55,;-3.44,-27.55,;-3.44,-29.09,;-4.77,-29.86,;-4.78,-31.4,;-3.44,-32.17,;-6.11,-32.17,;-2.11,-29.86,;-2.11,-31.4,;-.77,-29.09,;.56,-29.86,;.56,-31.4,;1.89,-32.18,;1.88,-33.71,;3.22,-34.48,;4.55,-33.71,;5.89,-34.48,;4.55,-32.17,;3.23,-31.41,;1.89,-29.1,;1.9,-27.56,;3.23,-29.87,;4.56,-29.1,;4.56,-27.56,;5.9,-26.79,;7.23,-27.56,;5.9,-25.25,;5.89,-29.87,;5.89,-31.41,;7.23,-29.1,;8.56,-29.87,;8.56,-31.41,;9.89,-32.18,;11.14,-31.19,;12.38,-32.18,;11.9,-33.65,;12.66,-34.97,;11.9,-36.29,;10.36,-36.29,;9.6,-34.96,;10.37,-33.64,;9.9,-29.1,;9.9,-27.56,;11.23,-29.88,;12.56,-29.11,;12.57,-27.57,;13.9,-26.8,;15.23,-27.57,;13.9,-25.26,;13.9,-29.88,;13.9,-31.42,;15.23,-29.11,;16.56,-29.88,;16.56,-31.42,;17.9,-29.11,;17.9,-27.57,;19.23,-29.88,;20.57,-29.12,;20.57,-27.58,;21.9,-26.81,;23.23,-27.58,;24.57,-26.82,;24.57,-25.27,;23.23,-24.5,;21.9,-25.27,;21.9,-29.89,;21.9,-31.43,;23.23,-29.12,;24.57,-29.89,;25.9,-29.12,;25.9,-27.58,;27.23,-29.89,;28.57,-29.12,;28.57,-27.58,;29.9,-26.82,;29.91,-25.28,;31.24,-27.59,;29.9,-29.9,;29.9,-31.44,;31.24,-29.13,;32.57,-29.9,;32.57,-31.44,;33.9,-32.21,;33.9,-33.75,;35.23,-34.52,;35.23,-36.06,;36.56,-36.83,;33.9,-36.83,;33.9,-29.13,;33.91,-27.59,;35.24,-29.9,;36.57,-29.13,;36.57,-27.59,;37.91,-26.82,;39.24,-27.6,;40.57,-26.84,;40.57,-25.3,;39.23,-24.52,;37.9,-25.3,;37.9,-29.9,;39.24,-29.13,;37.9,-31.44,)| Show InChI InChI=1S/C78H107N21O17/c1-41(2)30-50(79)77(116)99-29-15-23-61(99)76(115)98-60(38-64(82)103)75(114)94-56(34-46-24-26-48(100)27-25-46)72(111)97-59(37-63(81)102)74(113)95-57(35-47-39-87-51-21-13-12-20-49(47)51)73(112)96-58(36-62(80)101)70(109)89-43(5)67(106)93-55(33-45-18-10-7-11-19-45)68(107)88-40-65(104)90-54(31-42(3)4)71(110)91-52(22-14-28-86-78(84)85)69(108)92-53(66(83)105)32-44-16-8-6-9-17-44/h6-13,16-21,24-27,39,41-43,50,52-61,87,100H,14-15,22-23,28-38,40,79H2,1-5H3,(H2,80,101)(H2,81,102)(H2,82,103)(H2,83,105)(H,88,107)(H,89,109)(H,90,104)(H,91,110)(H,92,108)(H,93,106)(H,94,114)(H,95,113)(H,96,112)(H,97,111)(H,98,115)(H4,84,85,86)/t43-,50-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50203486

(2-(4-(4-(2-isopropoxyphenyl)piperazin-1-yl)cyclohe...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2ccncc2C1=O |(16.6,-44.54,;15.83,-43.21,;14.29,-43.21,;16.6,-41.88,;15.83,-40.54,;14.3,-40.54,;13.53,-39.22,;14.29,-37.89,;15.83,-37.88,;16.6,-39.21,;18.14,-39.22,;18.91,-40.55,;20.44,-40.56,;21.22,-39.23,;20.45,-37.89,;18.9,-37.88,;22.76,-39.23,;23.52,-40.57,;25.06,-40.58,;25.84,-39.25,;25.07,-37.91,;23.53,-37.9,;27.38,-39.25,;28.28,-38.01,;27.81,-36.54,;29.74,-38.49,;31.07,-37.73,;32.4,-38.5,;32.4,-40.05,;31.06,-40.81,;29.73,-40.03,;28.27,-40.5,;27.79,-41.96,)| Show InChI InChI=1S/C26H32N4O3/c1-18(2)33-24-6-4-3-5-23(24)29-15-13-28(14-16-29)19-7-9-20(10-8-19)30-25(31)21-11-12-27-17-22(21)26(30)32/h3-6,11-12,17-20H,7-10,13-16H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1d receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50211356
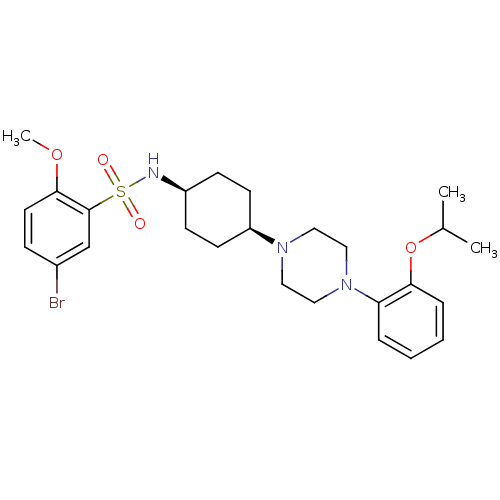
(5-bromo-N-((1s,4s)-4-(4-(2-isopropoxyphenyl)pipera...)Show SMILES COc1ccc(Br)cc1S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCN(CC1)c1ccccc1OC(C)C |wU:16.20,13.13,(6.82,.34,;7.59,1.68,;6.82,3.02,;7.59,4.35,;6.82,5.68,;5.28,5.68,;4.51,7.02,;4.51,4.35,;5.28,3.02,;4.51,1.68,;3.17,2.45,;5.84,.91,;3.74,.34,;2.2,.34,;1.43,-.98,;-.11,-.98,;-.88,.34,;-.11,1.68,;1.43,1.68,;-2.42,.34,;-3.2,-.98,;-4.74,-.98,;-5.51,.34,;-4.74,1.68,;-3.2,1.68,;-7.05,.34,;-7.82,-.98,;-9.36,-.98,;-10.13,.34,;-9.36,1.68,;-7.82,1.68,;-7.05,3.02,;-7.82,4.35,;-7.05,5.68,;-9.36,4.35,)| Show InChI InChI=1S/C26H36BrN3O4S/c1-19(2)34-24-7-5-4-6-23(24)30-16-14-29(15-17-30)22-11-9-21(10-12-22)28-35(31,32)26-18-20(27)8-13-25(26)33-3/h4-8,13,18-19,21-22,28H,9-12,14-17H2,1-3H3/t21-,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1D receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211350
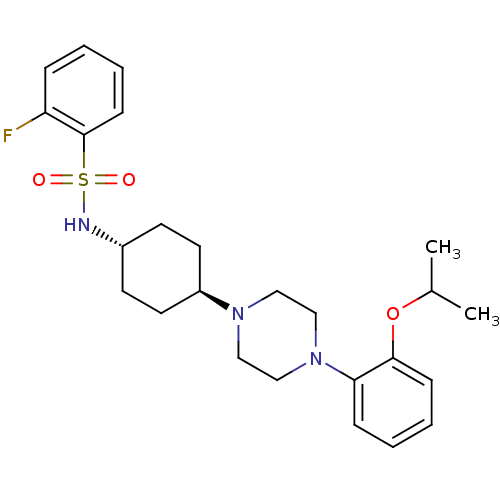
(2-fluoro-N-((1r,4r)-4-(4-(2-isopropoxyphenyl)piper...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccccc1F |wU:16.17,wD:19.24,(-6.34,-28.26,;-7.12,-29.59,;-8.66,-29.57,;-6.37,-30.93,;-7.16,-32.25,;-8.7,-32.23,;-9.48,-33.56,;-8.72,-34.91,;-7.18,-34.92,;-6.4,-33.6,;-4.87,-33.62,;-4.11,-34.95,;-2.57,-34.97,;-1.79,-33.65,;-2.54,-32.31,;-4.08,-32.3,;-.26,-33.67,;.53,-32.35,;2.07,-32.37,;2.82,-33.72,;2.03,-35.04,;.5,-35.02,;4.38,-33.75,;5.15,-32.42,;3.82,-31.65,;6.48,-33.19,;5.91,-31.08,;5.14,-29.76,;5.89,-28.42,;7.44,-28.4,;8.22,-29.73,;7.46,-31.07,;8.24,-32.4,)| Show InChI InChI=1S/C25H34FN3O3S/c1-19(2)32-24-9-5-4-8-23(24)29-17-15-28(16-18-29)21-13-11-20(12-14-21)27-33(30,31)25-10-6-3-7-22(25)26/h3-10,19-21,27H,11-18H2,1-2H3/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211319
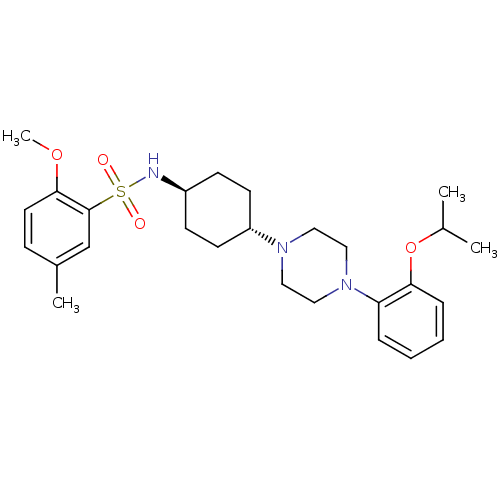
(CHEMBL233623 | N-((1r,4r)-4-(4-(2-isopropoxyphenyl...)Show SMILES COc1ccc(C)cc1S(=O)(=O)N[C@H]1CC[C@@H](CC1)N1CCN(CC1)c1ccccc1OC(C)C |wU:16.20,wD:13.13,(32.51,-20.49,;30.97,-20.49,;30.2,-19.15,;30.97,-17.83,;30.2,-16.49,;28.66,-16.49,;27.89,-15.15,;27.89,-17.83,;28.66,-19.15,;27.89,-20.49,;26.56,-19.72,;29.22,-21.26,;27.12,-21.83,;25.58,-21.83,;24.81,-20.49,;23.27,-20.49,;22.5,-21.83,;23.27,-23.15,;24.81,-23.15,;20.96,-21.83,;20.19,-23.15,;18.65,-23.15,;17.88,-21.83,;18.65,-20.49,;20.19,-20.49,;16.34,-21.83,;15.57,-23.15,;14.03,-23.15,;13.26,-21.83,;14.03,-20.49,;15.57,-20.49,;16.34,-19.15,;15.57,-17.83,;16.34,-16.49,;14.03,-17.83,)| Show InChI InChI=1S/C27H39N3O4S/c1-20(2)34-25-8-6-5-7-24(25)30-17-15-29(16-18-30)23-12-10-22(11-13-23)28-35(31,32)27-19-21(3)9-14-26(27)33-4/h5-9,14,19-20,22-23,28H,10-13,15-18H2,1-4H3/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50213511
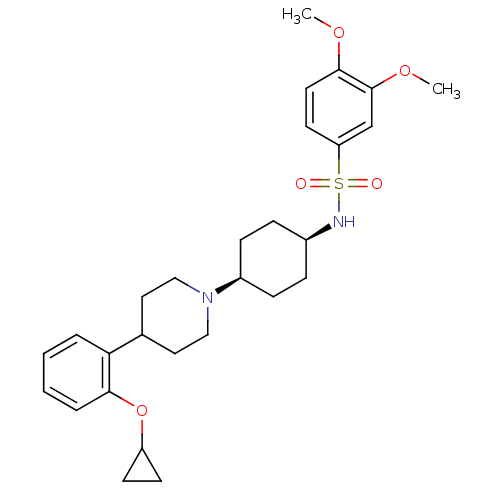
(CHEMBL229085 | N-((1s,4s)-4-(4-(2-cyclopropoxyphen...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCC(CC1)c1ccccc1OC1CC1 |wU:17.21,14.14,(10.4,-18.11,;8.86,-18.12,;8.11,-19.46,;6.55,-19.48,;5.8,-20.82,;6.58,-22.14,;8.13,-22.14,;8.89,-20.8,;10.43,-20.78,;11.21,-22.11,;5.82,-23.49,;4.47,-22.75,;7.17,-24.23,;5.1,-24.85,;3.57,-24.91,;2.85,-26.27,;1.31,-26.33,;.49,-25.02,;1.2,-23.66,;2.75,-23.6,;-1.04,-25.08,;-1.76,-26.44,;-3.29,-26.51,;-4.12,-25.21,;-3.41,-23.84,;-1.87,-23.78,;-5.66,-25.27,;-6.37,-26.64,;-7.91,-26.71,;-8.73,-25.41,;-8.03,-24.04,;-6.49,-23.97,;-5.79,-22.6,;-6.62,-21.31,;-6.69,-19.77,;-7.99,-20.6,)| Show InChI InChI=1S/C28H38N2O5S/c1-33-27-14-13-24(19-28(27)34-2)36(31,32)29-21-7-9-22(10-8-21)30-17-15-20(16-18-30)25-5-3-4-6-26(25)35-23-11-12-23/h3-6,13-14,19-23,29H,7-12,15-18H2,1-2H3/t21-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1a receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50213511

(CHEMBL229085 | N-((1s,4s)-4-(4-(2-cyclopropoxyphen...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCC(CC1)c1ccccc1OC1CC1 |wU:17.21,14.14,(10.4,-18.11,;8.86,-18.12,;8.11,-19.46,;6.55,-19.48,;5.8,-20.82,;6.58,-22.14,;8.13,-22.14,;8.89,-20.8,;10.43,-20.78,;11.21,-22.11,;5.82,-23.49,;4.47,-22.75,;7.17,-24.23,;5.1,-24.85,;3.57,-24.91,;2.85,-26.27,;1.31,-26.33,;.49,-25.02,;1.2,-23.66,;2.75,-23.6,;-1.04,-25.08,;-1.76,-26.44,;-3.29,-26.51,;-4.12,-25.21,;-3.41,-23.84,;-1.87,-23.78,;-5.66,-25.27,;-6.37,-26.64,;-7.91,-26.71,;-8.73,-25.41,;-8.03,-24.04,;-6.49,-23.97,;-5.79,-22.6,;-6.62,-21.31,;-6.69,-19.77,;-7.99,-20.6,)| Show InChI InChI=1S/C28H38N2O5S/c1-33-27-14-13-24(19-28(27)34-2)36(31,32)29-21-7-9-22(10-8-21)30-17-15-20(16-18-30)25-5-3-4-6-26(25)35-23-11-12-23/h3-6,13-14,19-23,29H,7-12,15-18H2,1-2H3/t21-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1a receptor |
Bioorg Med Chem Lett 17: 6123-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.051
BindingDB Entry DOI: 10.7270/Q2C82925 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM82517

(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1b receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50213529

(5-chloro-2-fluoro-N-((1s,4s)-4-(4-(2-(2,2,2-triflu...)Show SMILES Fc1ccc(Cl)cc1S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCC(CC1)c1ccccc1OCC(F)(F)F |wU:15.19,12.12,(8.56,-46.34,;7.79,-45.01,;8.56,-43.69,;7.8,-42.34,;6.24,-42.34,;5.47,-41.01,;5.47,-43.69,;6.23,-45.01,;5.46,-46.35,;4.14,-45.59,;6.82,-47.13,;4.71,-47.66,;3.13,-47.66,;2.37,-48.98,;.85,-48.98,;.08,-47.63,;.84,-46.31,;2.37,-46.35,;-1.49,-47.63,;-2.26,-48.96,;-3.79,-48.96,;-4.56,-47.61,;-3.79,-46.28,;-2.26,-46.31,;-6.12,-47.61,;-6.89,-48.95,;-8.43,-48.95,;-9.2,-47.6,;-8.42,-46.28,;-6.89,-46.28,;-6.12,-44.93,;-6.89,-43.6,;-6.12,-42.27,;-5.36,-40.92,;-4.78,-43.03,;-7.46,-41.5,)| Show InChI InChI=1S/C25H29ClF4N2O3S/c26-18-5-10-22(27)24(15-18)36(33,34)31-19-6-8-20(9-7-19)32-13-11-17(12-14-32)21-3-1-2-4-23(21)35-16-25(28,29)30/h1-5,10,15,17,19-20,31H,6-9,11-14,16H2/t19-,20+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1d receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211336
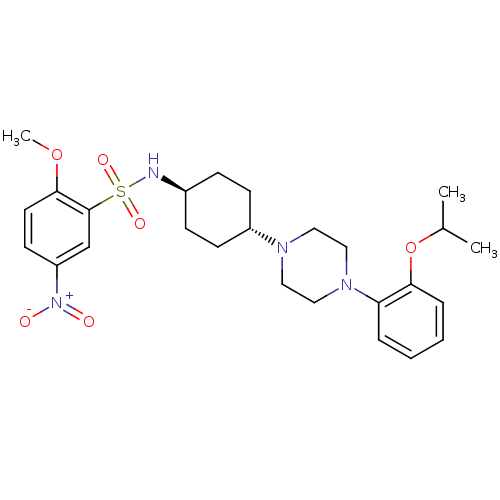
(CHEMBL234422 | N-((1r,4r)-4-(4-(2-isopropoxyphenyl...)Show SMILES COc1ccc(cc1S(=O)(=O)N[C@H]1CC[C@@H](CC1)N1CCN(CC1)c1ccccc1OC(C)C)[N+]([O-])=O |wU:15.19,wD:12.12,(29.55,-22.35,;30.32,-21.01,;29.55,-19.68,;30.32,-18.35,;29.55,-17.01,;28.01,-17.01,;27.24,-18.35,;28.01,-19.68,;27.24,-21.01,;25.9,-20.24,;28.57,-21.78,;26.47,-22.35,;24.93,-22.35,;24.16,-21.01,;22.62,-21.01,;21.85,-22.35,;22.62,-23.68,;24.16,-23.68,;20.31,-22.35,;19.53,-23.68,;17.99,-23.68,;17.22,-22.35,;17.99,-21.01,;19.53,-21.01,;15.68,-22.35,;14.91,-23.68,;13.37,-23.68,;12.6,-22.35,;13.37,-21.01,;14.91,-21.01,;15.68,-19.68,;14.91,-18.35,;15.68,-17.01,;13.37,-18.35,;27.24,-15.67,;28.01,-14.35,;25.7,-15.67,)| Show InChI InChI=1S/C26H36N4O6S/c1-19(2)36-24-7-5-4-6-23(24)29-16-14-28(15-17-29)21-10-8-20(9-11-21)27-37(33,34)26-18-22(30(31)32)12-13-25(26)35-3/h4-7,12-13,18-21,27H,8-11,14-17H2,1-3H3/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50203482
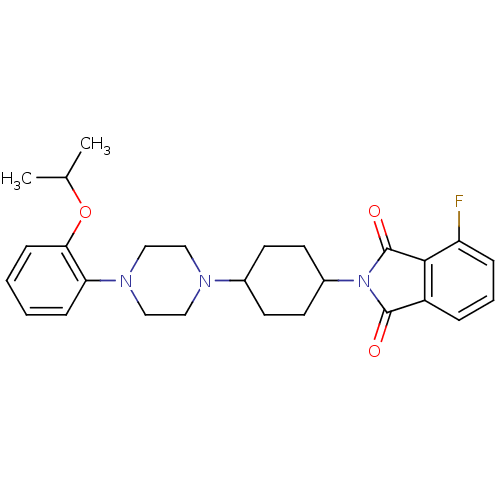
(4-fluoro-2-(4-(4-(2-isopropoxyphenyl)piperazin-1-y...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2cccc(F)c2C1=O |(16.25,-12.88,;15.48,-11.55,;13.94,-11.55,;16.25,-10.21,;15.48,-8.88,;13.95,-8.88,;13.18,-7.55,;13.94,-6.23,;15.48,-6.22,;16.25,-7.55,;17.79,-7.55,;18.56,-8.89,;20.09,-8.89,;20.87,-7.56,;20.1,-6.23,;18.55,-6.22,;22.41,-7.57,;23.17,-8.91,;24.71,-8.91,;25.49,-7.58,;24.72,-6.25,;23.18,-6.24,;27.03,-7.59,;27.92,-8.83,;27.44,-10.3,;29.38,-8.37,;30.71,-9.14,;32.05,-8.38,;32.05,-6.84,;30.72,-6.07,;30.73,-4.53,;29.39,-6.82,;27.93,-6.34,;27.46,-4.88,)| Show InChI InChI=1S/C27H32FN3O3/c1-18(2)34-24-9-4-3-8-23(24)30-16-14-29(15-17-30)19-10-12-20(13-11-19)31-26(32)21-6-5-7-22(28)25(21)27(31)33/h3-9,18-20H,10-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1a receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50203482

(4-fluoro-2-(4-(4-(2-isopropoxyphenyl)piperazin-1-y...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2cccc(F)c2C1=O |(16.25,-12.88,;15.48,-11.55,;13.94,-11.55,;16.25,-10.21,;15.48,-8.88,;13.95,-8.88,;13.18,-7.55,;13.94,-6.23,;15.48,-6.22,;16.25,-7.55,;17.79,-7.55,;18.56,-8.89,;20.09,-8.89,;20.87,-7.56,;20.1,-6.23,;18.55,-6.22,;22.41,-7.57,;23.17,-8.91,;24.71,-8.91,;25.49,-7.58,;24.72,-6.25,;23.18,-6.24,;27.03,-7.59,;27.92,-8.83,;27.44,-10.3,;29.38,-8.37,;30.71,-9.14,;32.05,-8.38,;32.05,-6.84,;30.72,-6.07,;30.73,-4.53,;29.39,-6.82,;27.93,-6.34,;27.46,-4.88,)| Show InChI InChI=1S/C27H32FN3O3/c1-18(2)34-24-9-4-3-8-23(24)30-16-14-29(15-17-30)19-10-12-20(13-11-19)31-26(32)21-6-5-7-22(28)25(21)27(31)33/h3-9,18-20H,10-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1a receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211345
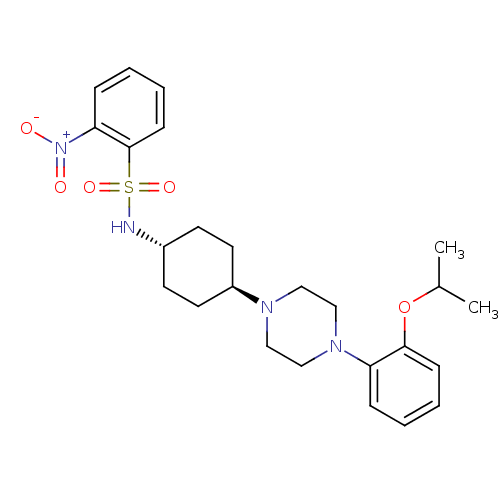
(CHEMBL232593 | N-((1r,4r)-4-(4-(2-isopropoxyphenyl...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccccc1[N+]([O-])=O |wU:16.17,wD:19.24,(-5.99,-39.63,;-6.77,-40.96,;-8.31,-40.94,;-6.02,-42.3,;-6.81,-43.62,;-8.35,-43.61,;-9.13,-44.93,;-8.37,-46.28,;-6.83,-46.29,;-6.05,-44.97,;-4.52,-44.99,;-3.76,-46.32,;-2.22,-46.34,;-1.44,-45.02,;-2.19,-43.68,;-3.73,-43.67,;.09,-45.04,;.88,-43.72,;2.42,-43.74,;3.17,-45.09,;2.38,-46.41,;.85,-46.39,;4.73,-45.12,;5.5,-43.79,;4.17,-43.02,;6.83,-44.56,;6.26,-42.45,;5.49,-41.13,;6.24,-39.79,;7.79,-39.78,;8.57,-41.1,;7.81,-42.44,;8.59,-43.78,;7.83,-45.12,;10.13,-43.77,)| Show InChI InChI=1S/C25H34N4O5S/c1-19(2)34-24-9-5-3-7-22(24)28-17-15-27(16-18-28)21-13-11-20(12-14-21)26-35(32,33)25-10-6-4-8-23(25)29(30)31/h3-10,19-21,26H,11-18H2,1-2H3/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203803
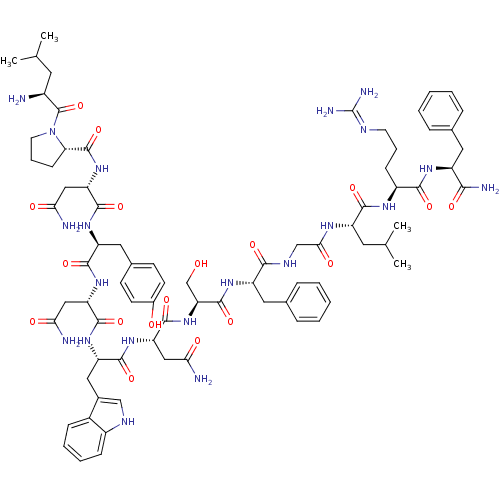
((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.110,wD:66.69,24.24,95.99,16.16,44.45,(-13.2,3.34,;-13.16,1.8,;-14.49,1.01,;-11.81,1.05,;-11.81,-.5,;-13.14,-1.27,;-10.47,-1.27,;-10.47,-2.81,;-9.14,-.51,;-8.66,.95,;-7.12,.95,;-6.65,-.51,;-7.9,-1.42,;-7.9,-2.96,;-9.24,-3.73,;-6.57,-3.73,;-6.57,-5.27,;-7.91,-6.04,;-7.91,-7.58,;-6.57,-8.35,;-9.24,-8.35,;-5.24,-6.04,;-5.24,-7.58,;-3.9,-5.27,;-2.57,-6.04,;-2.57,-7.59,;-1.24,-8.36,;-1.25,-9.89,;.08,-10.66,;1.42,-9.9,;2.75,-10.67,;1.42,-8.35,;.09,-7.59,;-1.24,-5.28,;-1.24,-3.74,;.1,-6.05,;1.43,-5.28,;1.43,-3.74,;2.77,-2.97,;4.1,-3.74,;2.77,-1.43,;2.76,-6.05,;2.76,-7.59,;4.1,-5.28,;5.43,-6.05,;5.43,-7.59,;6.76,-8.36,;8.01,-7.37,;9.25,-8.36,;8.77,-9.83,;9.53,-11.15,;8.77,-12.47,;7.23,-12.47,;6.47,-11.14,;7.24,-9.82,;6.77,-5.28,;6.77,-3.74,;8.1,-6.06,;9.43,-5.29,;9.43,-3.75,;10.77,-2.98,;12.1,-3.75,;10.77,-1.44,;10.77,-6.06,;10.76,-7.6,;12.1,-5.29,;13.43,-6.06,;13.43,-7.6,;14.76,-8.37,;14.77,-5.29,;14.77,-3.75,;16.1,-6.06,;17.44,-5.3,;17.44,-3.76,;18.77,-2.99,;20.1,-3.76,;21.44,-3,;21.44,-1.45,;20.1,-.68,;18.77,-1.46,;18.77,-6.07,;18.77,-7.61,;20.1,-5.3,;21.44,-6.07,;22.77,-5.3,;22.77,-3.76,;24.1,-6.07,;25.44,-5.3,;25.44,-3.76,;26.77,-3,;26.78,-1.46,;28.11,-3.77,;26.77,-6.08,;26.77,-7.62,;28.1,-5.31,;29.44,-6.08,;29.44,-7.62,;30.77,-8.39,;30.77,-9.93,;32.1,-10.7,;32.1,-12.24,;33.43,-13.01,;30.76,-13.01,;30.77,-5.31,;30.77,-3.77,;32.1,-6.08,;33.44,-5.31,;33.44,-3.77,;34.78,-3,;36.1,-3.79,;37.44,-3.02,;37.44,-1.48,;36.1,-.7,;34.77,-1.48,;34.77,-6.08,;36.11,-5.32,;34.77,-7.62,)| Show InChI InChI=1S/C78H107N21O18/c1-41(2)29-49(79)77(117)99-28-14-22-61(99)76(116)97-59(37-64(82)104)73(113)93-55(33-45-23-25-47(101)26-24-45)70(110)95-57(35-62(80)102)72(112)94-56(34-46-38-87-50-20-12-11-19-48(46)50)71(111)96-58(36-63(81)103)74(114)98-60(40-100)75(115)92-54(32-44-17-9-6-10-18-44)67(107)88-39-65(105)89-53(30-42(3)4)69(109)90-51(21-13-27-86-78(84)85)68(108)91-52(66(83)106)31-43-15-7-5-8-16-43/h5-12,15-20,23-26,38,41-42,49,51-61,87,100-101H,13-14,21-22,27-37,39-40,79H2,1-4H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM50203803

((2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]py...)Show SMILES CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:58.61,4.4,72.75,87.91,36.37,12.13,106.110,wD:66.69,24.24,95.99,16.16,44.45,(-13.2,3.34,;-13.16,1.8,;-14.49,1.01,;-11.81,1.05,;-11.81,-.5,;-13.14,-1.27,;-10.47,-1.27,;-10.47,-2.81,;-9.14,-.51,;-8.66,.95,;-7.12,.95,;-6.65,-.51,;-7.9,-1.42,;-7.9,-2.96,;-9.24,-3.73,;-6.57,-3.73,;-6.57,-5.27,;-7.91,-6.04,;-7.91,-7.58,;-6.57,-8.35,;-9.24,-8.35,;-5.24,-6.04,;-5.24,-7.58,;-3.9,-5.27,;-2.57,-6.04,;-2.57,-7.59,;-1.24,-8.36,;-1.25,-9.89,;.08,-10.66,;1.42,-9.9,;2.75,-10.67,;1.42,-8.35,;.09,-7.59,;-1.24,-5.28,;-1.24,-3.74,;.1,-6.05,;1.43,-5.28,;1.43,-3.74,;2.77,-2.97,;4.1,-3.74,;2.77,-1.43,;2.76,-6.05,;2.76,-7.59,;4.1,-5.28,;5.43,-6.05,;5.43,-7.59,;6.76,-8.36,;8.01,-7.37,;9.25,-8.36,;8.77,-9.83,;9.53,-11.15,;8.77,-12.47,;7.23,-12.47,;6.47,-11.14,;7.24,-9.82,;6.77,-5.28,;6.77,-3.74,;8.1,-6.06,;9.43,-5.29,;9.43,-3.75,;10.77,-2.98,;12.1,-3.75,;10.77,-1.44,;10.77,-6.06,;10.76,-7.6,;12.1,-5.29,;13.43,-6.06,;13.43,-7.6,;14.76,-8.37,;14.77,-5.29,;14.77,-3.75,;16.1,-6.06,;17.44,-5.3,;17.44,-3.76,;18.77,-2.99,;20.1,-3.76,;21.44,-3,;21.44,-1.45,;20.1,-.68,;18.77,-1.46,;18.77,-6.07,;18.77,-7.61,;20.1,-5.3,;21.44,-6.07,;22.77,-5.3,;22.77,-3.76,;24.1,-6.07,;25.44,-5.3,;25.44,-3.76,;26.77,-3,;26.78,-1.46,;28.11,-3.77,;26.77,-6.08,;26.77,-7.62,;28.1,-5.31,;29.44,-6.08,;29.44,-7.62,;30.77,-8.39,;30.77,-9.93,;32.1,-10.7,;32.1,-12.24,;33.43,-13.01,;30.76,-13.01,;30.77,-5.31,;30.77,-3.77,;32.1,-6.08,;33.44,-5.31,;33.44,-3.77,;34.78,-3,;36.1,-3.79,;37.44,-3.02,;37.44,-1.48,;36.1,-.7,;34.77,-1.48,;34.77,-6.08,;36.11,-5.32,;34.77,-7.62,)| Show InChI InChI=1S/C78H107N21O18/c1-41(2)29-49(79)77(117)99-28-14-22-61(99)76(116)97-59(37-64(82)104)73(113)93-55(33-45-23-25-47(101)26-24-45)70(110)95-57(35-62(80)102)72(112)94-56(34-46-38-87-50-20-12-11-19-48(46)50)71(111)96-58(36-63(81)103)74(114)98-60(40-100)75(115)92-54(32-44-17-9-6-10-18-44)67(107)88-39-65(105)89-53(30-42(3)4)69(109)90-51(21-13-27-86-78(84)85)68(108)91-52(66(83)106)31-43-15-7-5-8-16-43/h5-12,15-20,23-26,38,41-42,49,51-61,87,100-101H,13-14,21-22,27-37,39-40,79H2,1-4H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,106)(H,88,107)(H,89,105)(H,90,109)(H,91,108)(H,92,115)(H,93,113)(H,94,112)(H,95,110)(H,96,111)(H,97,116)(H,98,114)(H4,84,85,86)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211329
(CHEMBL234649 | N-((1s,4s)-4-(4-(2-isopropoxyphenyl...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@@H]1CC[C@@H](CC1)NS(=O)(=O)Cc1ccccc1 |wU:16.17,19.24,(-7.57,-30.94,;-8.35,-32.26,;-9.89,-32.24,;-7.6,-33.6,;-8.39,-34.93,;-9.92,-34.91,;-10.71,-36.24,;-9.95,-37.58,;-8.41,-37.59,;-7.63,-36.27,;-6.1,-36.29,;-5.34,-37.63,;-3.8,-37.65,;-3.02,-36.33,;-3.77,-34.99,;-5.31,-34.97,;-1.48,-36.35,;-.73,-37.69,;.8,-37.71,;1.59,-36.39,;.84,-35.05,;-.7,-35.02,;3.15,-36.43,;3.92,-35.1,;2.59,-34.32,;5.25,-35.86,;4.68,-33.76,;6.22,-33.74,;7,-35.07,;8.54,-35.06,;9.3,-33.72,;8.51,-32.39,;6.97,-32.41,)| Show InChI InChI=1S/C26H37N3O3S/c1-21(2)32-26-11-7-6-10-25(26)29-18-16-28(17-19-29)24-14-12-23(13-15-24)27-33(30,31)20-22-8-4-3-5-9-22/h3-11,21,23-24,27H,12-20H2,1-2H3/t23-,24+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM86848

(1-(2,5-difluorophenyl)-3-(4-(4-(2-isopropoxyphenyl...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)NC(=O)Nc1cc(F)ccc1F |(-10.67,-3.08,;-10.67,-4.62,;-9.34,-5.39,;-12,-5.39,;-12,-6.93,;-10.67,-7.7,;-10.67,-9.24,;-12,-10.01,;-13.34,-9.24,;-13.34,-7.7,;-14.67,-6.93,;-16,-7.7,;-17.34,-6.93,;-17.34,-5.39,;-16,-4.62,;-14.67,-5.39,;-18.67,-4.62,;-20.01,-5.39,;-21.34,-4.62,;-21.34,-3.08,;-20.01,-2.31,;-18.67,-3.08,;-22.67,-2.31,;-22.67,-.77,;-21.34,,;-24.01,,;-25.34,-.77,;-26.67,,;-28.01,-.77,;-29.34,,;-28.01,-2.31,;-26.67,-3.08,;-25.34,-2.31,;-24.01,-3.08,)| Show InChI InChI=1S/C26H34F2N4O2/c1-18(2)34-25-6-4-3-5-24(25)32-15-13-31(14-16-32)21-10-8-20(9-11-21)29-26(33)30-23-17-19(27)7-12-22(23)28/h3-7,12,17-18,20-21H,8-11,13-16H2,1-2H3,(H2,29,30,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50223565

(3,4-dimethoxy-N-((1s,4s)-4-(4-(2-(2,2,2-trifluoroe...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCN(CC1)c1ccccc1OCC(F)(F)F |wU:17.21,14.14,(32.39,2.57,;30.9,2.95,;29.83,1.85,;28.32,2.24,;27.24,1.13,;27.66,-.35,;29.16,-.74,;30.24,.36,;31.73,-.03,;32.14,-1.51,;26.57,-1.45,;25.37,-.49,;27.77,-2.42,;25.74,-2.75,;24.21,-2.68,;23.38,-3.98,;21.85,-3.91,;21.13,-2.55,;21.95,-1.25,;23.49,-1.32,;19.6,-2.48,;18.78,-3.78,;17.25,-3.72,;16.54,-2.36,;17.34,-1.06,;18.89,-1.12,;15,-2.3,;14.19,-3.61,;12.64,-3.55,;11.92,-2.18,;12.74,-.88,;14.29,-.95,;15.11,.35,;14.39,1.72,;15.21,3.02,;15.97,4.36,;16.53,2.23,;13.89,3.81,)| Show InChI InChI=1S/C26H34F3N3O5S/c1-35-24-12-11-21(17-25(24)36-2)38(33,34)30-19-7-9-20(10-8-19)31-13-15-32(16-14-31)22-5-3-4-6-23(22)37-18-26(27,28)29/h3-6,11-12,17,19-20,30H,7-10,13-16,18H2,1-2H3/t19-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1a receptor |
Bioorg Med Chem Lett 17: 6123-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.051
BindingDB Entry DOI: 10.7270/Q2C82925 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86844

(1-(5-chloro-2-methoxyphenyl)-3-(4-(4-(2-isopropoxy...)Show SMILES COc1ccc(Cl)cc1NC(=O)NC1CCC(CC1)N1CCN(CC1)c1ccccc1OC(C)C |(-24.01,-4.62,;-24.01,-3.08,;-25.34,-2.31,;-26.67,-3.08,;-28.01,-2.31,;-28.01,-.77,;-29.34,,;-26.67,,;-25.34,-.77,;-24.01,,;-22.67,-.77,;-21.34,,;-22.67,-2.31,;-21.34,-3.08,;-21.34,-4.62,;-20.01,-5.39,;-18.67,-4.62,;-18.67,-3.08,;-20.01,-2.31,;-17.34,-5.39,;-17.34,-6.93,;-16,-7.7,;-14.67,-6.93,;-14.67,-5.39,;-16,-4.62,;-13.34,-7.7,;-13.34,-9.24,;-12,-10.01,;-10.67,-9.24,;-10.67,-7.7,;-12,-6.93,;-12,-5.39,;-10.67,-4.62,;-10.67,-3.08,;-9.34,-5.39,)| Show InChI InChI=1S/C27H37ClN4O3/c1-19(2)35-26-7-5-4-6-24(26)32-16-14-31(15-17-32)22-11-9-21(10-12-22)29-27(33)30-23-18-20(28)8-13-25(23)34-3/h4-8,13,18-19,21-22H,9-12,14-17H2,1-3H3,(H2,29,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 640-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.068
BindingDB Entry DOI: 10.7270/Q27H1H5S |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [125]metastin from metastin receptor |
J Med Chem 50: 462-71 (2007)
Article DOI: 10.1021/jm0609824
BindingDB Entry DOI: 10.7270/Q2HT2P0K |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50213512
(5-chloro-N-((1s,4s)-4-(4-(2-cyclopropoxyphenyl)pip...)Show SMILES COc1ccc(Cl)cc1S(=O)(=O)N[C@H]1CC[C@H](CC1)N1CCC(CC1)c1ccccc1OC1CC1 |wU:16.20,13.13,(10.04,-34.29,;8.5,-34.3,;7.72,-32.97,;8.48,-31.63,;7.7,-30.3,;6.15,-30.32,;5.37,-29,;5.4,-31.66,;6.18,-32.98,;5.42,-34.32,;4.07,-33.59,;6.77,-35.07,;4.7,-35.69,;3.16,-35.74,;2.44,-37.1,;.91,-37.16,;.09,-35.86,;.8,-34.5,;2.34,-34.44,;-1.45,-35.92,;-2.16,-37.28,;-3.7,-37.35,;-4.52,-36.05,;-3.82,-34.68,;-2.28,-34.62,;-6.06,-36.11,;-6.77,-37.48,;-8.31,-37.55,;-9.14,-36.24,;-8.43,-34.88,;-6.9,-34.81,;-6.19,-33.44,;-7.02,-32.14,;-7.1,-30.6,;-8.39,-31.43,)| Show InChI InChI=1S/C27H35ClN2O4S/c1-33-26-13-6-20(28)18-27(26)35(31,32)29-21-7-9-22(10-8-21)30-16-14-19(15-17-30)24-4-2-3-5-25(24)34-23-11-12-23/h2-6,13,18-19,21-23,29H,7-12,14-17H2,1H3/t21-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1a receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211346
(4-fluoro-N-((1r,4r)-4-(4-(2-isopropoxyphenyl)piper...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1 |wU:16.17,wD:19.24,(16.2,-15.13,;15.43,-16.47,;13.89,-16.47,;16.21,-17.8,;15.44,-19.13,;13.9,-19.14,;13.14,-20.48,;13.92,-21.81,;15.46,-21.8,;16.22,-20.47,;17.75,-20.46,;18.53,-21.79,;20.07,-21.78,;20.83,-20.45,;20.06,-19.12,;18.52,-19.13,;22.37,-20.44,;23.13,-19.11,;24.67,-19.11,;25.44,-20.44,;24.67,-21.77,;23.14,-21.78,;27,-20.45,;27.75,-19.11,;26.41,-18.35,;29.09,-19.85,;28.49,-17.75,;30.04,-17.72,;30.78,-16.37,;29.97,-15.05,;30.71,-13.7,;28.43,-15.1,;27.7,-16.45,)| Show InChI InChI=1S/C25H34FN3O3S/c1-19(2)32-25-6-4-3-5-24(25)29-17-15-28(16-18-29)22-11-9-21(10-12-22)27-33(30,31)23-13-7-20(26)8-14-23/h3-8,13-14,19,21-22,27H,9-12,15-18H2,1-2H3/t21-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50213518
(3,4-difluoro-N-((1r,4r)-4-(4-(2-isopropoxyphenyl)p...)Show SMILES CC(C)Oc1ccccc1C1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)c(F)c1 |wU:16.17,wD:19.24,(14.85,-7.42,;14.15,-8.79,;12.61,-8.86,;14.98,-10.08,;14.27,-11.45,;12.74,-11.52,;12.03,-12.89,;12.86,-14.19,;14.4,-14.12,;15.11,-12.75,;16.65,-12.69,;17.47,-13.99,;19,-13.92,;19.72,-12.56,;18.89,-11.26,;17.35,-11.32,;21.26,-12.5,;21.97,-11.14,;23.51,-11.08,;24.33,-12.39,;23.61,-13.75,;22.08,-13.81,;25.87,-12.33,;26.59,-10.97,;25.24,-10.23,;27.94,-11.71,;27.35,-9.62,;26.57,-8.3,;27.32,-6.96,;28.87,-6.94,;29.63,-5.6,;29.65,-8.28,;31.19,-8.26,;28.89,-9.62,)| Show InChI InChI=1S/C26H34F2N2O3S/c1-18(2)33-26-6-4-3-5-23(26)19-13-15-30(16-14-19)21-9-7-20(8-10-21)29-34(31,32)22-11-12-24(27)25(28)17-22/h3-6,11-12,17-21,29H,7-10,13-16H2,1-2H3/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1a receptor |
Bioorg Med Chem Lett 17: 3930-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.098
BindingDB Entry DOI: 10.7270/Q2639PF1 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50211357
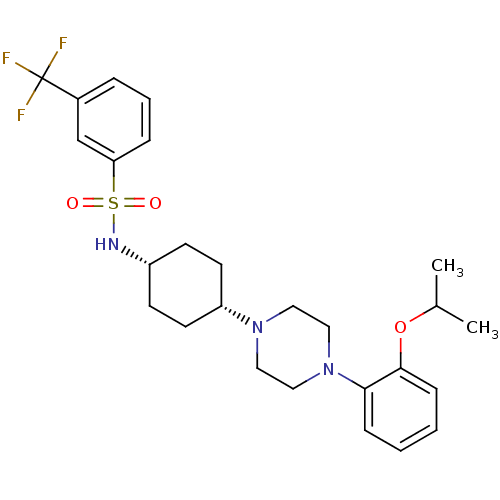
(CHEMBL233592 | N-((1s,4s)-4-(4-(2-isopropoxyphenyl...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@@H]1CC[C@@H](CC1)NS(=O)(=O)c1cccc(c1)C(F)(F)F |wU:16.17,19.24,(-7.37,-6.15,;-8.14,-7.48,;-9.68,-7.48,;-7.37,-8.81,;-8.14,-10.15,;-9.67,-10.15,;-10.44,-11.49,;-9.66,-12.82,;-8.13,-12.81,;-7.36,-11.48,;-5.83,-11.48,;-5.05,-12.81,;-3.51,-12.8,;-2.75,-11.47,;-3.52,-10.15,;-5.06,-10.15,;-1.22,-11.47,;-.45,-12.81,;1.09,-12.81,;1.86,-11.48,;1.09,-10.14,;-.45,-10.14,;3.42,-11.49,;4.18,-10.15,;2.83,-9.39,;5.51,-10.9,;4.92,-8.8,;4.13,-7.49,;4.86,-6.14,;6.41,-6.1,;7.21,-7.42,;6.47,-8.77,;8.75,-7.39,;10.28,-7.37,;8.72,-5.85,;8.78,-8.93,)| Show InChI InChI=1S/C26H34F3N3O3S/c1-19(2)35-25-9-4-3-8-24(25)32-16-14-31(15-17-32)22-12-10-21(11-13-22)30-36(33,34)23-7-5-6-20(18-23)26(27,28)29/h3-9,18-19,21-22,30H,10-17H2,1-2H3/t21-,22+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1D receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50203484

(6-(4-(4-(2-isopropoxyphenyl)piperazin-1-yl)cyclohe...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)C1CCC(CC1)N1C(=O)c2cccnc2C1=O |(-5.9,-44.57,;-6.67,-43.23,;-8.21,-43.23,;-5.9,-41.9,;-6.67,-40.57,;-8.21,-40.56,;-8.97,-39.24,;-8.21,-37.91,;-6.67,-37.91,;-5.9,-39.24,;-4.36,-39.24,;-3.6,-40.58,;-2.06,-40.58,;-1.29,-39.25,;-2.05,-37.91,;-3.6,-37.91,;.25,-39.26,;1.02,-40.59,;2.56,-40.6,;3.33,-39.27,;2.57,-37.93,;1.03,-37.93,;4.87,-39.28,;5.78,-38.03,;5.31,-36.56,;7.24,-38.51,;8.57,-37.75,;9.9,-38.52,;9.89,-40.07,;8.55,-40.83,;7.23,-40.05,;5.77,-40.52,;5.29,-41.98,)| Show InChI InChI=1S/C26H32N4O3/c1-18(2)33-23-8-4-3-7-22(23)29-16-14-28(15-17-29)19-9-11-20(12-10-19)30-25(31)21-6-5-13-27-24(21)26(30)32/h3-8,13,18-20H,9-12,14-17H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Displacement of [125]HEAT from human adrenergic alpha1d receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 1646-50 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.111
BindingDB Entry DOI: 10.7270/Q2PG1RC2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50211323
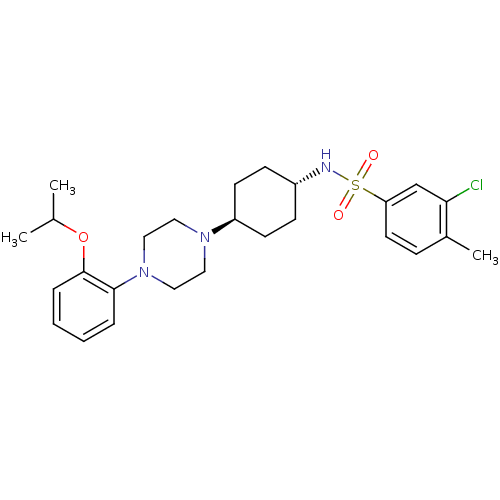
(3-chloro-N-((1r,4r)-4-(4-(2-isopropoxyphenyl)piper...)Show SMILES CC(C)Oc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(C)c(Cl)c1 |wU:16.17,wD:19.24,(14.8,-27.45,;14.03,-28.79,;12.49,-28.79,;14.8,-30.12,;14.03,-31.47,;12.49,-31.47,;11.72,-32.8,;12.5,-34.13,;14.02,-34.13,;14.8,-32.8,;16.35,-32.8,;17.11,-34.13,;18.66,-34.13,;19.43,-32.8,;18.67,-31.47,;17.13,-31.47,;20.98,-32.8,;21.75,-31.46,;23.29,-31.47,;24.05,-32.8,;23.28,-34.13,;21.75,-34.13,;25.61,-32.8,;26.37,-31.47,;25.04,-30.71,;27.72,-32.25,;27.14,-30.14,;26.38,-28.79,;27.14,-27.47,;28.67,-27.47,;29.45,-26.11,;29.44,-28.79,;30.99,-28.79,;28.67,-30.14,)| Show InChI InChI=1S/C26H36ClN3O3S/c1-19(2)33-26-7-5-4-6-25(26)30-16-14-29(15-17-30)22-11-9-21(10-12-22)28-34(31,32)23-13-8-20(3)24(27)18-23/h4-8,13,18-19,21-22,28H,9-12,14-17H2,1-3H3/t21-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned adrenergic alpha1A receptor |
Bioorg Med Chem Lett 17: 3292-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.008
BindingDB Entry DOI: 10.7270/Q2PG1RDH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data