 Found 6259 hits with Last Name = 'miller' and Initial = 'r'
Found 6259 hits with Last Name = 'miller' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50167296
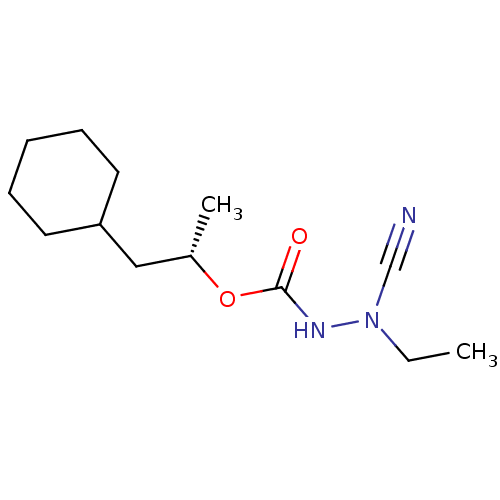
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-ethylhyd...)Show InChI InChI=1S/C13H23N3O2/c1-3-16(10-14)15-13(17)18-11(2)9-12-7-5-4-6-8-12/h11-12H,3-9H2,1-2H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167302

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isobutyl...)Show InChI InChI=1S/C15H27N3O2/c1-12(2)10-18(11-16)17-15(19)20-13(3)9-14-7-5-4-6-8-14/h12-14H,4-10H2,1-3H3,(H,17,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167295
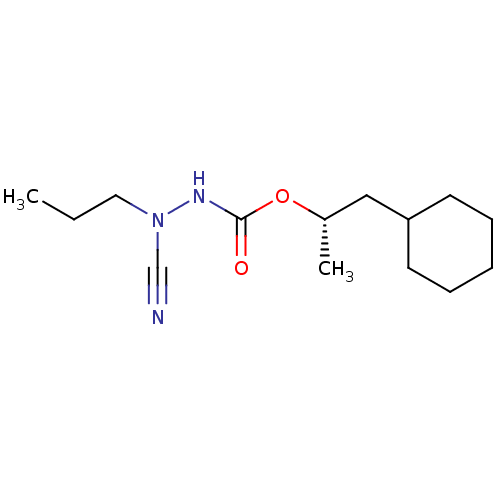
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-propylhy...)Show InChI InChI=1S/C14H25N3O2/c1-3-9-17(11-15)16-14(18)19-12(2)10-13-7-5-4-6-8-13/h12-13H,3-10H2,1-2H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167298
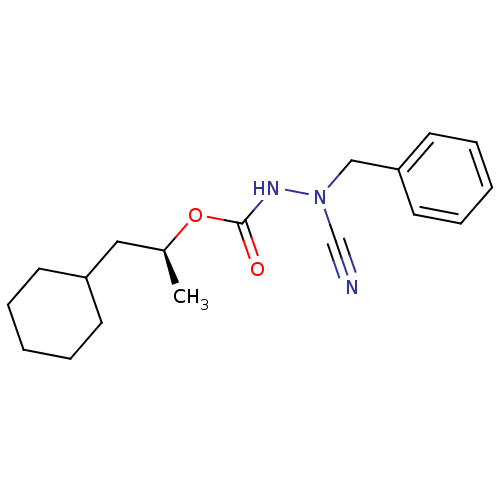
((1S)-2-cyclohexyl-1-methylethyl 2-benzyl-2-cyanohy...)Show InChI InChI=1S/C18H25N3O2/c1-15(12-16-8-4-2-5-9-16)23-18(22)20-21(14-19)13-17-10-6-3-7-11-17/h3,6-7,10-11,15-16H,2,4-5,8-9,12-13H2,1H3,(H,20,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167303
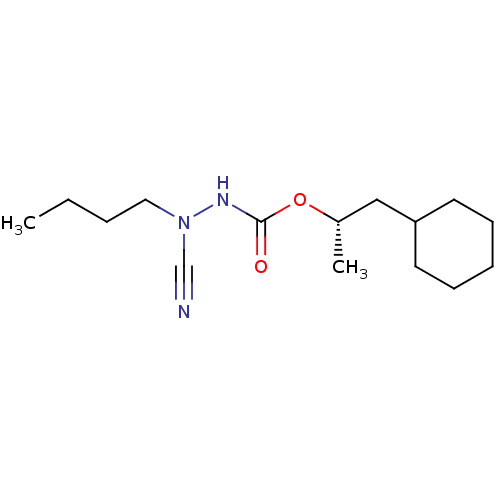
((1S)-2-cyclohexyl-1-methylethyl 2-butyl-2-cyanohyd...)Show InChI InChI=1S/C15H27N3O2/c1-3-4-10-18(12-16)17-15(19)20-13(2)11-14-8-6-5-7-9-14/h13-14H,3-11H2,1-2H3,(H,17,19)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167289
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167290
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50167289

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319536
((R)-2-(4-methylpiperidin-1-yl)-6-(2-(2-methylpyrro...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)N1CCC(C)CC1 |r| Show InChI InChI=1S/C22H31N3/c1-17-9-13-25(14-10-17)22-8-6-20-16-19(5-7-21(20)23-22)11-15-24-12-3-4-18(24)2/h5-8,16-18H,3-4,9-15H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50174619
(CHEMBL197747 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(Nc3ccc(cn3)[N+]([O-])=O)ccc2o1 Show InChI InChI=1S/C20H22N4O3/c1-14-3-2-9-23(14)10-8-18-12-15-11-16(4-6-19(15)27-18)22-20-7-5-17(13-21-20)24(25)26/h4-7,11-14H,2-3,8-10H2,1H3,(H,21,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Rattus norvegicus) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against rat cathepsin K |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50167289

((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50174627

(CHEMBL199245 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show InChI InChI=1S/C19H22N4O/c1-14-3-2-7-23(14)8-6-18-10-15-9-16(4-5-19(15)24-18)22-17-11-20-13-21-12-17/h4-5,9-14,22H,2-3,6-8H2,1H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50319536

((R)-2-(4-methylpiperidin-1-yl)-6-(2-(2-methylpyrro...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)N1CCC(C)CC1 |r| Show InChI InChI=1S/C22H31N3/c1-17-9-13-25(14-10-17)22-8-6-20-16-19(5-7-21(20)23-22)11-15-24-12-3-4-18(24)2/h5-8,16-18H,3-4,9-15H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from rat cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Dog) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319552
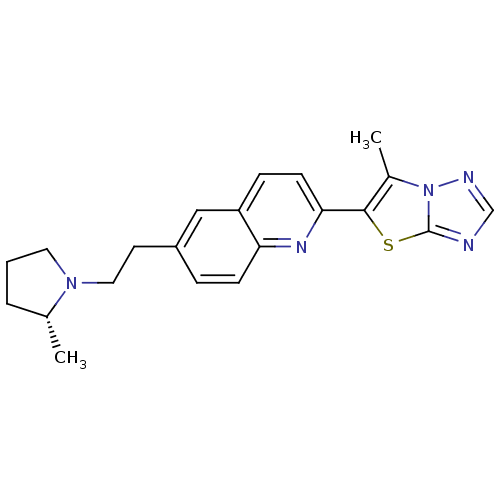
((R)-6-methyl-5-(6-(2-(2-methylpyrrolidin-1-yl)ethy...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1sc2ncnn2c1C |r| Show InChI InChI=1S/C21H23N5S/c1-14-4-3-10-25(14)11-9-16-5-7-18-17(12-16)6-8-19(24-18)20-15(2)26-21(27-20)22-13-23-26/h5-8,12-14H,3-4,9-11H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319549
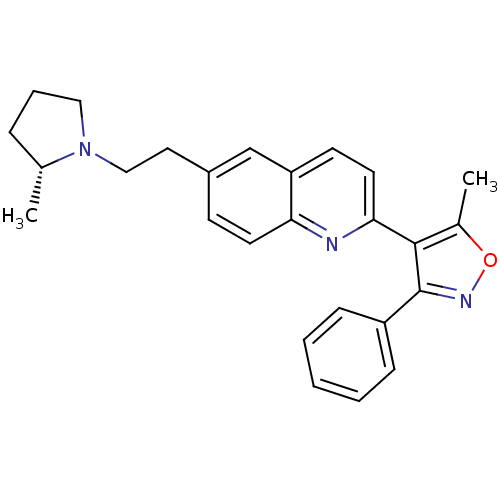
((R)-5-methyl-4-(6-(2-(2-methylpyrrolidin-1-yl)ethy...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1c(C)onc1-c1ccccc1 |r| Show InChI InChI=1S/C26H27N3O/c1-18-7-6-15-29(18)16-14-20-10-12-23-22(17-20)11-13-24(27-23)25-19(2)30-28-26(25)21-8-4-3-5-9-21/h3-5,8-13,17-18H,6-7,14-16H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319509
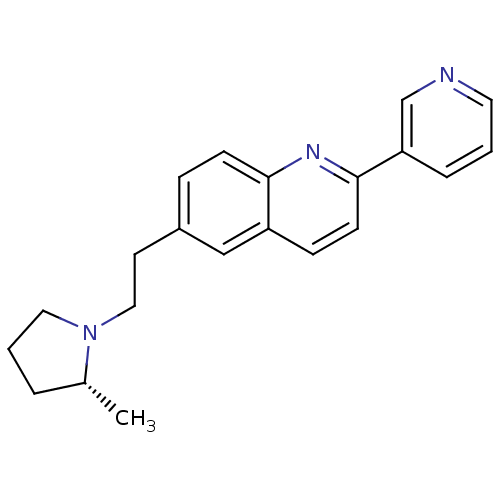
((R)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)-2-(pyridi...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cccnc1 |r| Show InChI InChI=1S/C21H23N3/c1-16-4-3-12-24(16)13-10-17-6-8-20-18(14-17)7-9-21(23-20)19-5-2-11-22-15-19/h2,5-9,11,14-16H,3-4,10,12-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319517
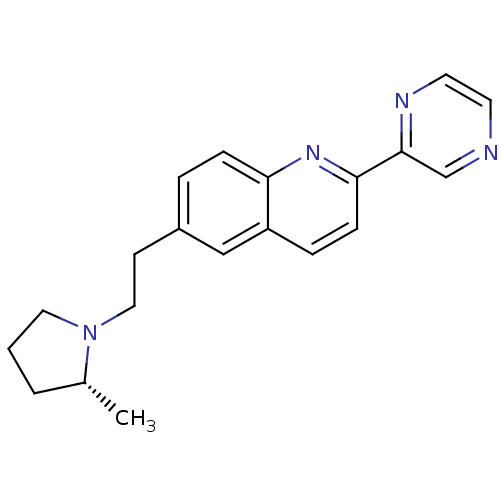
((R)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)-2-(pyrazi...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnccn1 |r| Show InChI InChI=1S/C20H22N4/c1-15-3-2-11-24(15)12-8-16-4-6-18-17(13-16)5-7-19(23-18)20-14-21-9-10-22-20/h4-7,9-10,13-15H,2-3,8,11-12H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50174621

(CHEMBL196467 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show InChI InChI=1S/C19H22N4O/c1-14-3-2-9-23(14)10-6-17-12-15-11-16(4-5-18(15)24-17)22-19-13-20-7-8-21-19/h4-5,7-8,11-14H,2-3,6,9-10H2,1H3,(H,21,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026
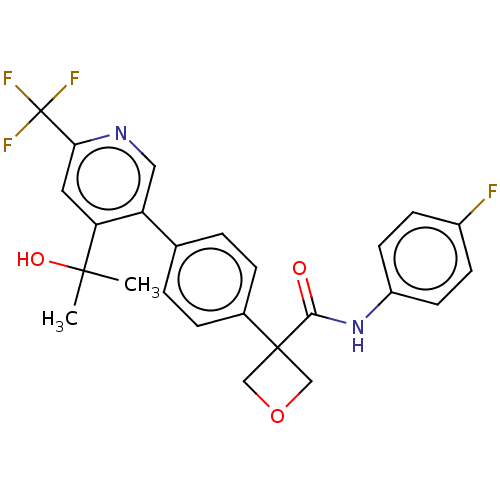
(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50174619

(CHEMBL197747 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(Nc3ccc(cn3)[N+]([O-])=O)ccc2o1 Show InChI InChI=1S/C20H22N4O3/c1-14-3-2-9-23(14)10-8-18-12-15-11-16(4-6-19(15)27-18)22-20-7-5-17(13-21-20)24(25)26/h4-7,11-14H,2-3,8-10H2,1H3,(H,21,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200642

((S)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...)Show SMILES C[C@H]1CCCN1CCc1ccc2cc(ccc2c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24N2/c1-18-3-2-13-26(18)14-12-19-4-9-24-16-23(11-10-22(24)15-19)21-7-5-20(17-25)6-8-21/h4-11,15-16,18H,2-3,12-14H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells |
Bioorg Med Chem Lett 17: 1443-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.073
BindingDB Entry DOI: 10.7270/Q2416WR3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319554
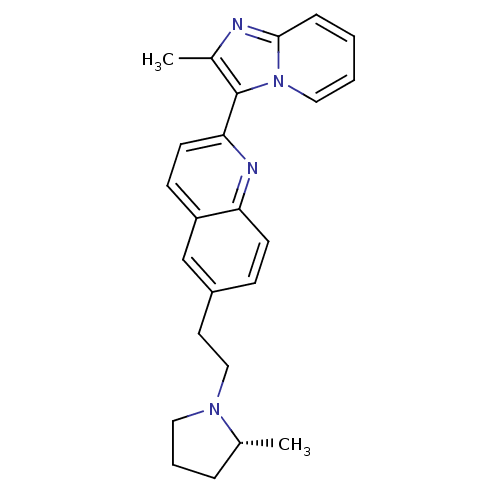
((R)-2-(2-methylimidazo[1,2-a]pyridin-3-yl)-6-(2-(2...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1c(C)nc2ccccn12 |r| Show InChI InChI=1S/C24H26N4/c1-17-6-5-13-27(17)15-12-19-8-10-21-20(16-19)9-11-22(26-21)24-18(2)25-23-7-3-4-14-28(23)24/h3-4,7-11,14,16-17H,5-6,12-13,15H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319538
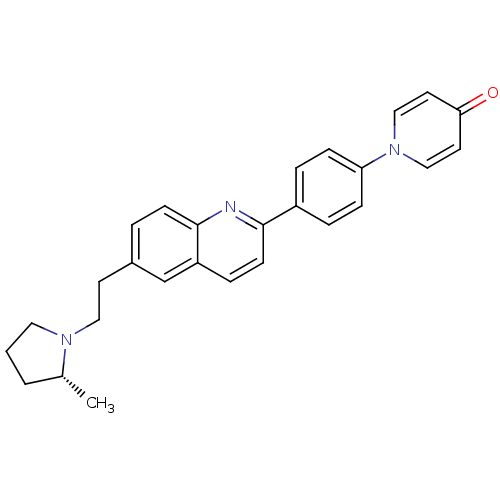
((R)-1-(4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)quin...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1ccc(cc1)-n1ccc(=O)cc1 |r| Show InChI InChI=1S/C27H27N3O/c1-20-3-2-15-29(20)16-12-21-4-10-27-23(19-21)7-11-26(28-27)22-5-8-24(9-6-22)30-17-13-25(31)14-18-30/h4-11,13-14,17-20H,2-3,12,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50174613
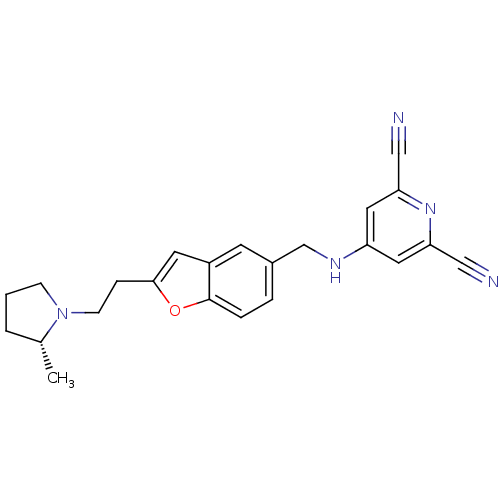
(4-({2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(CNc3cc(nc(c3)C#N)C#N)ccc2o1 Show InChI InChI=1S/C23H23N5O/c1-16-3-2-7-28(16)8-6-22-10-18-9-17(4-5-23(18)29-22)15-26-19-11-20(13-24)27-21(12-19)14-25/h4-5,9-12,16H,2-3,6-8,15H2,1H3,(H,26,27)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50174637
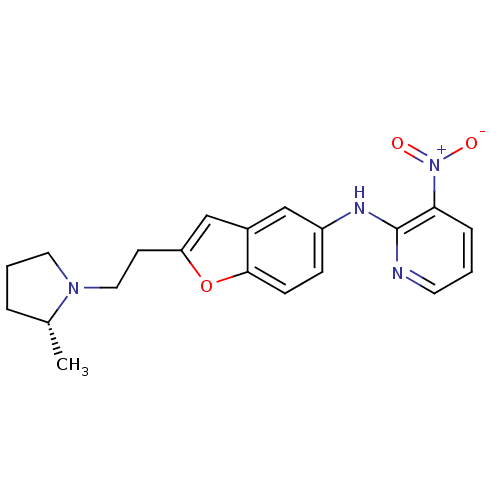
(CHEMBL196294 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(Nc3ncccc3[N+]([O-])=O)ccc2o1 Show InChI InChI=1S/C20H22N4O3/c1-14-4-3-10-23(14)11-8-17-13-15-12-16(6-7-19(15)27-17)22-20-18(24(25)26)5-2-9-21-20/h2,5-7,9,12-14H,3-4,8,10-11H2,1H3,(H,21,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319512
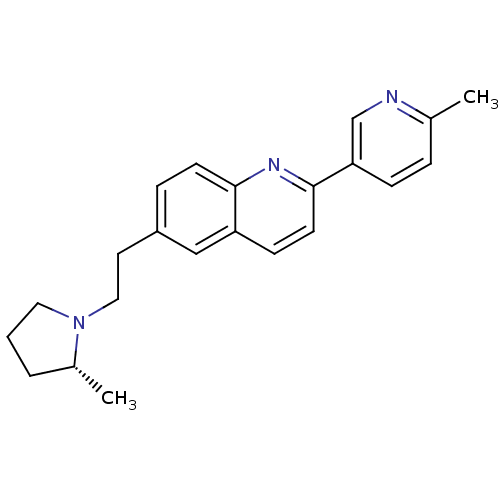
((R)-2-(6-methylpyridin-3-yl)-6-(2-(2-methylpyrroli...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1ccc(C)nc1 |r| Show InChI InChI=1S/C22H25N3/c1-16-5-7-20(15-23-16)22-10-8-19-14-18(6-9-21(19)24-22)11-13-25-12-3-4-17(25)2/h5-10,14-15,17H,3-4,11-13H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
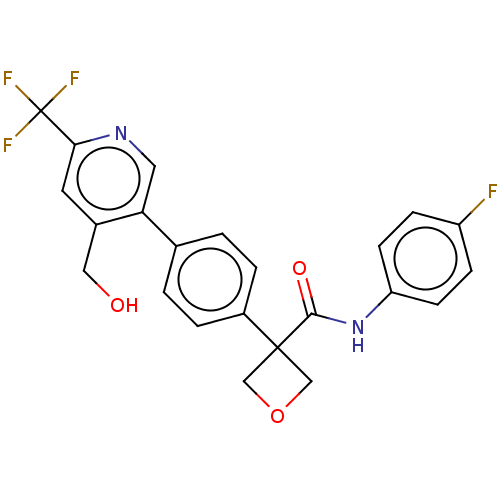
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319547
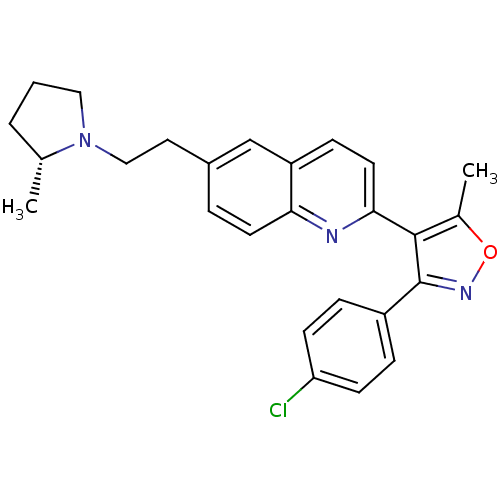
((R)-3-(4-chlorophenyl)-5-methyl-4-(6-(2-(2-methylp...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1c(C)onc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H26ClN3O/c1-17-4-3-14-30(17)15-13-19-5-11-23-21(16-19)8-12-24(28-23)25-18(2)31-29-26(25)20-6-9-22(27)10-7-20/h5-12,16-17H,3-4,13-15H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319528
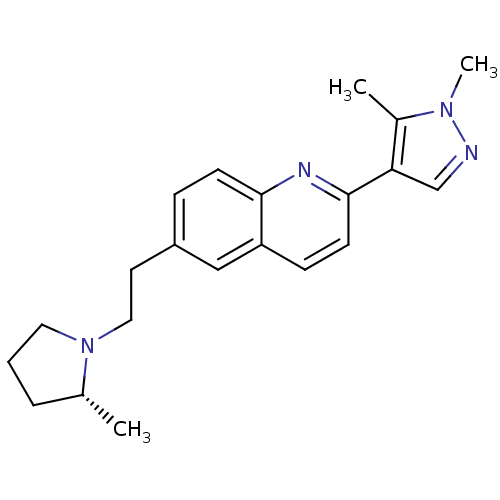
((R)-2-(1,5-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnn(C)c1C |r| Show InChI InChI=1S/C21H26N4/c1-15-5-4-11-25(15)12-10-17-6-8-20-18(13-17)7-9-21(23-20)19-14-22-24(3)16(19)2/h6-9,13-15H,4-5,10-12H2,1-3H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200641
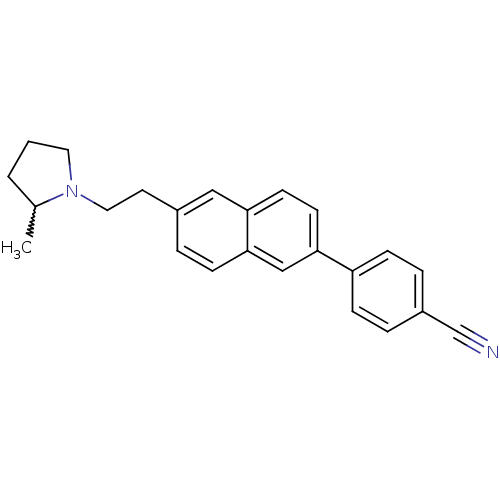
(4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphthalen-...)Show SMILES CC1CCCN1CCc1ccc2cc(ccc2c1)-c1ccc(cc1)C#N |w:1.0| Show InChI InChI=1S/C24H24N2/c1-18-3-2-13-26(18)14-12-19-4-9-24-16-23(11-10-22(24)15-19)21-7-5-20(17-25)6-8-21/h4-11,15-16,18H,2-3,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells |
Bioorg Med Chem Lett 17: 1443-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.073
BindingDB Entry DOI: 10.7270/Q2416WR3 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319508
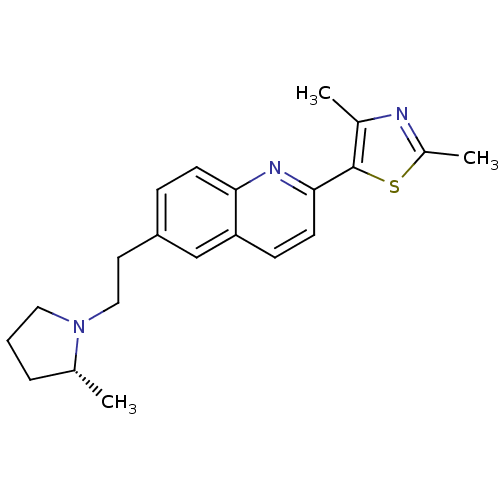
((R)-2,4-dimethyl-5-(6-(2-(2-methylpyrrolidin-1-yl)...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1sc(C)nc1C |r| Show InChI InChI=1S/C21H25N3S/c1-14-5-4-11-24(14)12-10-17-6-8-19-18(13-17)7-9-20(23-19)21-15(2)22-16(3)25-21/h6-9,13-14H,4-5,10-12H2,1-3H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224191
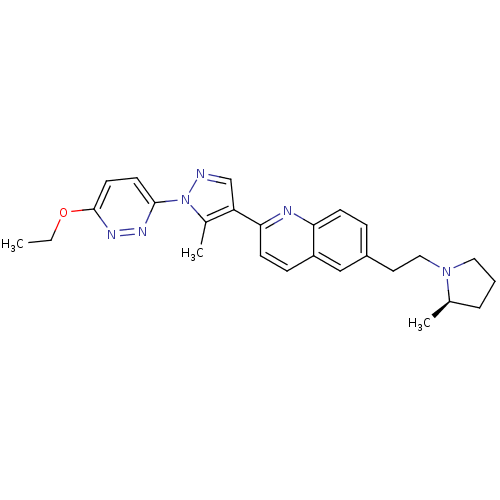
((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...)Show SMILES CCOc1ccc(nn1)-n1ncc(c1C)-c1ccc2cc(CCN3CCC[C@H]3C)ccc2n1 Show InChI InChI=1S/C26H30N6O/c1-4-33-26-12-11-25(29-30-26)32-19(3)22(17-27-32)24-10-8-21-16-20(7-9-23(21)28-24)13-15-31-14-5-6-18(31)2/h7-12,16-18H,4-6,13-15H2,1-3H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells |
J Med Chem 50: 5439-48 (2007)
Article DOI: 10.1021/jm0705051
BindingDB Entry DOI: 10.7270/Q25M65G0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319533
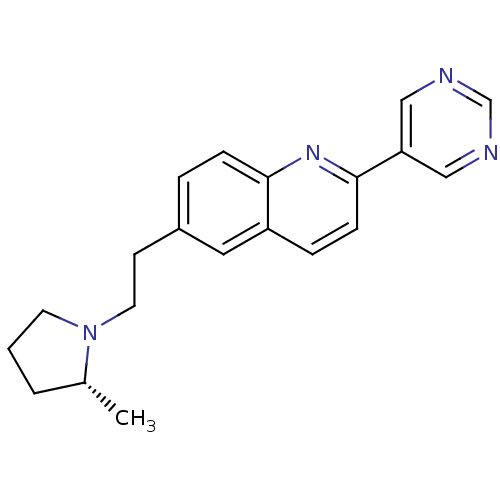
((R)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)-2-(pyrimi...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cncnc1 |r| Show InChI InChI=1S/C20H22N4/c1-15-3-2-9-24(15)10-8-16-4-6-19-17(11-16)5-7-20(23-19)18-12-21-14-22-13-18/h4-7,11-15H,2-3,8-10H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224191

((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...)Show SMILES CCOc1ccc(nn1)-n1ncc(c1C)-c1ccc2cc(CCN3CCC[C@H]3C)ccc2n1 Show InChI InChI=1S/C26H30N6O/c1-4-33-26-12-11-25(29-30-26)32-19(3)22(17-27-32)24-10-8-21-16-20(7-9-23(21)28-24)13-15-31-14-5-6-18(31)2/h7-12,16-18H,4-6,13-15H2,1-3H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319566
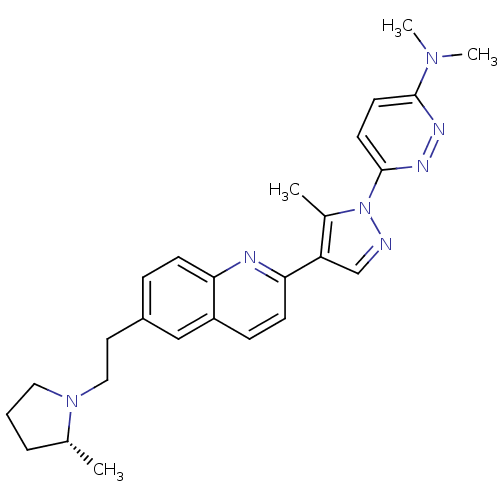
((R)-N,N-dimethyl-6-(5-methyl-4-(6-(2-(2-methylpyrr...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnn(c1C)-c1ccc(nn1)N(C)C |r| Show InChI InChI=1S/C26H31N7/c1-18-6-5-14-32(18)15-13-20-7-9-23-21(16-20)8-10-24(28-23)22-17-27-33(19(22)2)26-12-11-25(29-30-26)31(3)4/h7-12,16-18H,5-6,13-15H2,1-4H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50174615
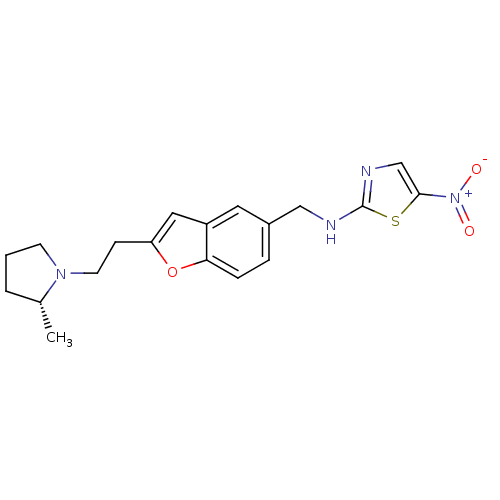
(CHEMBL424842 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(CNc3ncc(s3)[N+]([O-])=O)ccc2o1 Show InChI InChI=1S/C19H22N4O3S/c1-13-3-2-7-22(13)8-6-16-10-15-9-14(4-5-17(15)26-16)11-20-19-21-12-18(27-19)23(24)25/h4-5,9-10,12-13H,2-3,6-8,11H2,1H3,(H,20,21)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319527
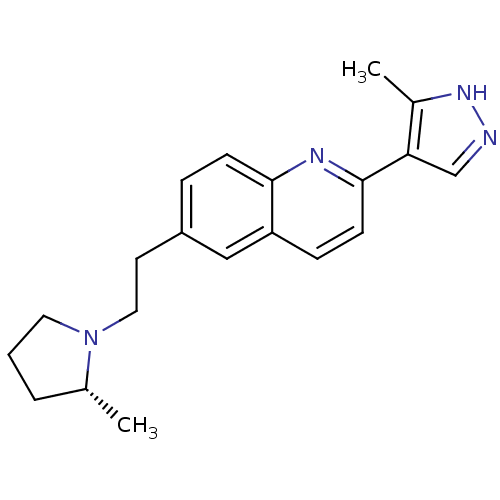
((R)-2-(3-methyl-1H-pyrazol-4-yl)-6-(2-(2-methylpyr...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cn[nH]c1C |r| Show InChI InChI=1S/C20H24N4/c1-14-4-3-10-24(14)11-9-16-5-7-19-17(12-16)6-8-20(22-19)18-13-21-23-15(18)2/h5-8,12-14H,3-4,9-11H2,1-2H3,(H,21,23)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27208
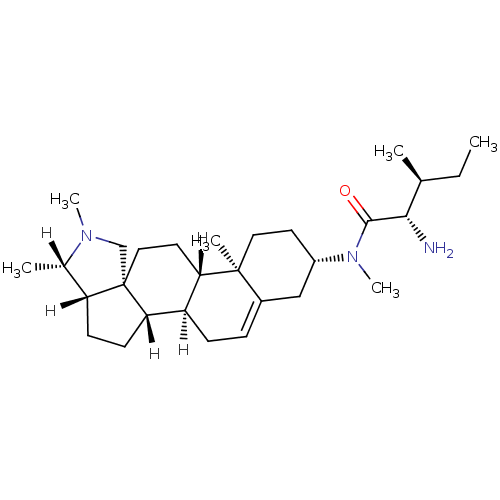
((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@@H](N)[C@@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19-,21-,22+,23+,24-,25-,26-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50174620
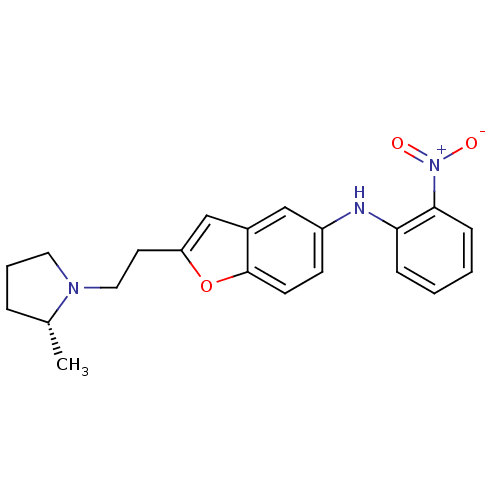
(CHEMBL371258 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(Nc3ccccc3[N+]([O-])=O)ccc2o1 Show InChI InChI=1S/C21H23N3O3/c1-15-5-4-11-23(15)12-10-18-14-16-13-17(8-9-21(16)27-18)22-19-6-2-3-7-20(19)24(25)26/h2-3,6-9,13-15,22H,4-5,10-12H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine |
J Med Chem 48: 6482-90 (2005)
Article DOI: 10.1021/jm0504398
BindingDB Entry DOI: 10.7270/Q25D8RDT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200646
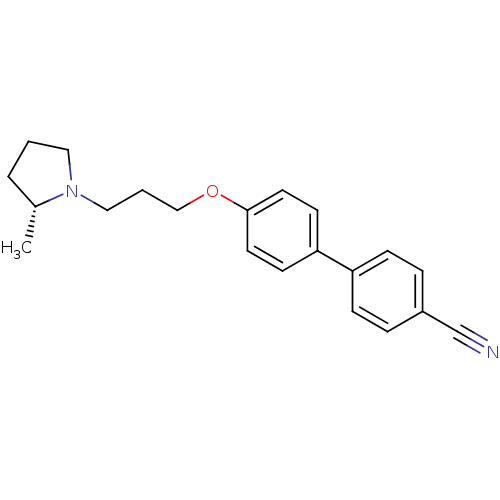
(4'-[3-((R)-2-methyl-pyrrolidin-1-yl)-propoxy]-biph...)Show InChI InChI=1S/C21H24N2O/c1-17-4-2-13-23(17)14-3-15-24-21-11-9-20(10-12-21)19-7-5-18(16-22)6-8-19/h5-12,17H,2-4,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells |
Bioorg Med Chem Lett 17: 1443-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.073
BindingDB Entry DOI: 10.7270/Q2416WR3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50319510
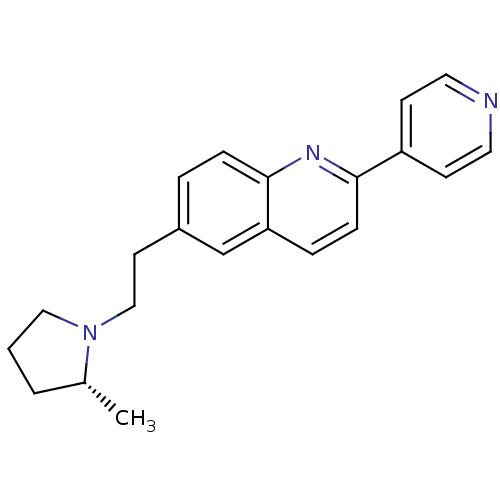
((R)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)-2-(pyridi...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1ccncc1 |r| Show InChI InChI=1S/C21H23N3/c1-16-3-2-13-24(16)14-10-17-4-6-21-19(15-17)5-7-20(23-21)18-8-11-22-12-9-18/h4-9,11-12,15-16H,2-3,10,13-14H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor |
Bioorg Med Chem Lett 20: 3295-300 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.045
BindingDB Entry DOI: 10.7270/Q2XW4JZN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data