 Found 1402 hits with Last Name = 'milkiewicz' and Initial = 'kl'
Found 1402 hits with Last Name = 'milkiewicz' and Initial = 'kl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231280
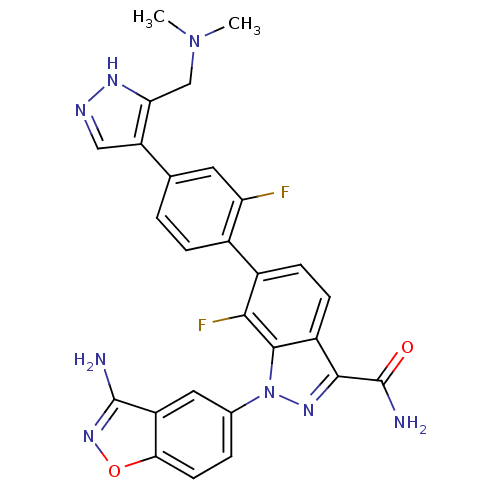
(1-(3-cyano-4-fluorophenyl)-6-[4-(3-dimethylaminome...)Show SMILES CN(C)Cc1[nH]ncc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H22F2N8O2/c1-36(2)12-21-19(11-32-33-21)13-3-5-15(20(28)9-13)16-6-7-17-24(27(31)38)34-37(25(17)23(16)29)14-4-8-22-18(10-14)26(30)35-39-22/h3-11H,12H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231282
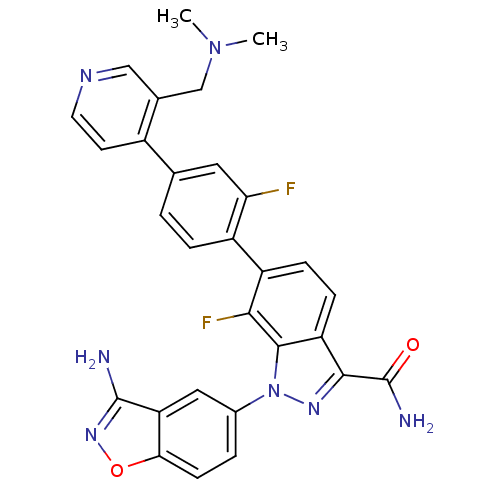
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(3-dimethyla...)Show SMILES CN(C)Cc1cnccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-13-34-10-9-18(16)15-3-5-19(23(30)11-15)20-6-7-21-26(29(33)39)35-38(27(21)25(20)31)17-4-8-24-22(12-17)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231275
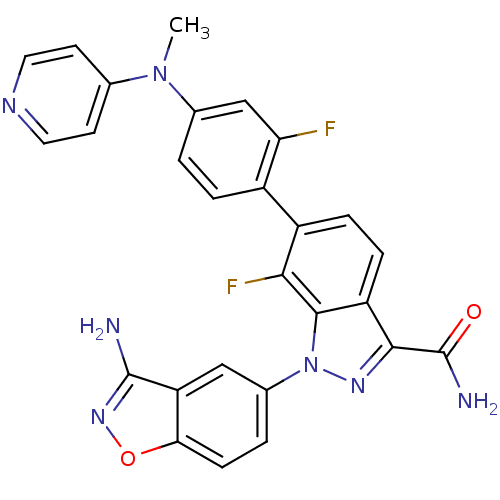
(1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...)Show SMILES CN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H19F2N7O2/c1-35(14-8-10-32-11-9-14)15-2-4-17(21(28)13-15)18-5-6-19-24(27(31)37)33-36(25(19)23(18)29)16-3-7-22-20(12-16)26(30)34-38-22/h2-13H,1H3,(H2,30,34)(H2,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231284
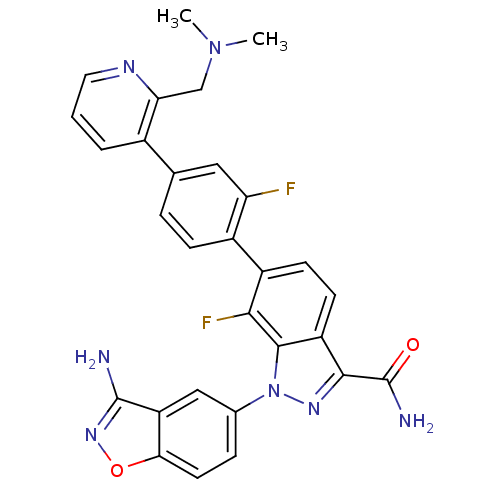
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(2-dimethyla...)Show SMILES CN(C)Cc1ncccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-23-17(4-3-11-34-23)15-5-7-18(22(30)12-15)19-8-9-20-26(29(33)39)35-38(27(20)25(19)31)16-6-10-24-21(13-16)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231271
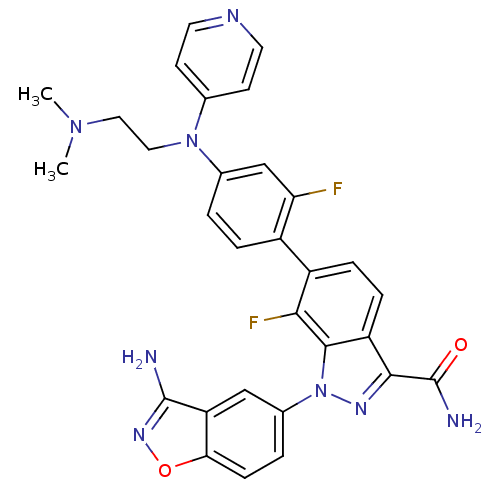
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H26F2N8O2/c1-38(2)13-14-39(17-9-11-35-12-10-17)18-3-5-20(24(31)16-18)21-6-7-22-27(30(34)41)36-40(28(22)26(21)32)19-4-8-25-23(15-19)29(33)37-42-25/h3-12,15-16H,13-14H2,1-2H3,(H2,33,37)(H2,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231279
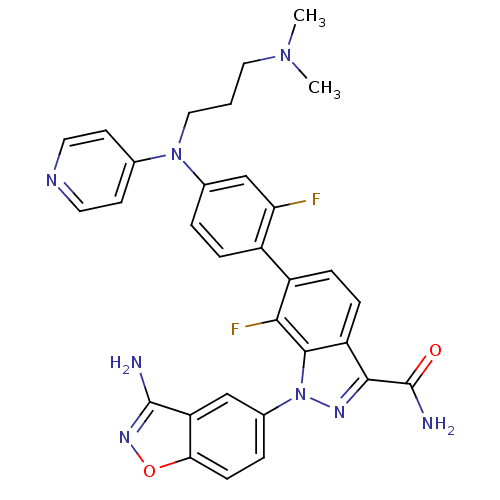
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(3-dimethyl...)Show SMILES CN(C)CCCN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C31H28F2N8O2/c1-39(2)14-3-15-40(18-10-12-36-13-11-18)19-4-6-21(25(32)17-19)22-7-8-23-28(31(35)42)37-41(29(23)27(22)33)20-5-9-26-24(16-20)30(34)38-43-26/h4-13,16-17H,3,14-15H2,1-2H3,(H2,34,38)(H2,35,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231283
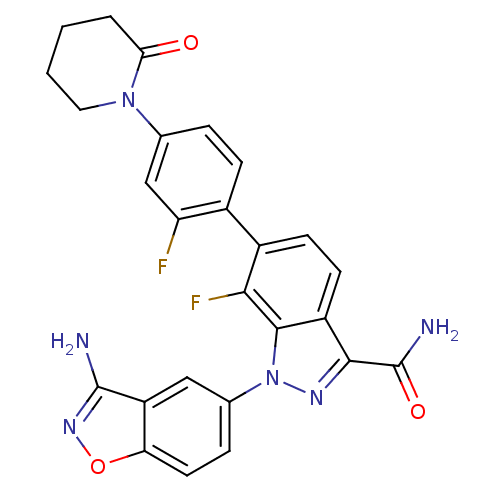
(1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...)Show SMILES NC(=O)c1nn(-c2ccc3onc(N)c3c2)c2c(F)c(ccc12)-c1ccc(cc1F)N1CCCCC1=O Show InChI InChI=1S/C26H20F2N6O3/c27-19-12-13(33-10-2-1-3-21(33)35)4-6-15(19)16-7-8-17-23(26(30)36)31-34(24(17)22(16)28)14-5-9-20-18(11-14)25(29)32-37-20/h4-9,11-12H,1-3,10H2,(H2,29,32)(H2,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231270

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-(2'-dimethylami...)Show SMILES CN(C)Cc1ccccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H24F2N6O2/c1-37(2)15-17-5-3-4-6-19(17)16-7-9-20(24(31)13-16)21-10-11-22-27(30(34)39)35-38(28(22)26(21)32)18-8-12-25-23(14-18)29(33)36-40-25/h3-14H,15H2,1-2H3,(H2,33,36)(H2,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231272
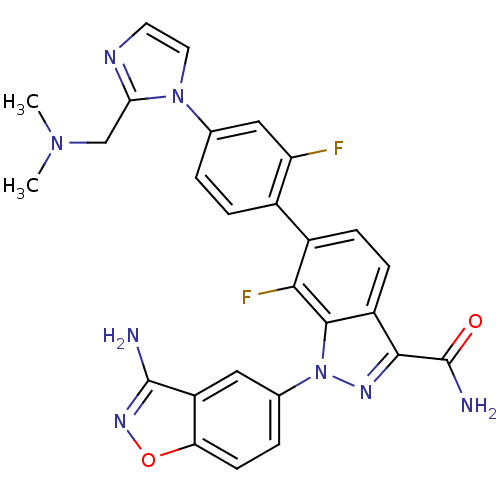
(1-(3-amino-1,2-benzisoxazol-5-yl)-6-(4-{2-[(dimeth...)Show SMILES CN(C)Cc1nccn1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H22F2N8O2/c1-35(2)13-22-32-9-10-36(22)14-3-5-16(20(28)12-14)17-6-7-18-24(27(31)38)33-37(25(18)23(17)29)15-4-8-21-19(11-15)26(30)34-39-21/h3-12H,13H2,1-2H3,(H2,30,34)(H2,31,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 15.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231269
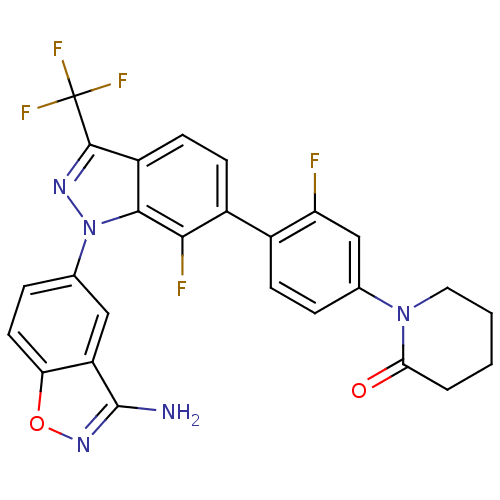
(1-{4-[1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-3-...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2ccc(c(F)c12)-c1ccc(cc1F)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C26H18F5N5O2/c27-19-12-13(35-10-2-1-3-21(35)37)4-6-15(19)16-7-8-17-23(22(16)28)36(33-24(17)26(29,30)31)14-5-9-20-18(11-14)25(32)34-38-20/h4-9,11-12H,1-3,10H2,(H2,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231274
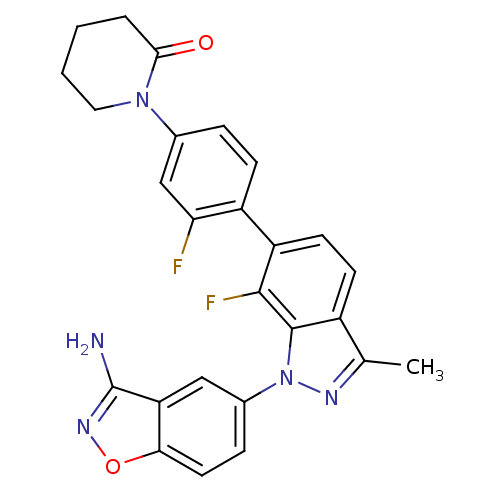
(1-{4-[1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-3-...)Show SMILES Cc1nn(-c2ccc3onc(N)c3c2)c2c(F)c(ccc12)-c1ccc(cc1F)N1CCCCC1=O Show InChI InChI=1S/C26H21F2N5O2/c1-14-17-8-9-19(18-7-5-15(13-21(18)27)32-11-3-2-4-23(32)34)24(28)25(17)33(30-14)16-6-10-22-20(12-16)26(29)31-35-22/h5-10,12-13H,2-4,11H2,1H3,(H2,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231286
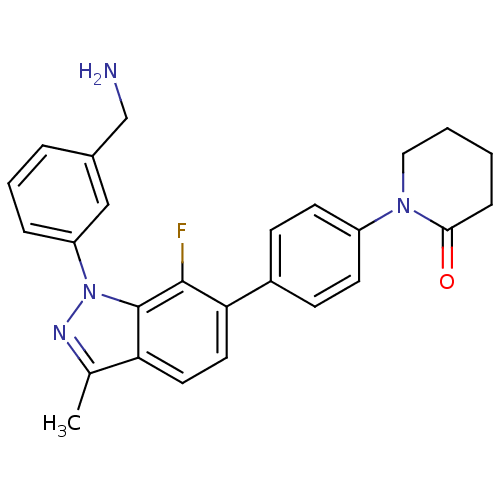
(1-{4-[1-(3-aminomethylphenyl)-7-fluoro-3-methyl-1H...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2c(F)c(ccc12)-c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C26H25FN4O/c1-17-22-12-13-23(19-8-10-20(11-9-19)30-14-3-2-7-24(30)32)25(27)26(22)31(29-17)21-6-4-5-18(15-21)16-28/h4-6,8-13,15H,2-3,7,14,16,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231268
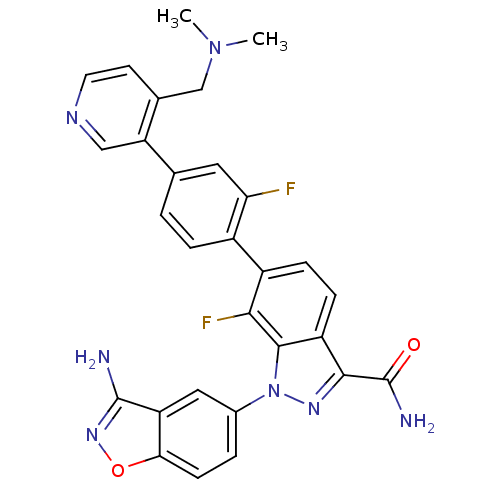
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(4-dimethyla...)Show SMILES CN(C)Cc1ccncc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-9-10-34-13-22(16)15-3-5-18(23(30)11-15)19-6-7-20-26(29(33)39)35-38(27(20)25(19)31)17-4-8-24-21(12-17)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231278
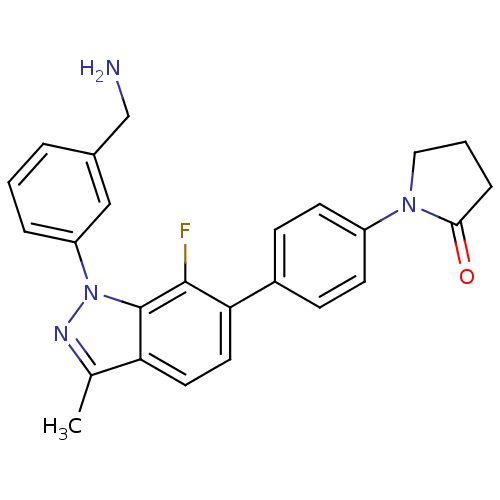
(1-{4-[1-(3-aminomethylphenyl)-7-fluoro-3-methyl-1H...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2c(F)c(ccc12)-c1ccc(cc1)N1CCCC1=O Show InChI InChI=1S/C25H23FN4O/c1-16-21-11-12-22(18-7-9-19(10-8-18)29-13-3-6-23(29)31)24(26)25(21)30(28-16)20-5-2-4-17(14-20)15-27/h2,4-5,7-12,14H,3,6,13,15,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231281

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1cccnc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H26F2N8O2/c1-38(2)12-13-39(19-4-3-11-35-16-19)17-5-7-20(24(31)15-17)21-8-9-22-27(30(34)41)36-40(28(22)26(21)32)18-6-10-25-23(14-18)29(33)37-42-25/h3-11,14-16H,12-13H2,1-2H3,(H2,33,37)(H2,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231267
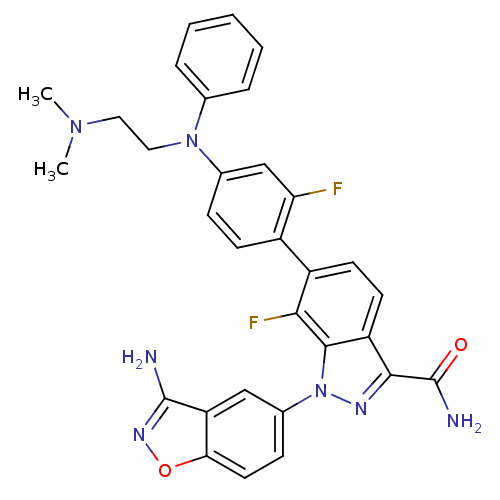
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1ccccc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C31H27F2N7O2/c1-38(2)14-15-39(18-6-4-3-5-7-18)19-8-10-21(25(32)17-19)22-11-12-23-28(31(35)41)36-40(29(23)27(22)33)20-9-13-26-24(16-20)30(34)37-42-26/h3-13,16-17H,14-15H2,1-2H3,(H2,34,37)(H2,35,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231266

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(3-dimethyla...)Show SMILES CN(C)Cc1cccnc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-4-3-11-34-25(16)15-5-7-18(22(30)12-15)19-8-9-20-26(29(33)39)35-38(27(20)24(19)31)17-6-10-23-21(13-17)28(32)36-40-23/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231273
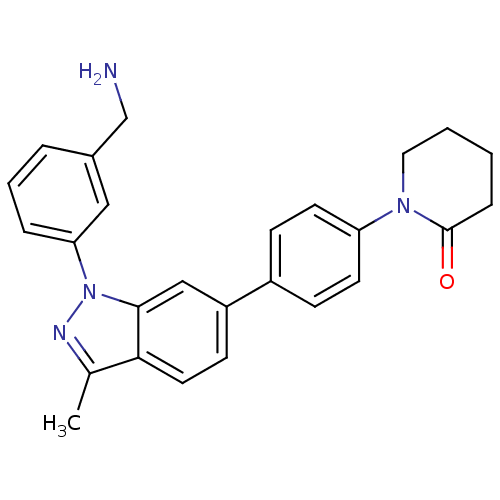
(1-{4-[1-(3-aminomethylphenyl)-3-methyl-1H-indazol-...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2cc(ccc12)-c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C26H26N4O/c1-18-24-13-10-21(16-25(24)30(28-18)23-6-4-5-19(15-23)17-27)20-8-11-22(12-9-20)29-14-3-2-7-26(29)31/h4-6,8-13,15-16H,2-3,7,14,17,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231265
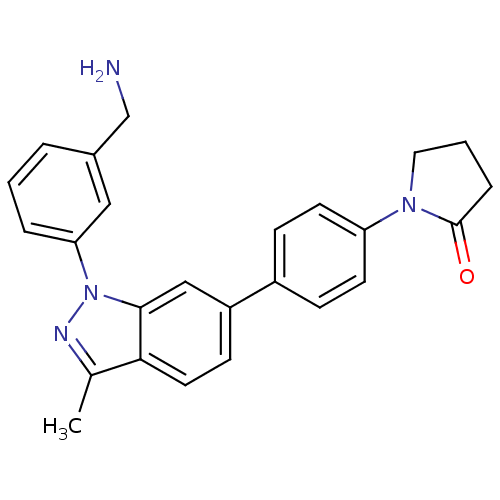
(1-{4-[1-(3-aminomethylphenyl)-3-methyl-1H-indazol-...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2cc(ccc12)-c1ccc(cc1)N1CCCC1=O Show InChI InChI=1S/C25H24N4O/c1-17-23-12-9-20(19-7-10-21(11-8-19)28-13-3-6-25(28)30)15-24(23)29(27-17)22-5-2-4-18(14-22)16-26/h2,4-5,7-12,14-15H,3,6,13,16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361211
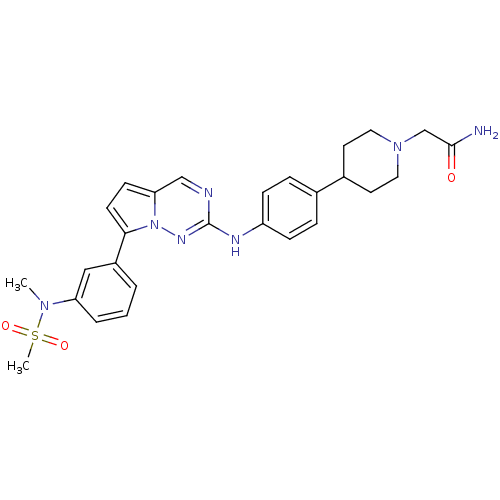
(CHEMBL1934340)Show SMILES CN(c1cccc(c1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(CC(N)=O)CC3)nn12)S(C)(=O)=O Show InChI InChI=1S/C27H31N7O3S/c1-32(38(2,36)37)23-5-3-4-21(16-23)25-11-10-24-17-29-27(31-34(24)25)30-22-8-6-19(7-9-22)20-12-14-33(15-13-20)18-26(28)35/h3-11,16-17,20H,12-15,18H2,1-2H3,(H2,28,35)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358895
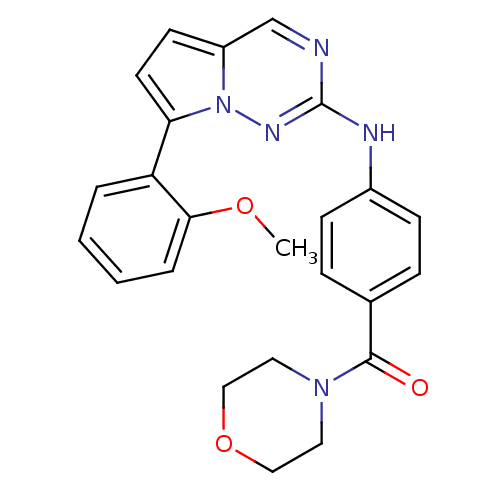
(CHEMBL1923571)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(cc3)C(=O)N3CCOCC3)nn12 Show InChI InChI=1S/C24H23N5O3/c1-31-22-5-3-2-4-20(22)21-11-10-19-16-25-24(27-29(19)21)26-18-8-6-17(7-9-18)23(30)28-12-14-32-15-13-28/h2-11,16H,12-15H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50353052

(CHEMBL1822515)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(cc3)N3CCN(C)CC3)nn12 Show InChI InChI=1S/C24H26N6O/c1-28-13-15-29(16-14-28)19-9-7-18(8-10-19)26-24-25-17-20-11-12-22(30(20)27-24)21-5-3-4-6-23(21)31-2/h3-12,17H,13-16H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50353052

(CHEMBL1822515)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(cc3)N3CCN(C)CC3)nn12 Show InChI InChI=1S/C24H26N6O/c1-28-13-15-29(16-14-28)19-9-7-18(8-10-19)26-24-25-17-20-11-12-22(30(20)27-24)21-5-3-4-6-23(21)31-2/h3-12,17H,13-16H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50353049
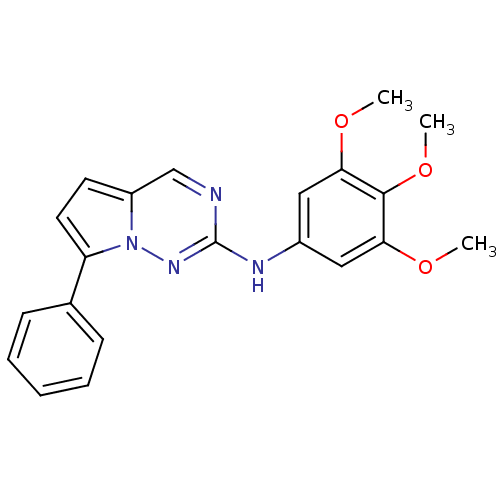
(CHEMBL1822511)Show SMILES COc1cc(Nc2ncc3ccc(-c4ccccc4)n3n2)cc(OC)c1OC Show InChI InChI=1S/C21H20N4O3/c1-26-18-11-15(12-19(27-2)20(18)28-3)23-21-22-13-16-9-10-17(25(16)24-21)14-7-5-4-6-8-14/h4-13H,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361210
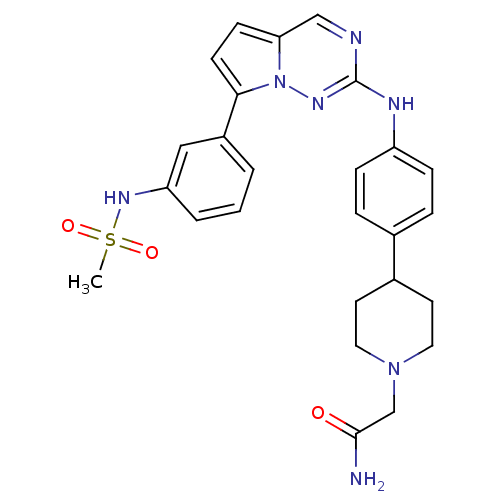
(CHEMBL1934339)Show SMILES CS(=O)(=O)Nc1cccc(c1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(CC(N)=O)CC3)nn12 Show InChI InChI=1S/C26H29N7O3S/c1-37(35,36)31-22-4-2-3-20(15-22)24-10-9-23-16-28-26(30-33(23)24)29-21-7-5-18(6-8-21)19-11-13-32(14-12-19)17-25(27)34/h2-10,15-16,19,31H,11-14,17H2,1H3,(H2,27,34)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
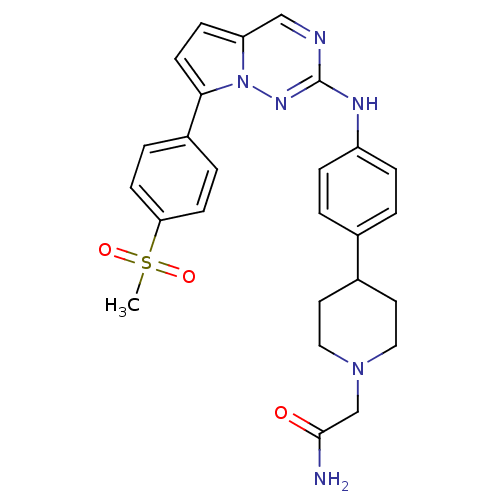
(Homo sapiens (Human)) | BDBM50361213
(CHEMBL1934342)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(CC(N)=O)CC3)nn12 Show InChI InChI=1S/C26H28N6O3S/c1-36(34,35)23-9-4-20(5-10-23)24-11-8-22-16-28-26(30-32(22)24)29-21-6-2-18(3-7-21)19-12-14-31(15-13-19)17-25(27)33/h2-11,16,19H,12-15,17H2,1H3,(H2,27,33)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358897

(CHEMBL1923573)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(cc3)N3CCOCC3)nn12 Show InChI InChI=1S/C23H23N5O2/c1-29-22-5-3-2-4-20(22)21-11-10-19-16-24-23(26-28(19)21)25-17-6-8-18(9-7-17)27-12-14-30-15-13-27/h2-11,16H,12-15H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
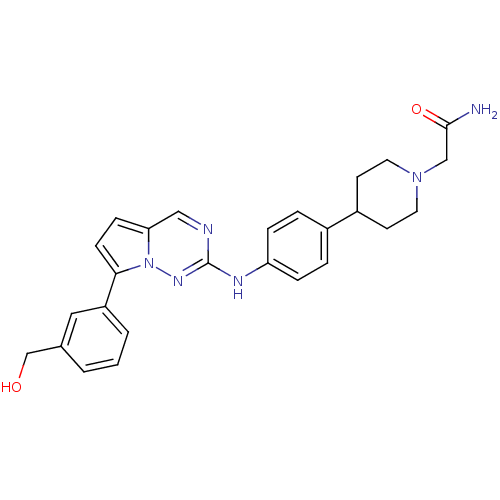
(Homo sapiens (Human)) | BDBM50361212
(CHEMBL1934341)Show SMILES NC(=O)CN1CCC(CC1)c1ccc(Nc2ncc3ccc(-c4cccc(CO)c4)n3n2)cc1 Show InChI InChI=1S/C26H28N6O2/c27-25(34)16-31-12-10-20(11-13-31)19-4-6-22(7-5-19)29-26-28-15-23-8-9-24(32(23)30-26)21-3-1-2-18(14-21)17-33/h1-9,14-15,20,33H,10-13,16-17H2,(H2,27,34)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
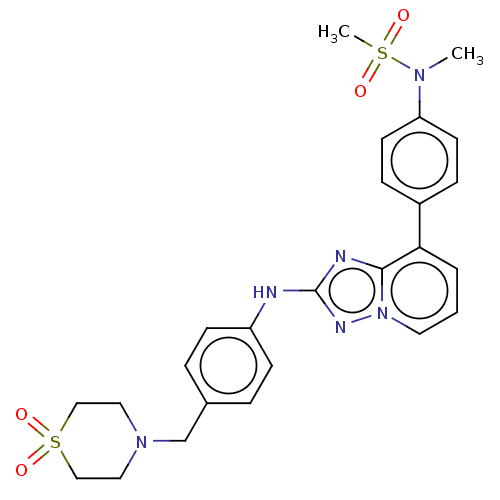
(Homo sapiens (Human)) | BDBM99618
(US8501936, 119)Show SMILES CN(c1ccc(cc1)-c1cccn2nc(Nc3ccc(CN4CCS(=O)(=O)CC4)cc3)nc12)S(C)(=O)=O Show InChI InChI=1S/C25H28N6O4S2/c1-29(36(2,32)33)22-11-7-20(8-12-22)23-4-3-13-31-24(23)27-25(28-31)26-21-9-5-19(6-10-21)18-30-14-16-37(34,35)17-15-30/h3-13H,14-18H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
US Patent
| Assay Description
The ability of compounds to inhibit the kinase activity of baculovirus-expressed human FAK and JAK kinase using the time-resolved fluorescence (TRF) ... |
US Patent US8501936 (2013)
BindingDB Entry DOI: 10.7270/Q22Z1455 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
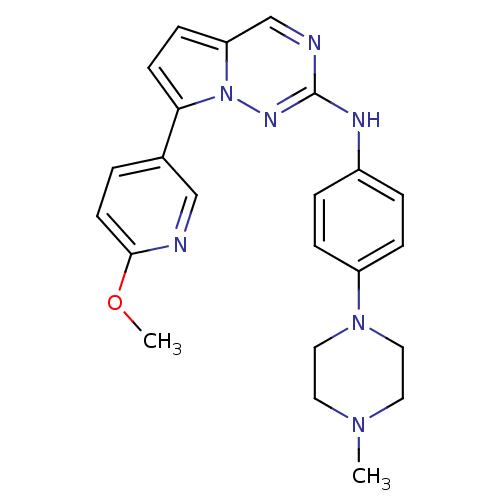
(Homo sapiens (Human)) | BDBM50358912
(CHEMBL1923588)Show SMILES COc1ccc(cn1)-c1ccc2cnc(Nc3ccc(cc3)N3CCN(C)CC3)nn12 Show InChI InChI=1S/C23H25N7O/c1-28-11-13-29(14-12-28)19-6-4-18(5-7-19)26-23-25-16-20-8-9-21(30(20)27-23)17-3-10-22(31-2)24-15-17/h3-10,15-16H,11-14H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358909

(CHEMBL1923585)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3ccc(-c4cccc(c4)S(=O)(=O)NC(C)(C)C)n3n2)cc1 Show InChI InChI=1S/C27H33N7O2S/c1-27(2,3)31-37(35,36)24-7-5-6-20(18-24)25-13-12-23-19-28-26(30-34(23)25)29-21-8-10-22(11-9-21)33-16-14-32(4)15-17-33/h5-13,18-19,31H,14-17H2,1-4H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358907
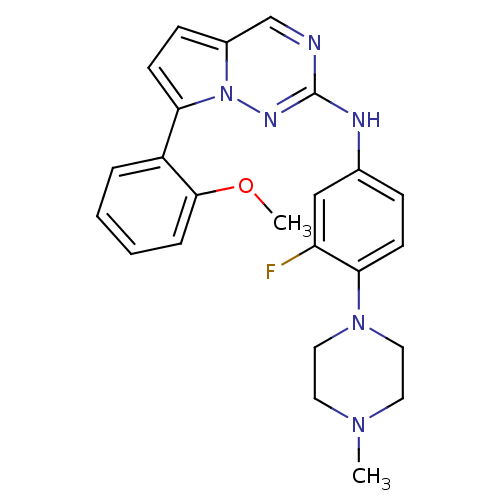
(CHEMBL1923583)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(N4CCN(C)CC4)c(F)c3)nn12 Show InChI InChI=1S/C24H25FN6O/c1-29-11-13-30(14-12-29)22-9-7-17(15-20(22)25)27-24-26-16-18-8-10-21(31(18)28-24)19-5-3-4-6-23(19)32-2/h3-10,15-16H,11-14H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358899
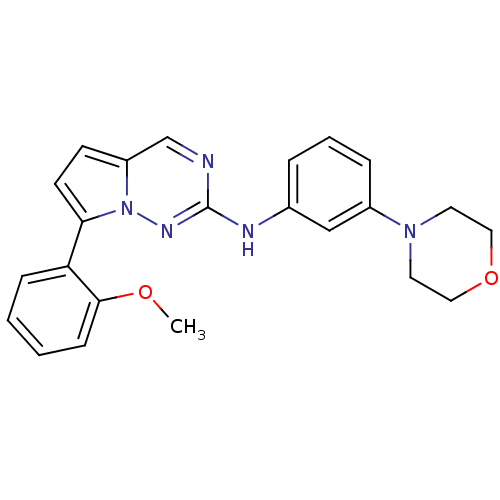
(CHEMBL1923575)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3cccc(c3)N3CCOCC3)nn12 Show InChI InChI=1S/C23H23N5O2/c1-29-22-8-3-2-7-20(22)21-10-9-19-16-24-23(26-28(19)21)25-17-5-4-6-18(15-17)27-11-13-30-14-12-27/h2-10,15-16H,11-14H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358904

(CHEMBL1923580)Show SMILES C[C@H](O)CN1CCN(CC1)c1ccc(Nc2ncc3ccc(-c4cccc(c4)S(=O)(=O)NC(C)(C)C)n3n2)cc1F |r| Show InChI InChI=1S/C29H36FN7O3S/c1-20(38)19-35-12-14-36(15-13-35)27-10-8-22(17-25(27)30)32-28-31-18-23-9-11-26(37(23)33-28)21-6-5-7-24(16-21)41(39,40)34-29(2,3)4/h5-11,16-18,20,34,38H,12-15,19H2,1-4H3,(H,32,33)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358914

(CHEMBL1923590)Show SMILES COc1ccc(cn1)-c1ccc2cnc(Nc3ccc(N4CCN(C)CC4)c(Cl)c3)nn12 Show InChI InChI=1S/C23H24ClN7O/c1-29-9-11-30(12-10-29)21-6-4-17(13-19(21)24)27-23-26-15-18-5-7-20(31(18)28-23)16-3-8-22(32-2)25-14-16/h3-8,13-15H,9-12H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358913

(CHEMBL1923589)Show SMILES COc1ccc(cn1)-c1ccc2cnc(Nc3ccc(N4CCN(C)CC4)c(F)c3)nn12 Show InChI InChI=1S/C23H24FN7O/c1-29-9-11-30(12-10-29)21-6-4-17(13-19(21)24)27-23-26-15-18-5-7-20(31(18)28-23)16-3-8-22(32-2)25-14-16/h3-8,13-15H,9-12H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM99529
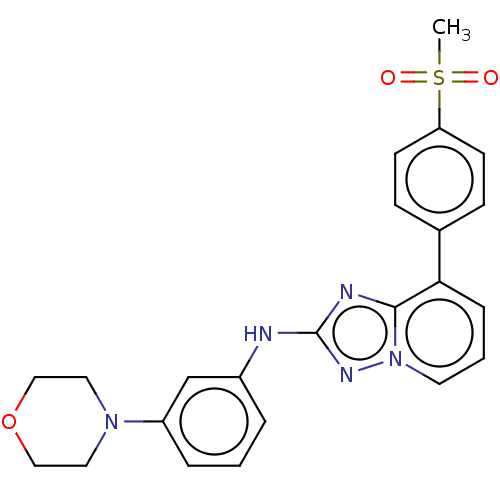
(CHEMBL2062802 | US8501936, 27)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cccn2nc(Nc3cccc(c3)N3CCOCC3)nc12 Show InChI InChI=1S/C23H23N5O3S/c1-32(29,30)20-9-7-17(8-10-20)21-6-3-11-28-22(21)25-23(26-28)24-18-4-2-5-19(16-18)27-12-14-31-15-13-27/h2-11,16H,12-15H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
US Patent
| Assay Description
The ability of compounds to inhibit the kinase activity of baculovirus-expressed human FAK and JAK kinase using the time-resolved fluorescence (TRF) ... |
US Patent US8501936 (2013)
BindingDB Entry DOI: 10.7270/Q22Z1455 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM99683

(US8501936, 184)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cccn2nc(Nc3cccc(c3)C3CCN(CC(N)=O)CC3)nc12 Show InChI InChI=1S/C26H28N6O3S/c1-36(34,35)22-9-7-19(8-10-22)23-6-3-13-32-25(23)29-26(30-32)28-21-5-2-4-20(16-21)18-11-14-31(15-12-18)17-24(27)33/h2-10,13,16,18H,11-12,14-15,17H2,1H3,(H2,27,33)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
US Patent
| Assay Description
The ability of compounds to inhibit the kinase activity of baculovirus-expressed human FAK and JAK kinase using the time-resolved fluorescence (TRF) ... |
US Patent US8501936 (2013)
BindingDB Entry DOI: 10.7270/Q22Z1455 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM99689
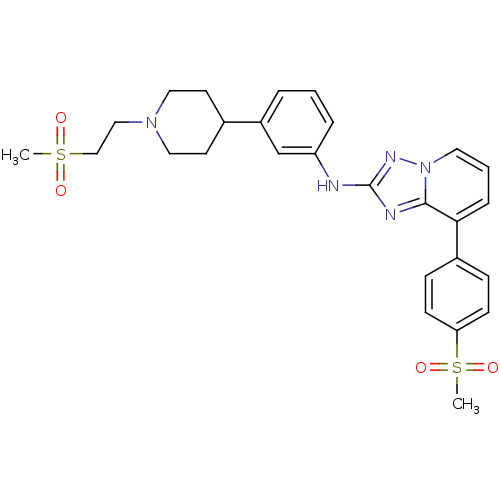
(US8501936, 190)Show SMILES CS(=O)(=O)CCN1CCC(CC1)c1cccc(Nc2nc3c(cccn3n2)-c2ccc(cc2)S(C)(=O)=O)c1 Show InChI InChI=1S/C27H31N5O4S2/c1-37(33,34)18-17-31-15-12-20(13-16-31)22-5-3-6-23(19-22)28-27-29-26-25(7-4-14-32(26)30-27)21-8-10-24(11-9-21)38(2,35)36/h3-11,14,19-20H,12-13,15-18H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
US Patent
| Assay Description
The ability of compounds to inhibit the kinase activity of baculovirus-expressed human FAK and JAK kinase using the time-resolved fluorescence (TRF) ... |
US Patent US8501936 (2013)
BindingDB Entry DOI: 10.7270/Q22Z1455 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358898

(CHEMBL1923574)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(cc3)N3CCN(C[C@H](C)O)CC3)nn12 |r| Show InChI InChI=1S/C26H30N6O2/c1-19(33)18-30-13-15-31(16-14-30)21-9-7-20(8-10-21)28-26-27-17-22-11-12-24(32(22)29-26)23-5-3-4-6-25(23)34-2/h3-12,17,19,33H,13-16,18H2,1-2H3,(H,28,29)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358894

(CHEMBL1923570)Show InChI InChI=1S/C20H18N4O2/c1-25-16-10-7-14(8-11-16)22-20-21-13-15-9-12-18(24(15)23-20)17-5-3-4-6-19(17)26-2/h3-13H,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358903
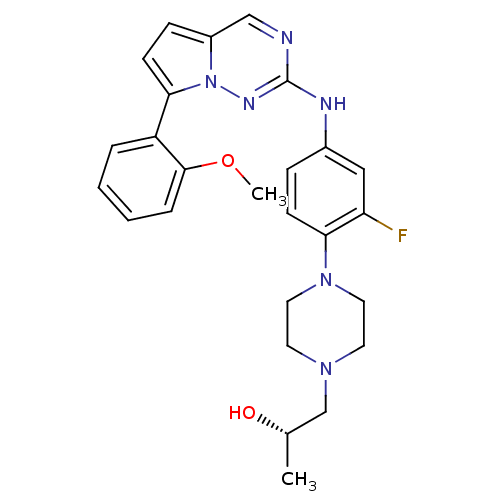
(CHEMBL1923579)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(N4CCN(C[C@H](C)O)CC4)c(F)c3)nn12 |r| Show InChI InChI=1S/C26H29FN6O2/c1-18(34)17-31-11-13-32(14-12-31)24-9-7-19(15-22(24)27)29-26-28-16-20-8-10-23(33(20)30-26)21-5-3-4-6-25(21)35-2/h3-10,15-16,18,34H,11-14,17H2,1-2H3,(H,29,30)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358893

(CHEMBL1923594)Show SMILES COc1ccc(cn1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(C[C@H](C)O)CC3)nn12 |r| Show InChI InChI=1S/C26H30N6O2/c1-18(33)17-31-13-11-20(12-14-31)19-3-6-22(7-4-19)29-26-28-16-23-8-9-24(32(23)30-26)21-5-10-25(34-2)27-15-21/h3-10,15-16,18,20,33H,11-14,17H2,1-2H3,(H,29,30)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358908
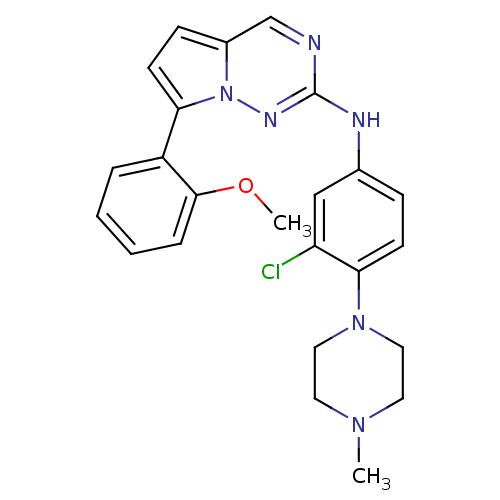
(CHEMBL1923584)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3ccc(N4CCN(C)CC4)c(Cl)c3)nn12 Show InChI InChI=1S/C24H25ClN6O/c1-29-11-13-30(14-12-29)22-9-7-17(15-20(22)25)27-24-26-16-18-8-10-21(31(18)28-24)19-5-3-4-6-23(19)32-2/h3-10,15-16H,11-14H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM99710

(US8501936, 212)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)n1cc(Nc2nc3c(cccn3n2)-c2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C26H31N7O4S/c1-26(2,3)37-25(34)31-14-11-20(12-15-31)33-17-19(16-27-33)28-24-29-23-22(6-5-13-32(23)30-24)18-7-9-21(10-8-18)38(4,35)36/h5-10,13,16-17,20H,11-12,14-15H2,1-4H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
US Patent
| Assay Description
The ability of compounds to inhibit the kinase activity of baculovirus-expressed human FAK and JAK kinase using the time-resolved fluorescence (TRF) ... |
US Patent US8501936 (2013)
BindingDB Entry DOI: 10.7270/Q22Z1455 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50361214
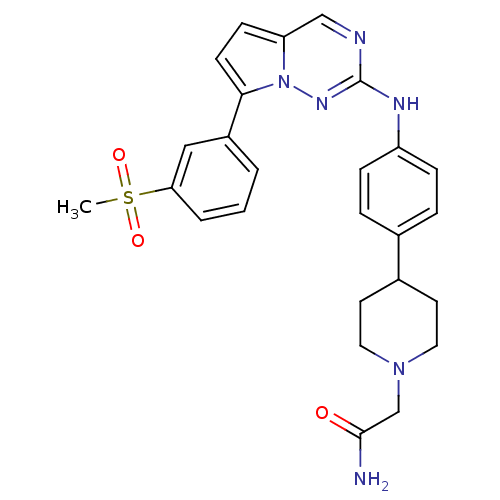
(CHEMBL1934343)Show SMILES CS(=O)(=O)c1cccc(c1)-c1ccc2cnc(Nc3ccc(cc3)C3CCN(CC(N)=O)CC3)nn12 Show InChI InChI=1S/C26H28N6O3S/c1-36(34,35)23-4-2-3-20(15-23)24-10-9-22-16-28-26(30-32(22)24)29-21-7-5-18(6-8-21)19-11-13-31(14-12-19)17-25(27)33/h2-10,15-16,19H,11-14,17H2,1H3,(H2,27,33)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 expressed in baculovirus expression system after 20 mins by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 133-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.049
BindingDB Entry DOI: 10.7270/Q2C829R4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50358901

(CHEMBL1923577)Show SMILES COc1ccccc1-c1ccc2cnc(Nc3cccc(c3)N3CCN(C[C@H](C)O)CC3)nn12 |r| Show InChI InChI=1S/C26H30N6O2/c1-19(33)18-30-12-14-31(15-13-30)21-7-5-6-20(16-21)28-26-27-17-22-10-11-24(32(22)29-26)23-8-3-4-9-25(23)34-2/h3-11,16-17,19,33H,12-15,18H2,1-2H3,(H,28,29)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant JAK2 using biotinyl-amino-hexanoyl-EQEDEPEGDYFEWLE-amide as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 7325-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.032
BindingDB Entry DOI: 10.7270/Q2GT5NM2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM99620
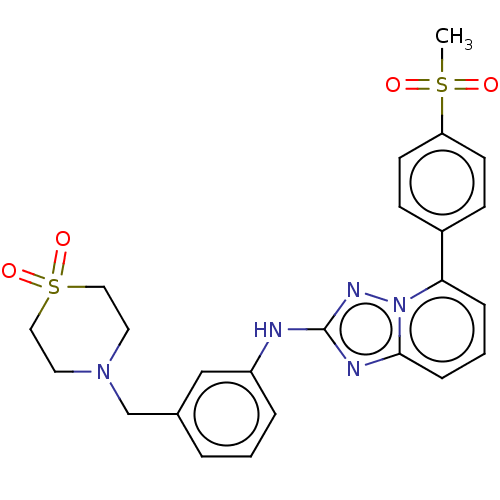
(US8501936, 121)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cccc2nc(Nc3cccc(CN4CCS(=O)(=O)CC4)c3)nn12 Show InChI InChI=1S/C24H25N5O4S2/c1-34(30,31)21-10-8-19(9-11-21)22-6-3-7-23-26-24(27-29(22)23)25-20-5-2-4-18(16-20)17-28-12-14-35(32,33)15-13-28/h2-11,16H,12-15,17H2,1H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
US Patent
| Assay Description
The ability of compounds to inhibit the kinase activity of baculovirus-expressed human FAK and JAK kinase using the time-resolved fluorescence (TRF) ... |
US Patent US8501936 (2013)
BindingDB Entry DOI: 10.7270/Q22Z1455 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data