

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50167296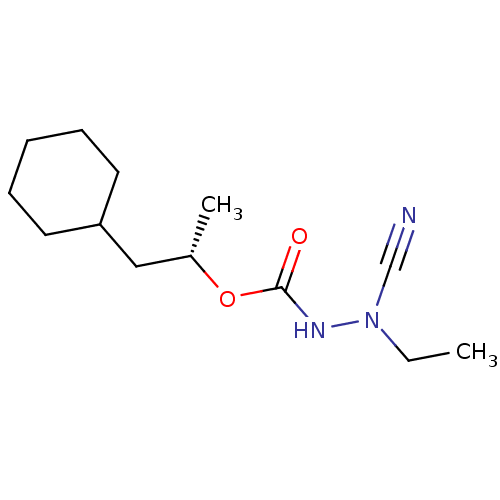 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-ethylhyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167302 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isobutyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167295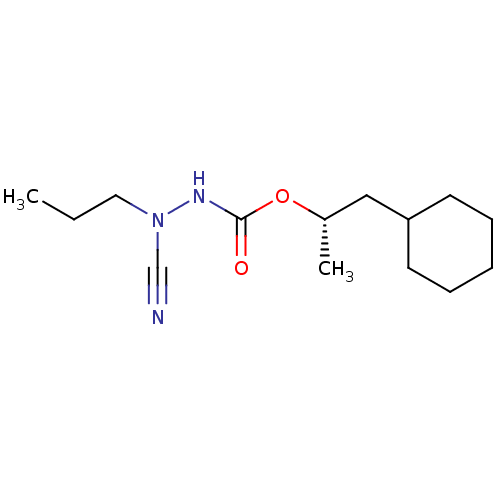 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-propylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167298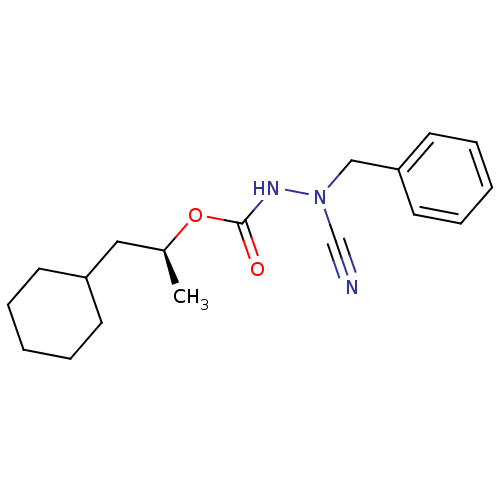 ((1S)-2-cyclohexyl-1-methylethyl 2-benzyl-2-cyanohy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167303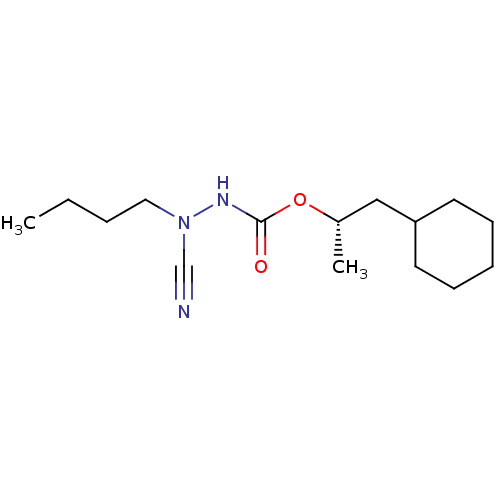 ((1S)-2-cyclohexyl-1-methylethyl 2-butyl-2-cyanohyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167289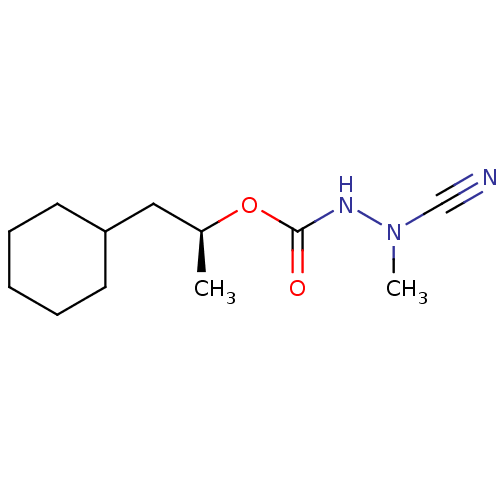 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167290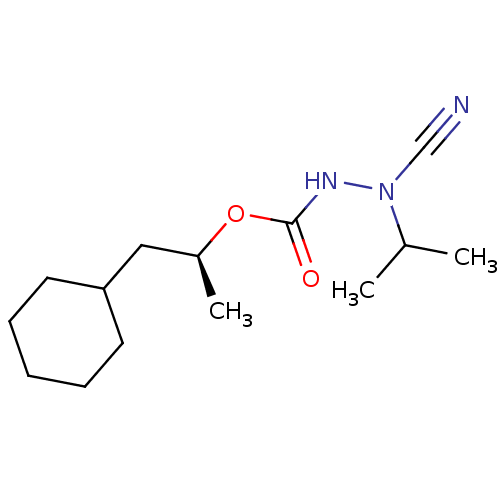 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167289 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50417287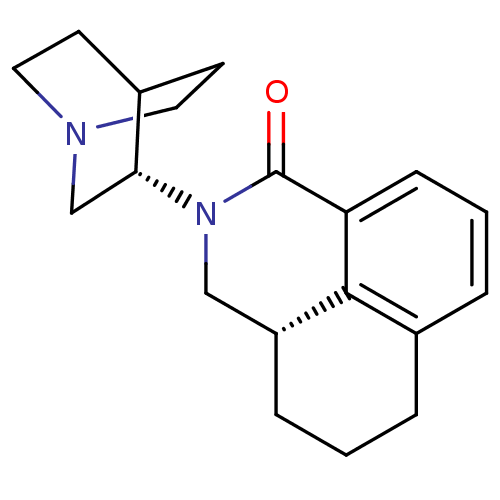 (Aloxi | Aurothioglucose | PALONOSETRON | PALONOSET...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against rat cathepsin K | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50167289 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427363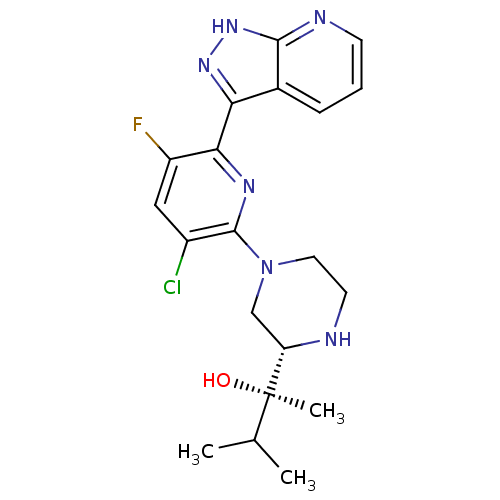 (CHEMBL2326002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427367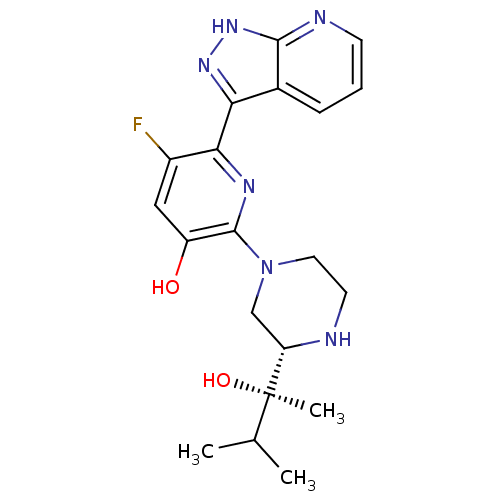 (CHEMBL2325998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427364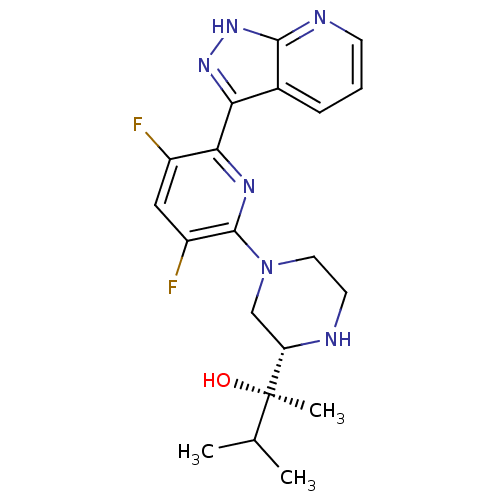 (CHEMBL2326001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427365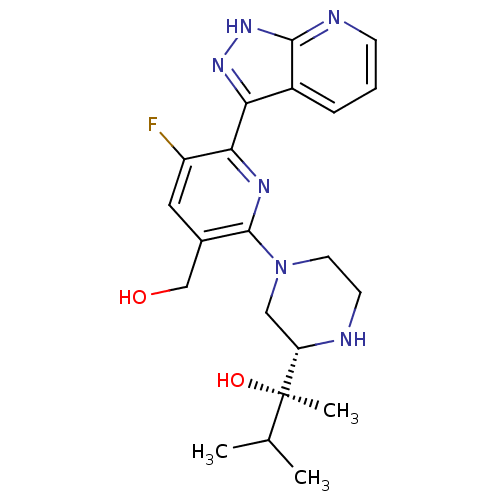 (CHEMBL2326000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000453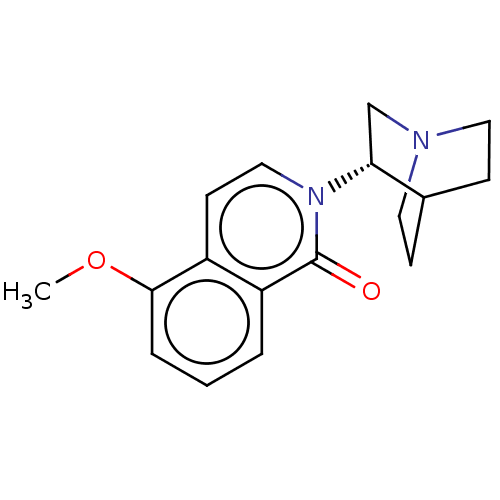 (CHEMBL540055) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000523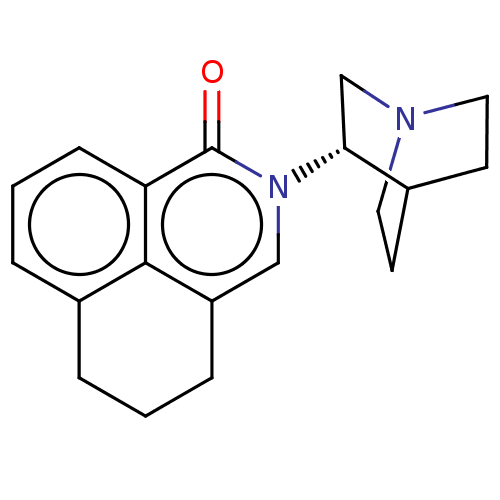 (CHEMBL544784) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427370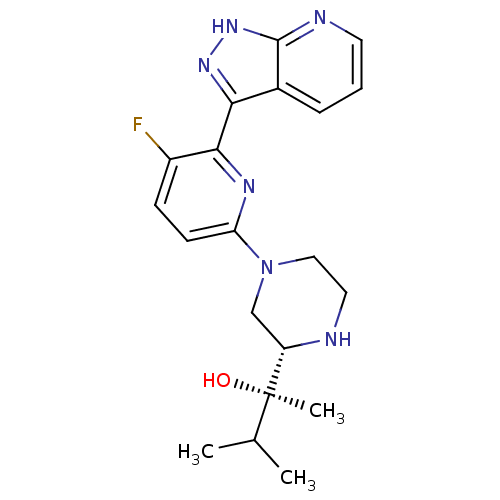 (CHEMBL2326007) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525600 (CHEMBL4469412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000471 (CHEMBL542900) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000450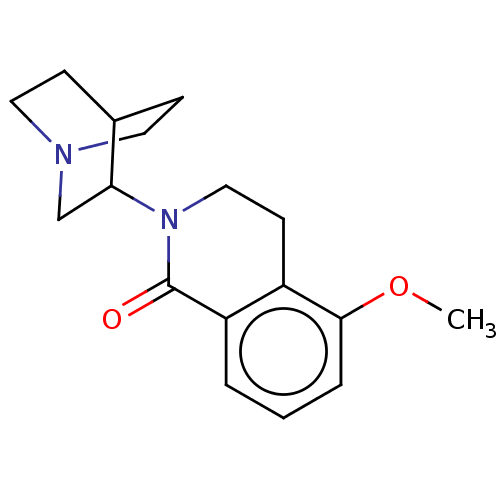 (CHEMBL542669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000457 (CHEMBL542904) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50056419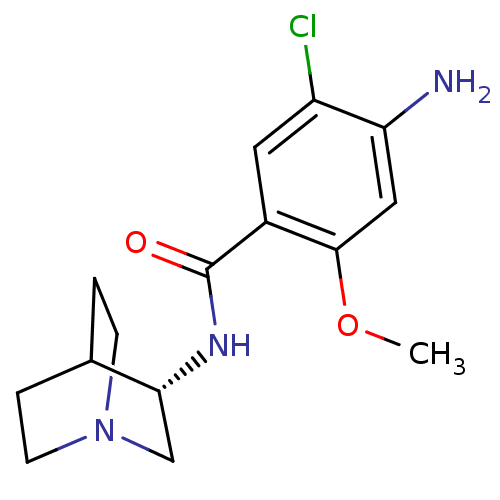 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50271820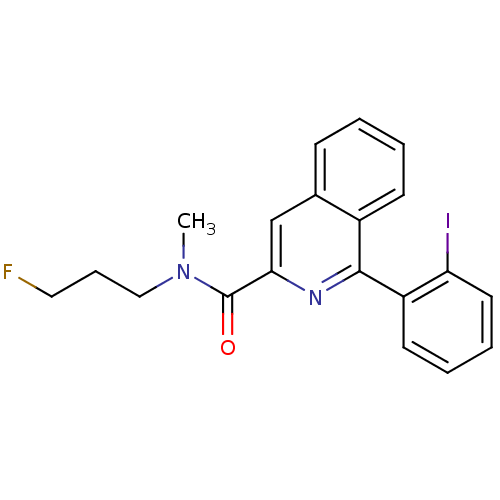 (1-(2-iodophenyl)-N-methyl-N-(3-fluoropropyl)-3-iso...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from PBR in rat kidney mitochondrial membrane | Bioorg Med Chem 16: 6145-55 (2008) Article DOI: 10.1016/j.bmc.2008.04.046 BindingDB Entry DOI: 10.7270/Q26Q1Z4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50411736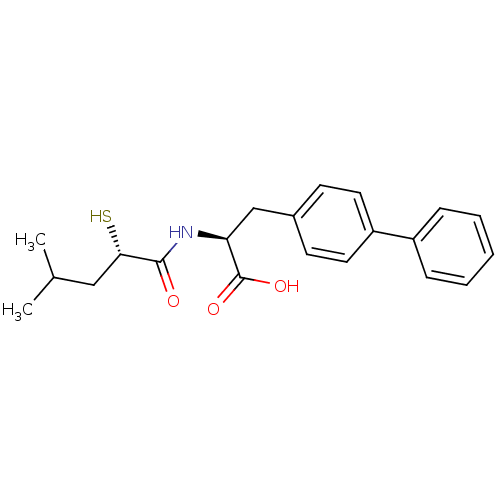 (CHEMBL271225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP by fluorescence assay | Bioorg Med Chem Lett 18: 732-7 (2008) Article DOI: 10.1016/j.bmcl.2007.11.048 BindingDB Entry DOI: 10.7270/Q2GT5PCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525603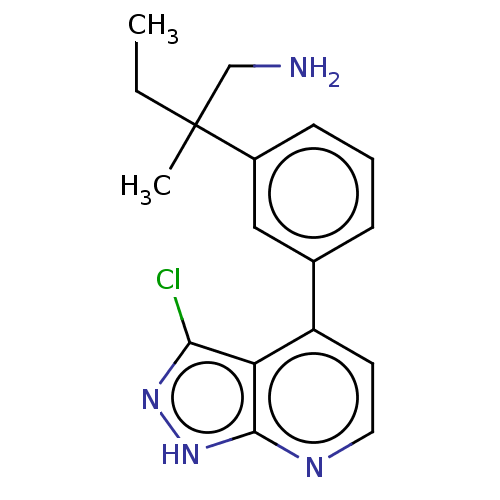 (CHEMBL4528271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000475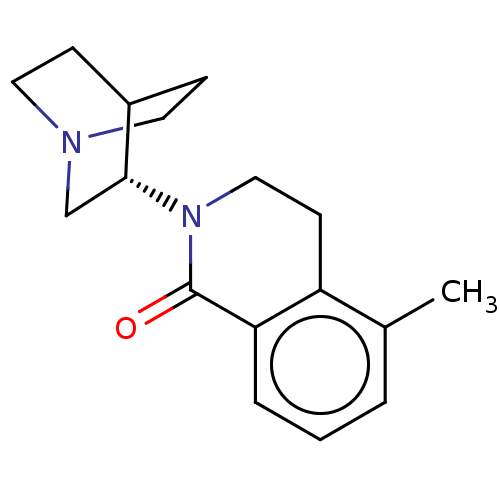 (CHEMBL555068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000460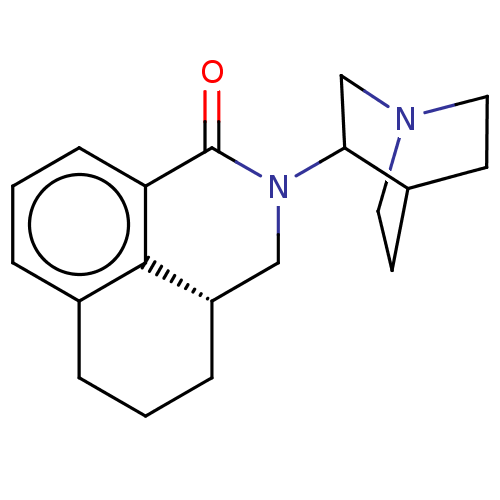 (CHEMBL540057) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525602 (CHEMBL4569479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000469 (CHEMBL542903) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000455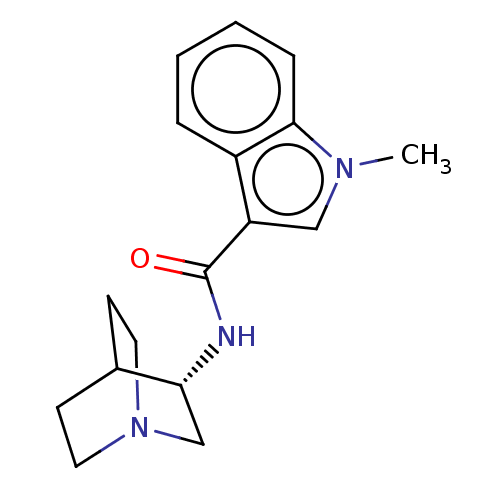 (CHEMBL2093898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50167290 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin H using L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000462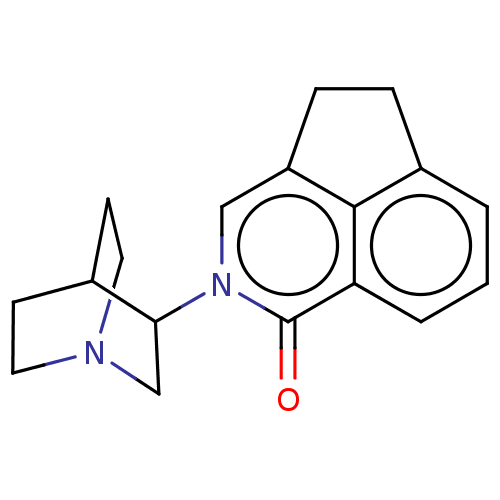 (CHEMBL545719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000445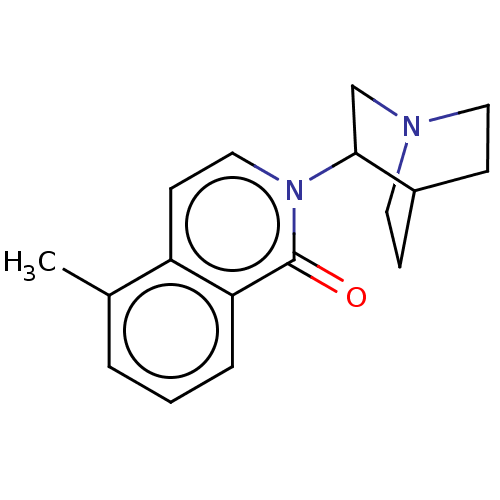 (CHEMBL555038) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471981 (CHEMBL446629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471983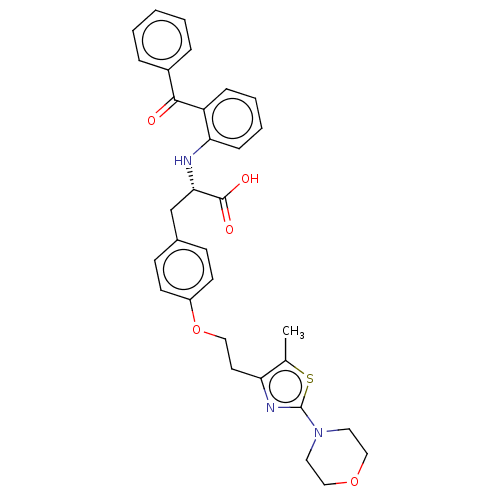 (CHEMBL149876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50449636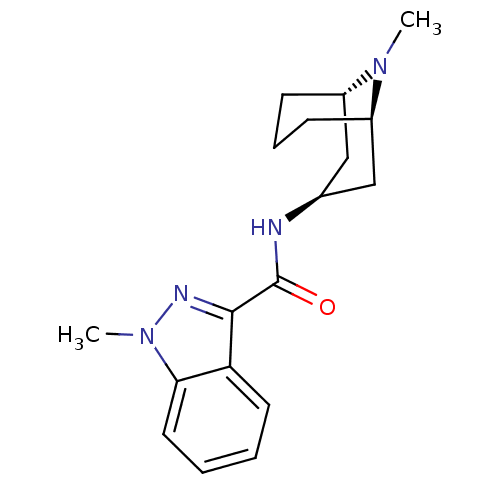 (BRL-43694 | GRANISETRON | Kytril | LY-278584 | San...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167290 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000446 (CHEMBL88565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50277679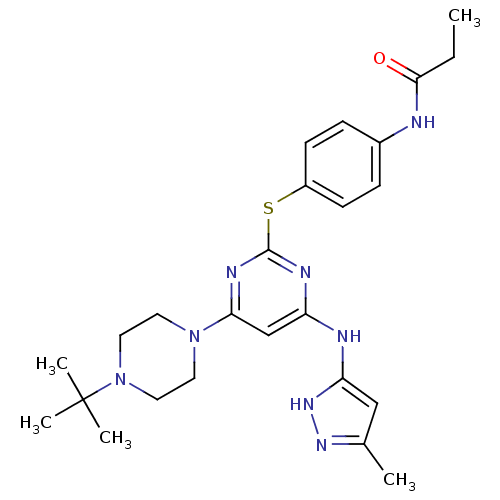 (CHEMBL484006 | N-(4-(4-(4-tert-butylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-B by time dependent kinetic study | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50411731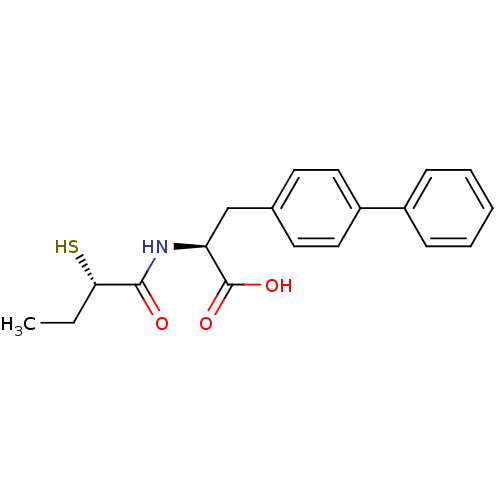 (CHEMBL257726) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP by fluorescence assay | Bioorg Med Chem Lett 18: 732-7 (2008) Article DOI: 10.1016/j.bmcl.2007.11.048 BindingDB Entry DOI: 10.7270/Q2GT5PCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135453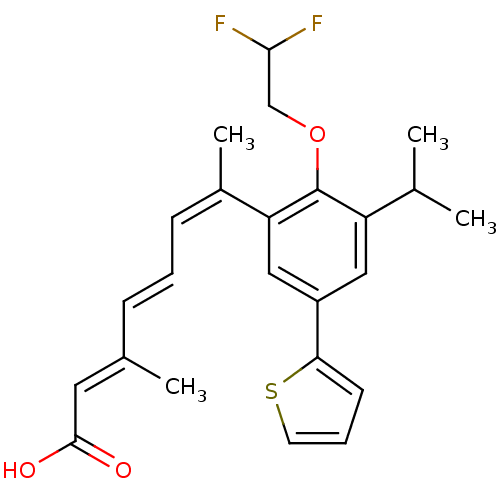 ((2E,4E,6Z)-7-[2-(2,2-Difluoro-ethoxy)-3-isopropyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135460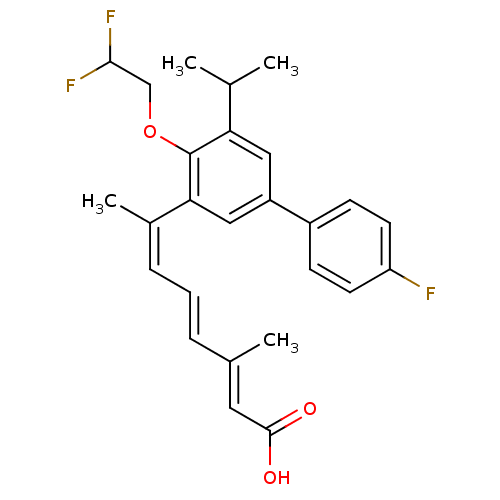 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-4'-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471980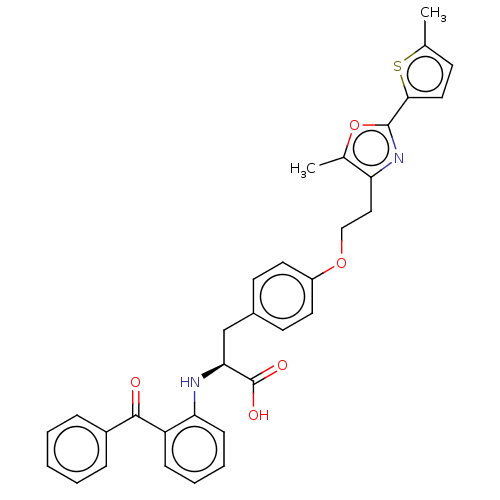 (CHEMBL147095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471978 (CHEMBL147090) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7310 total ) | Next | Last >> |