

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM50336997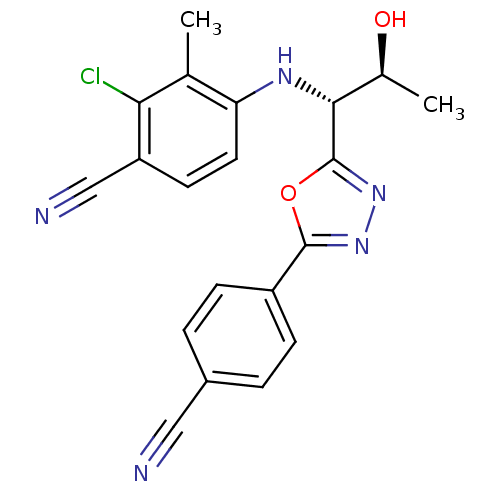 (2-chloro-4-((1R,2S)-1-(5-(4-cyanophenyl)-1,3,4-oxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay | ACS Med Chem Lett 2: 124-129 (2011) Article DOI: 10.1021/ml1002508 BindingDB Entry DOI: 10.7270/Q2JQ119N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay | ACS Med Chem Lett 2: 124-129 (2011) Article DOI: 10.1021/ml1002508 BindingDB Entry DOI: 10.7270/Q2JQ119N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM8885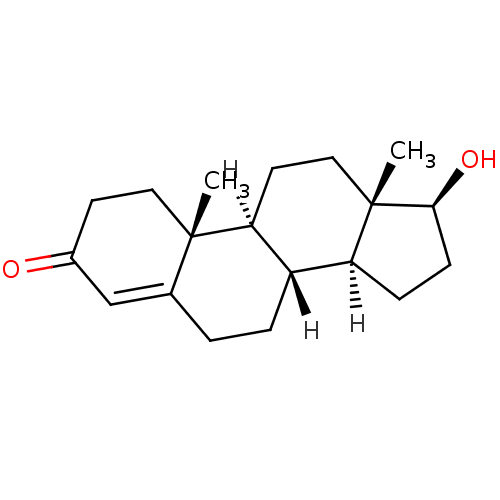 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay | ACS Med Chem Lett 2: 124-129 (2011) Article DOI: 10.1021/ml1002508 BindingDB Entry DOI: 10.7270/Q2JQ119N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112830 (US8629167, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112818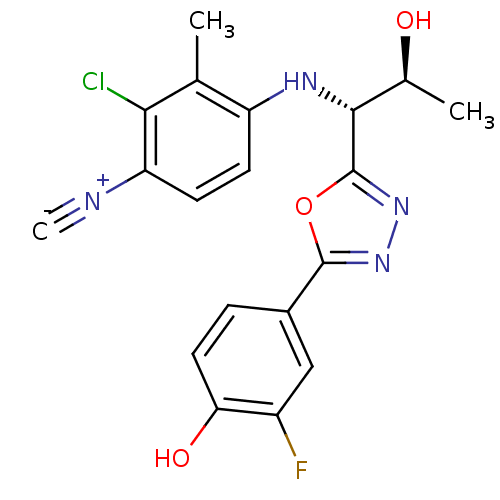 (US8629167, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112828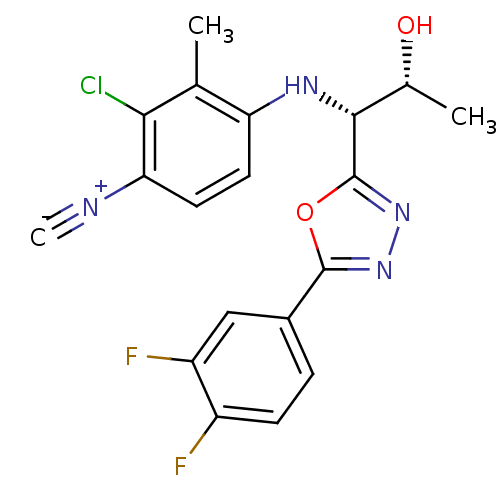 (US8629167, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112829 (US8629167, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM8903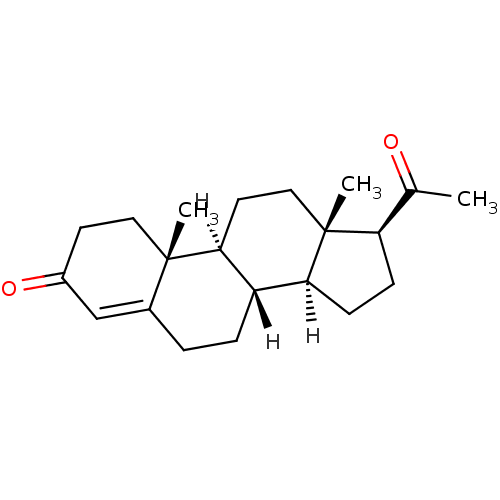 ((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | ACS Med Chem Lett 2: 124-129 (2011) Article DOI: 10.1021/ml1002508 BindingDB Entry DOI: 10.7270/Q2JQ119N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112826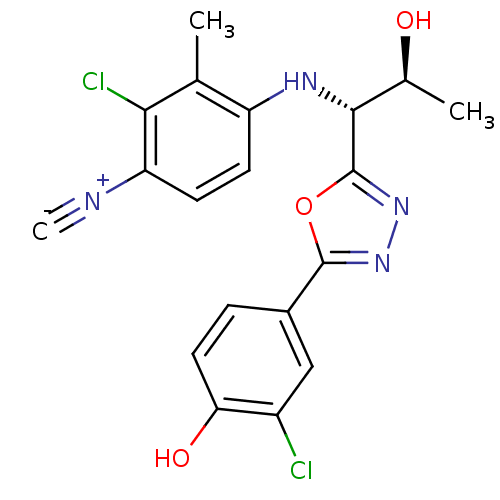 (US8629167, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112824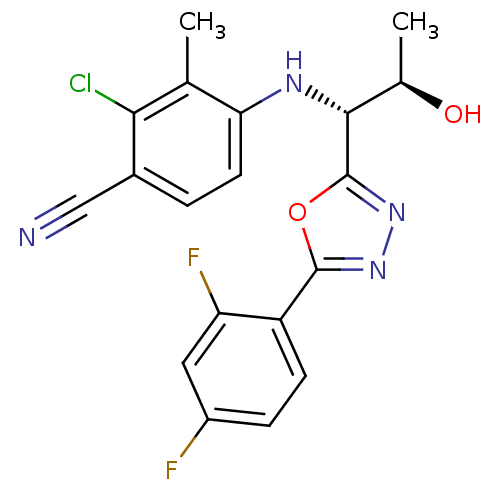 (US8629167, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112819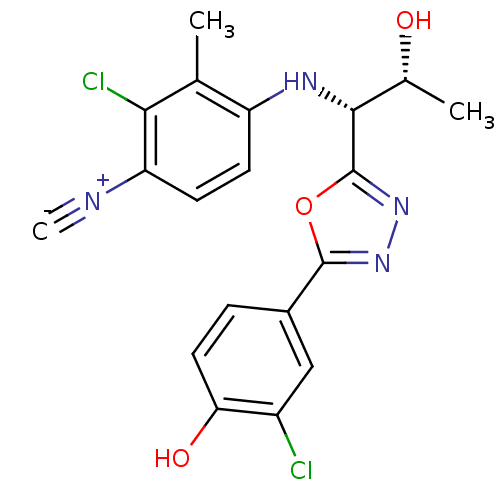 (US8629167, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152609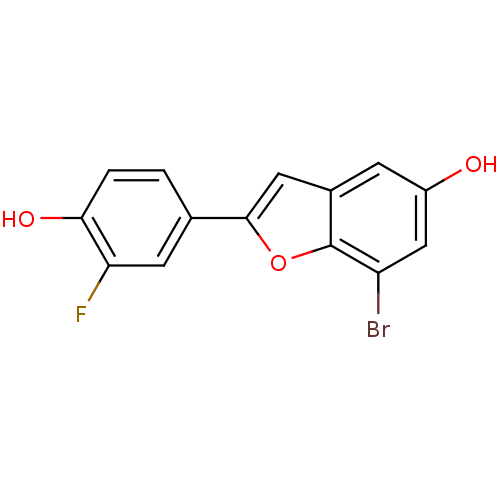 (7-Bromo-2-(3-fluoro-4-hydroxy-phenyl)-benzofuran-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112797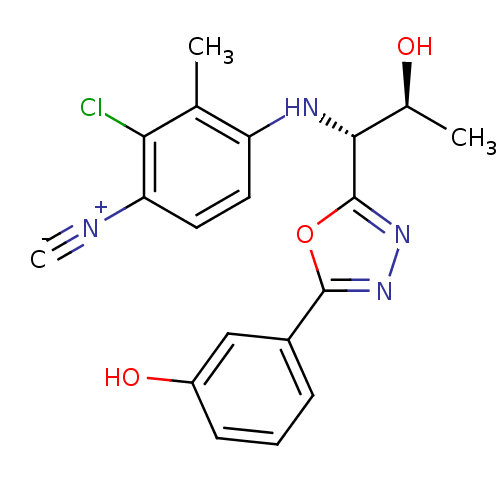 (US8629167, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50169743 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Antagonist effect on transcriptional activation in MCF-7 cells expressing estrogen receptor alpha | Bioorg Med Chem Lett 10: 147-51 (2000) BindingDB Entry DOI: 10.7270/Q2JH3MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112805 (US8629167, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152616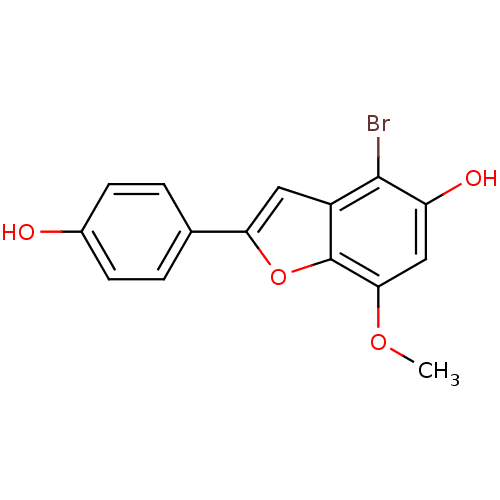 (4-Bromo-2-(4-hydroxy-phenyl)-7-methoxy-benzofuran-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112812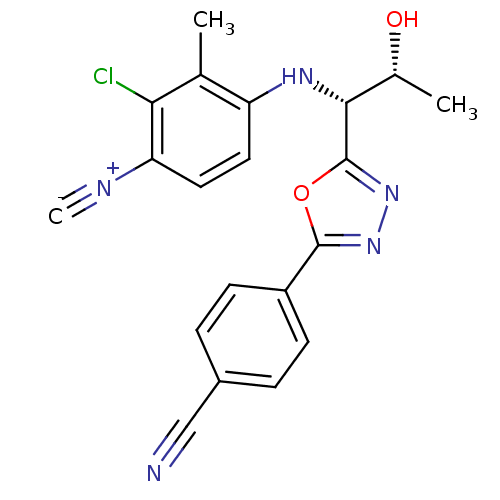 (US8629167, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiol | J Med Chem 44: 1654-7 (2001) BindingDB Entry DOI: 10.7270/Q2F47NDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Antagonist effect on transcriptional activation in MCF-7 cells expressing estrogen receptor alpha | Bioorg Med Chem Lett 10: 147-51 (2000) BindingDB Entry DOI: 10.7270/Q2JH3MQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112853 (US8629167, 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112852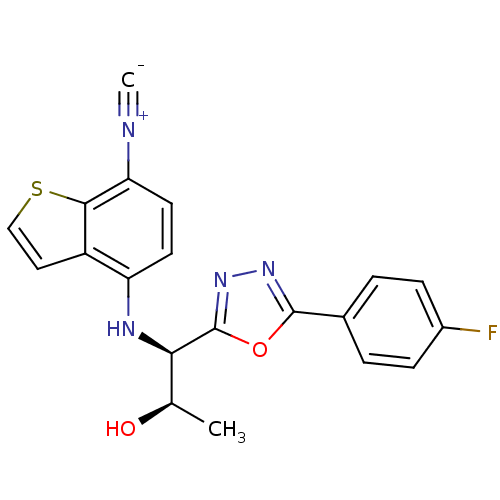 (US8629167, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM594883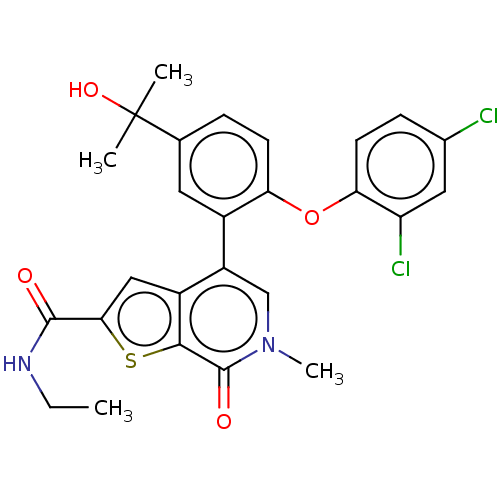 (US11584756, Compound 2 | US11584756, Example S-2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... | Citation and Details BindingDB Entry DOI: 10.7270/Q2K0786J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112865 (US8629167, 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112867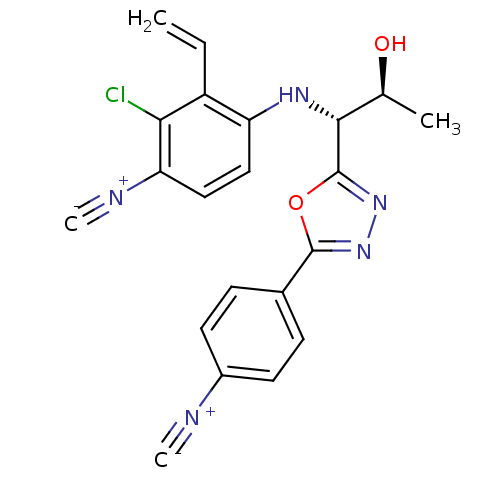 (US8629167, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112882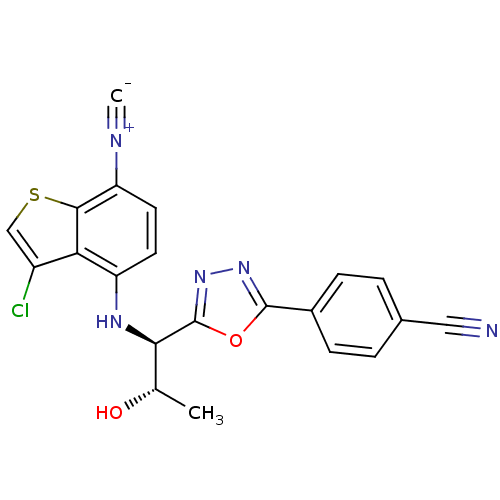 (US8629167, 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112836 (US8629167, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112841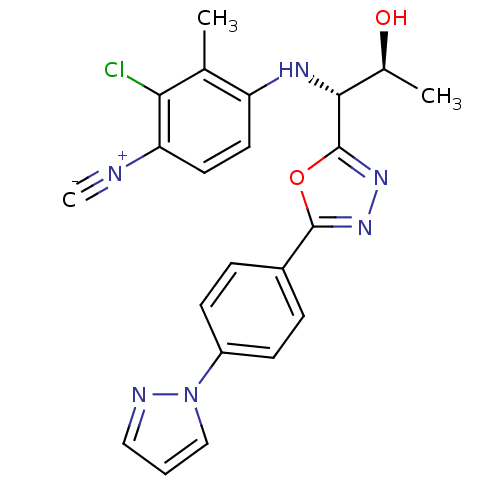 (US8629167, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112842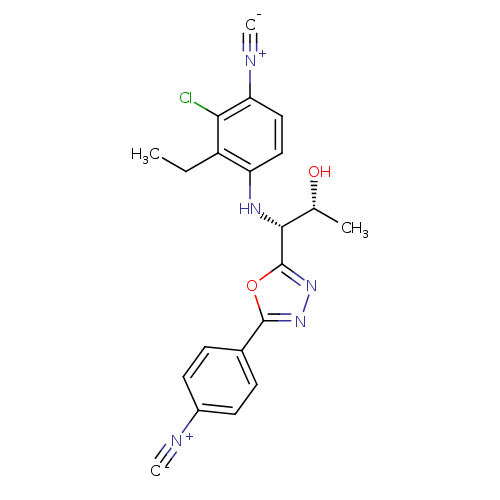 (US8629167, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112851 (US8629167, 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM112849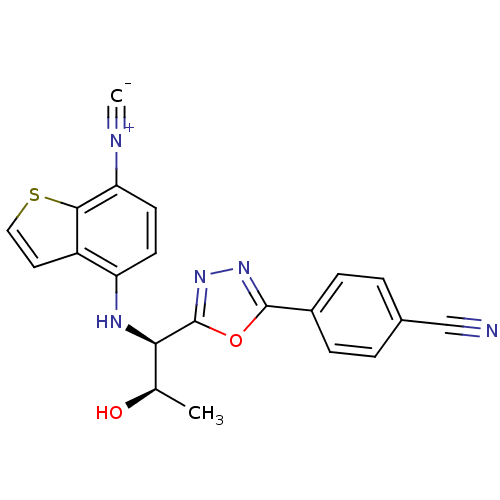 (US8629167, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Radius Health, Inc. US Patent | Assay Description In order to demonstrate the utility of the compounds of this invention, an androgen receptor binding assay was performed wherein many of the compound... | US Patent US8629167 (2014) BindingDB Entry DOI: 10.7270/Q2GT5KTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152628 (2-(3-Fluoro-4-hydroxy-phenyl)-5-hydroxy-benzofuran...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM594901 (US11584756, Compound 150 | US11584756, Example S-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... | Citation and Details BindingDB Entry DOI: 10.7270/Q2K0786J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM50154088 (7-Bromo-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | 7-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to rat ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50166755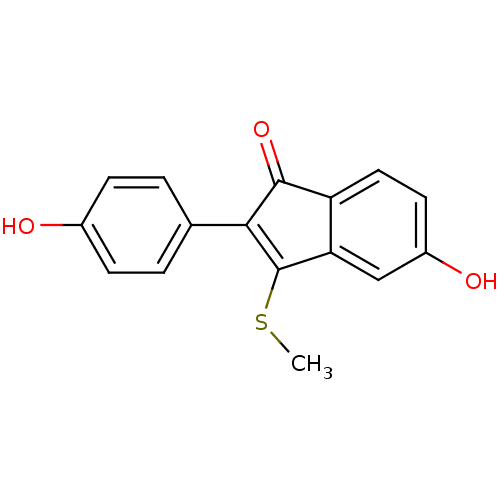 (5-Hydroxy-2-(4-hydroxy-phenyl)-3-methylsulfanyl-in...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human estrogen receptor beta | Bioorg Med Chem Lett 15: 3137-42 (2005) Article DOI: 10.1016/j.bmcl.2005.04.013 BindingDB Entry DOI: 10.7270/Q25Q4VMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154059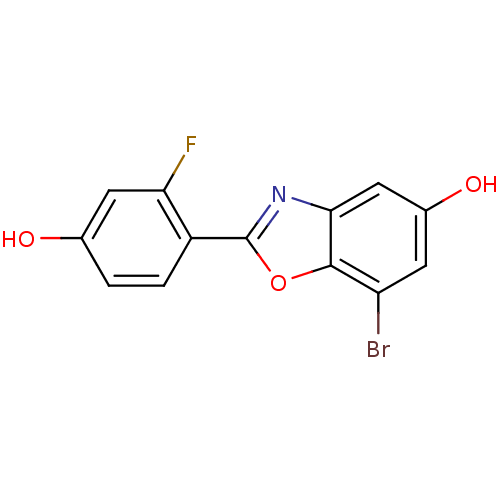 (7-Bromo-2-(2-fluoro-4-hydroxy-phenyl)-benzooxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154134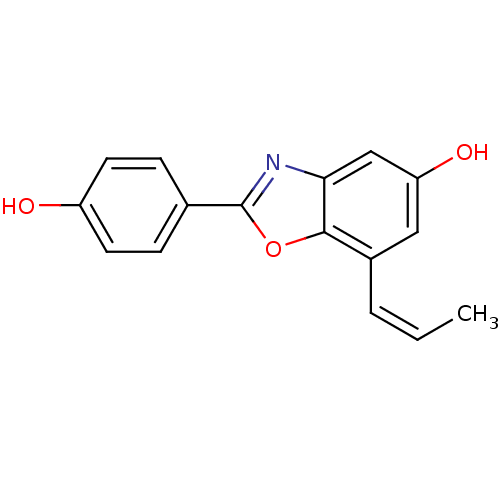 (2-(4-Hydroxy-phenyl)-7-propenyl-benzooxazol-5-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154078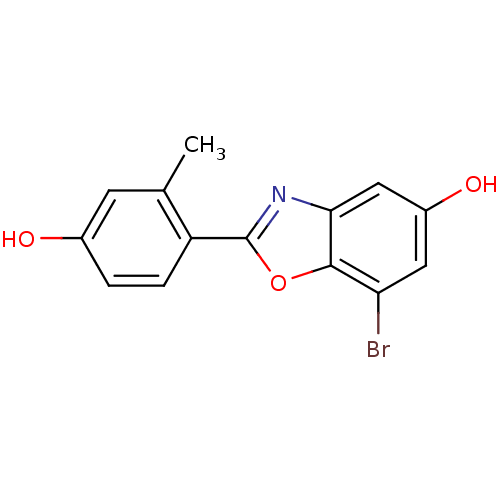 (7-Bromo-2-(4-hydroxy-2-methyl-phenyl)-benzooxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154137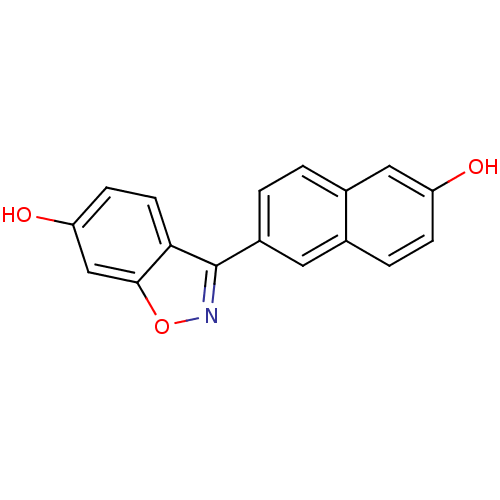 (3-(6-HYDROXY-NAPHTHALEN-2-YL)-BENZO[D]ISOOXAZOL-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50152629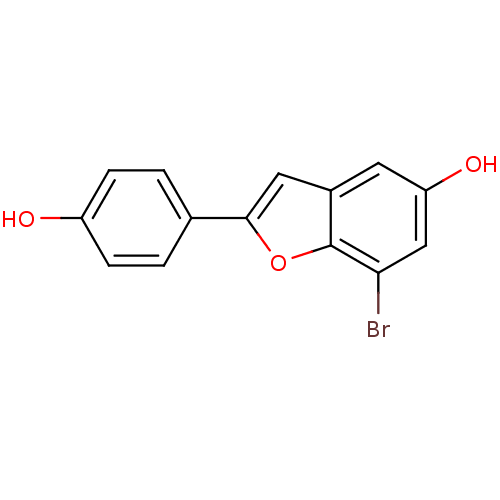 (7-Bromo-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor alpha | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50099587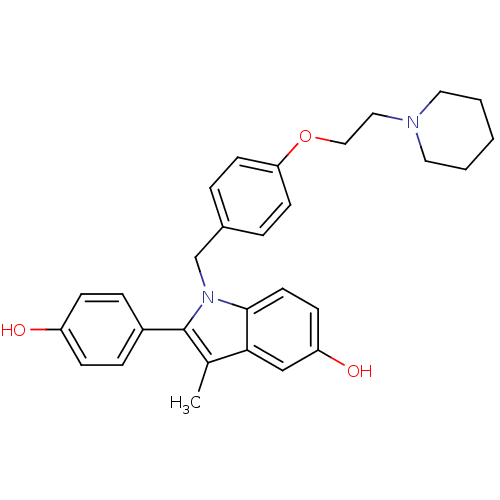 (2-(4-Hydroxy-phenyl)-3-methyl-1-[4-(2-piperidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiol | J Med Chem 44: 1654-7 (2001) BindingDB Entry DOI: 10.7270/Q2F47NDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154048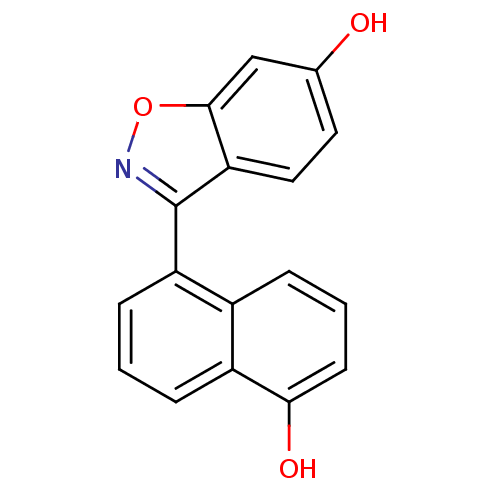 (3-(5-Hydroxy-naphthalen-1-yl)-benzo[d]isoxazol-6-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152626 (7-Chloro-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Mus musculus) | BDBM50154088 (7-Bromo-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | 7-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to mouse ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to rat ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50130177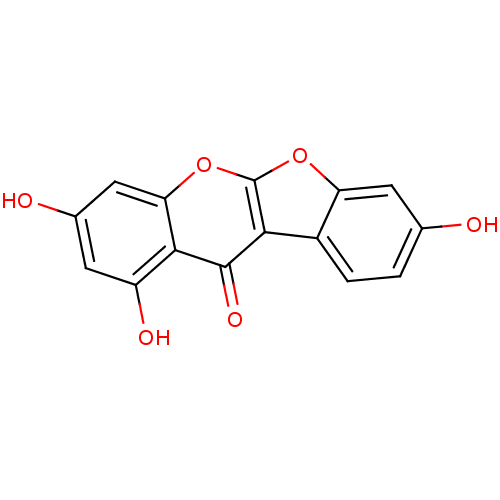 (2,6,8-Trihydroxy-10,11-dioxa-benzo[b]fluoren-5-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity towards human estrogen receptor beta (ERbeta) | Bioorg Med Chem Lett 13: 2399-403 (2003) BindingDB Entry DOI: 10.7270/Q2TB169D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50166756 (3-Bromo-5-hydroxy-2-(4-hydroxy-phenyl)-inden-1-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human estrogen receptor beta | Bioorg Med Chem Lett 15: 3137-42 (2005) Article DOI: 10.1016/j.bmcl.2005.04.013 BindingDB Entry DOI: 10.7270/Q25Q4VMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM594911 (US11584756, Compound 159 | US11584756, Example S-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... | Citation and Details BindingDB Entry DOI: 10.7270/Q2K0786J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20731 (4-bromo-6-(6-hydroxy-1,2-benzoxazol-3-yl)benzene-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM594896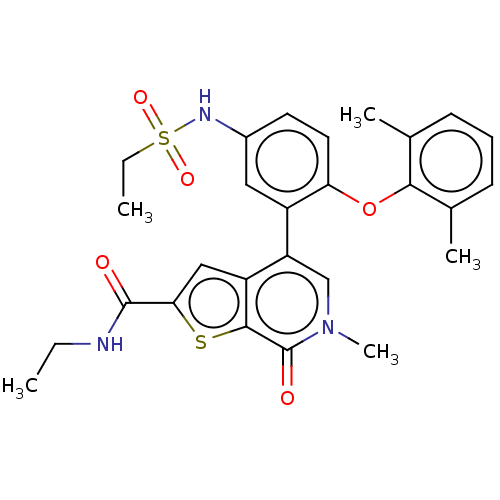 (US11584756, Compound 147 | US11584756, Example S-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... | Citation and Details BindingDB Entry DOI: 10.7270/Q2K0786J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154140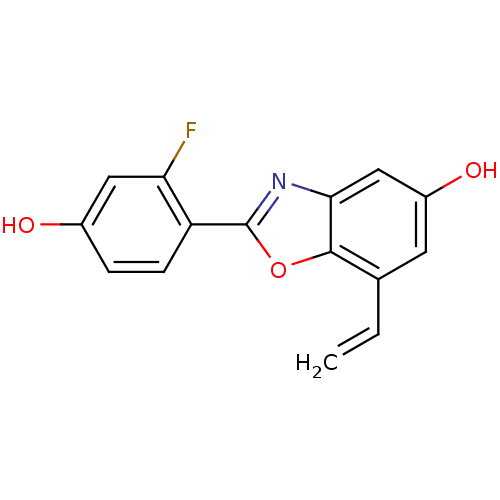 (2-(2-Fluoro-4-hydroxy-phenyl)-7-vinyl-benzooxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 718 total ) | Next | Last >> |