

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM233008 (H-D-Ser-Ala-Arg-NH-(CH2)8-NH2 (7)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM233006 (H-D-Ser-Ala-Arg-NH-(CH2)6-NH2 (5)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM233009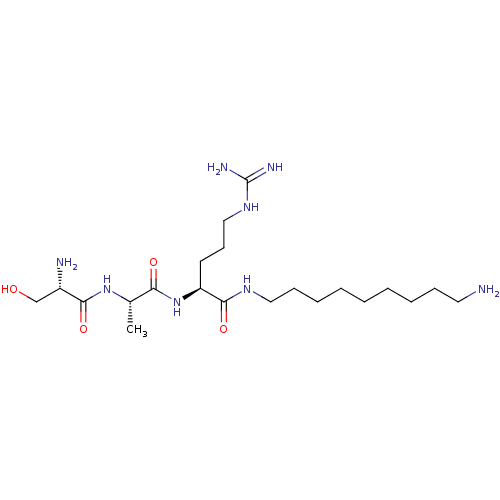 (H-D-Ser-Ala-Arg-NH-(CH2)9-NH2 (8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240793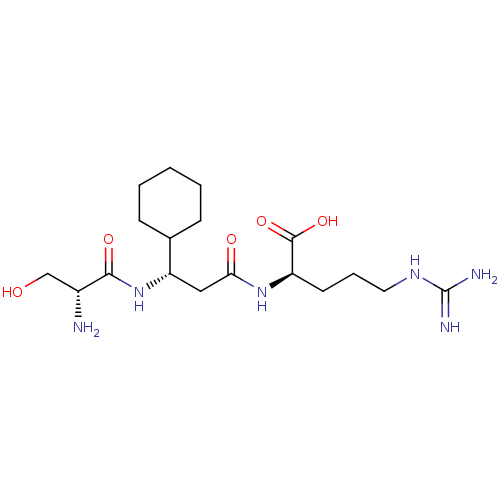 (H-D-Ser-Chg-Arg-OH (4)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240790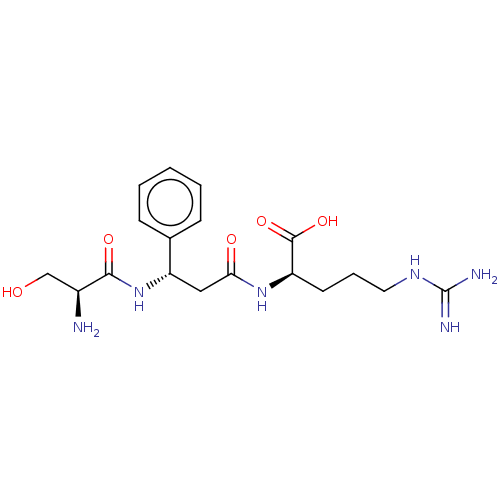 (H-D-Ser-Phg-Arg-OH (1)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240792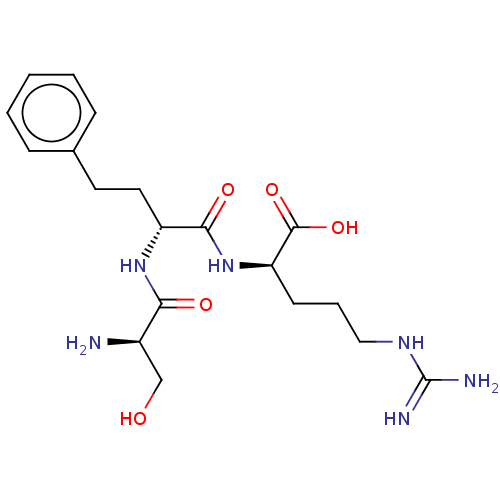 (H-D-Ser-homoPhe-Arg-OH (3)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233005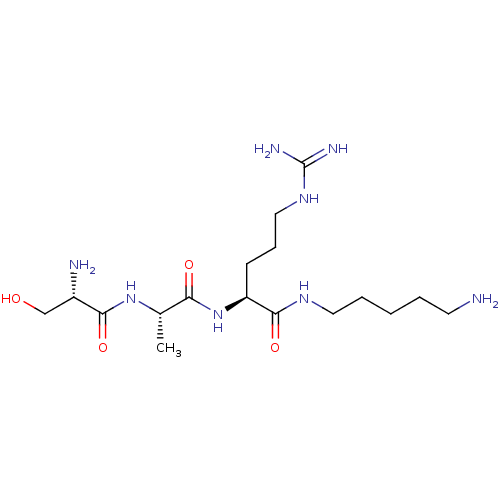 (H-D-Ser-Ala-Arg-NH-(CH2)5-NH2 (4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240793 (H-D-Ser-Chg-Arg-OH (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240797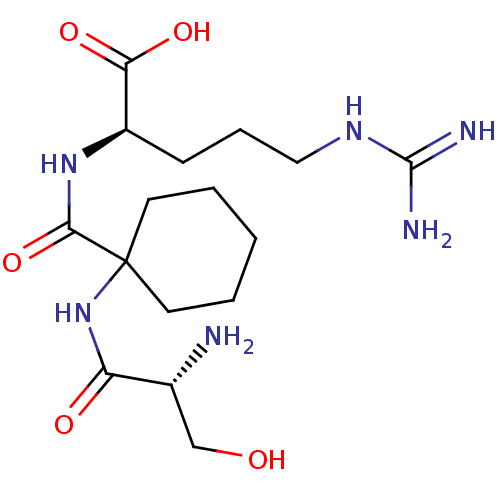 (H-D-Ser-1ACH-Arg-OH (8)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240796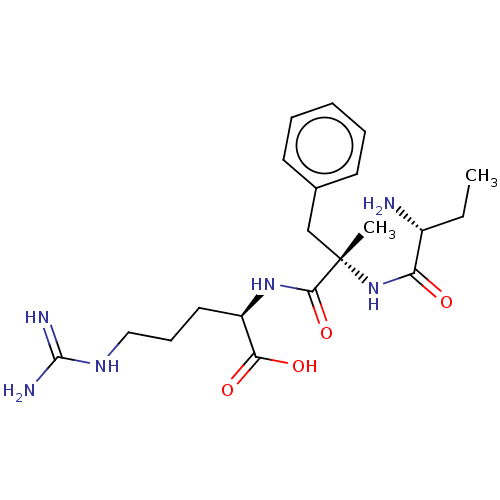 (H-D-Ser-αMePhe-Arg-OH (7)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240791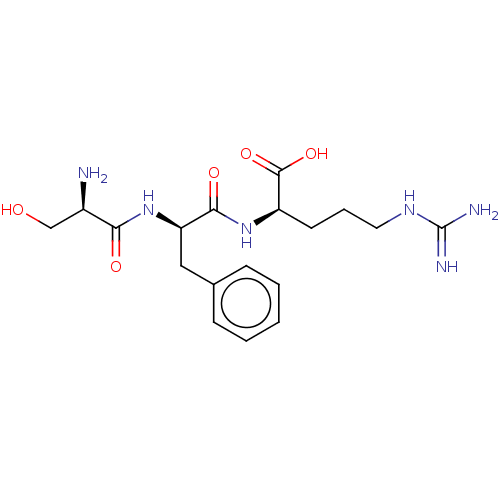 (H-D-Ser-Phe-Arg-OH (2)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240794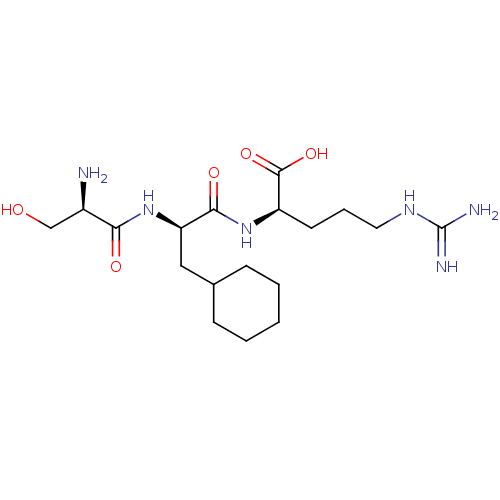 (H-D-Ser-Cha-Arg-OH (5)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM233008 (H-D-Ser-Ala-Arg-NH-(CH2)8-NH2 (7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM233012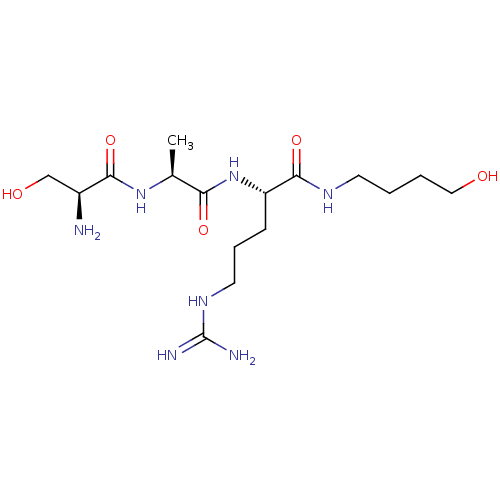 (H-D-Ser-Ala-Arg-NH-(CH2)4-OH (11)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM240795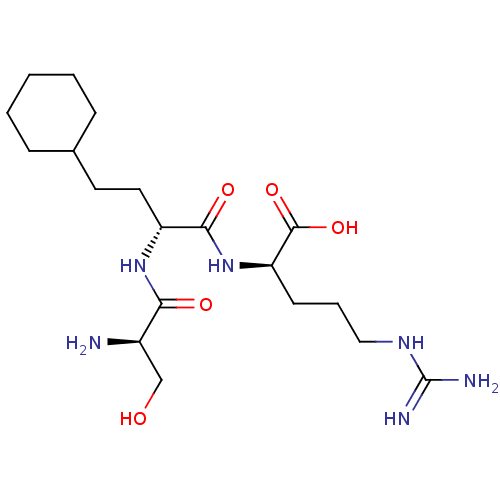 (H-D-Ser-homoCha-Arg-OH (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240791 (H-D-Ser-Phe-Arg-OH (2)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240796 (H-D-Ser-αMePhe-Arg-OH (7)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240797 (H-D-Ser-1ACH-Arg-OH (8)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM233011 (H-D-Ser-Ala-Arg-NH-(CH2)3-OH (10)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240792 (H-D-Ser-homoPhe-Arg-OH (3)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240795 (H-D-Ser-homoCha-Arg-OH (6)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240794 (H-D-Ser-Cha-Arg-OH (5)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240791 (H-D-Ser-Phe-Arg-OH (2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM240790 (H-D-Ser-Phg-Arg-OH (1)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.4 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240792 (H-D-Ser-homoPhe-Arg-OH (3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240797 (H-D-Ser-1ACH-Arg-OH (8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240790 (H-D-Ser-Phg-Arg-OH (1)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240795 (H-D-Ser-homoCha-Arg-OH (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240796 (H-D-Ser-αMePhe-Arg-OH (7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM233007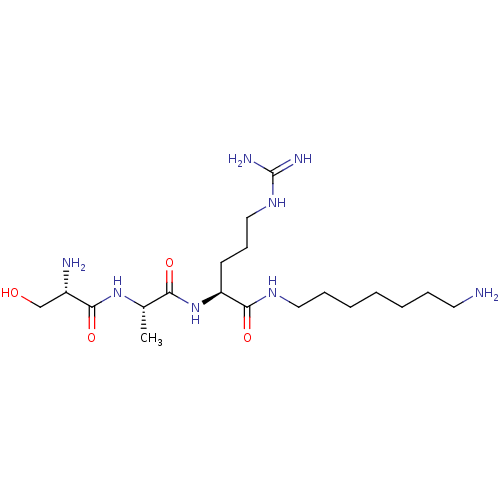 (H-D-Ser-Ala-Arg-NH-(CH2)7-NH2 (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240794 (H-D-Ser-Cha-Arg-OH (5)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM233005 (H-D-Ser-Ala-Arg-NH-(CH2)5-NH2 (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM240793 (H-D-Ser-Chg-Arg-OH (4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233008 (H-D-Ser-Ala-Arg-NH-(CH2)8-NH2 (7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM233009 (H-D-Ser-Ala-Arg-NH-(CH2)9-NH2 (8)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM233006 (H-D-Ser-Ala-Arg-NH-(CH2)6-NH2 (5)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233007 (H-D-Ser-Ala-Arg-NH-(CH2)7-NH2 (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240794 (H-D-Ser-Cha-Arg-OH (5)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240796 (H-D-Ser-αMePhe-Arg-OH (7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233009 (H-D-Ser-Ala-Arg-NH-(CH2)9-NH2 (8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240793 (H-D-Ser-Chg-Arg-OH (4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM233007 (H-D-Ser-Ala-Arg-NH-(CH2)7-NH2 (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240797 (H-D-Ser-1ACH-Arg-OH (8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240795 (H-D-Ser-homoCha-Arg-OH (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 4.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240790 (H-D-Ser-Phg-Arg-OH (1)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240791 (H-D-Ser-Phe-Arg-OH (2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM233012 (H-D-Ser-Ala-Arg-NH-(CH2)4-OH (11)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM240792 (H-D-Ser-homoPhe-Arg-OH (3)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en... | J Enzyme Inhib Med Chem 28: 639-43 (2013) Article DOI: 10.3109/14756366.2011.651463 BindingDB Entry DOI: 10.7270/Q2HQ3XTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |