

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537869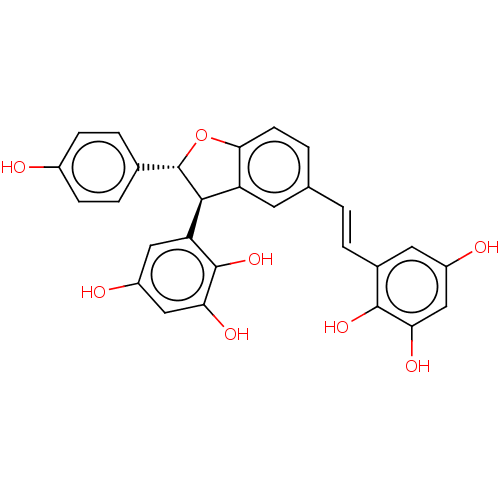 (CHEMBL4638367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate by Dixon plot analysis | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092532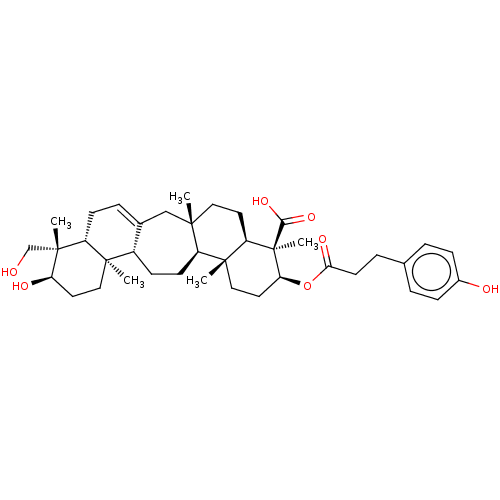 (CHEMBL3586200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537867 (CHEMBL4649760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate by Dixon plot analysis | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092540 (CHEMBL3586207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092531 (CHEMBL3586199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241345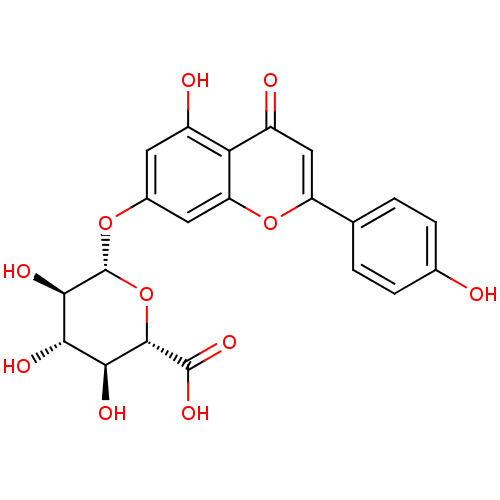 (Apigenin 7-O-β-D-glucuronide (9) | CHEMBL2542...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Catholic University of Daegu | Assay Description The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM4078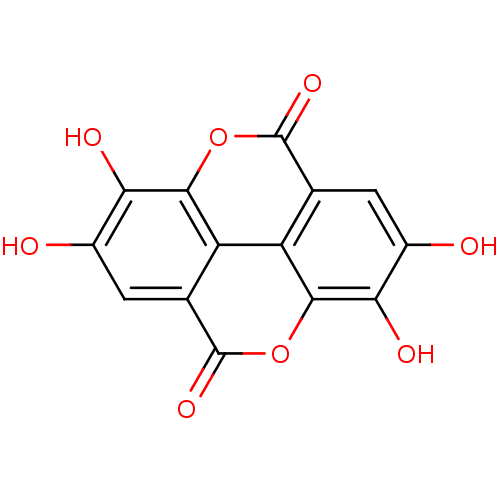 (6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Catholic University of Daegu | Assay Description The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226157 (PTP1B spring 7 (7)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 3.00E+3 | -32.8 | 4.80E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50060922 (CHEMBL3394769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis | J Nat Prod 78: 34-42 (2015) Article DOI: 10.1021/np5005856 BindingDB Entry DOI: 10.7270/Q2TX3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093525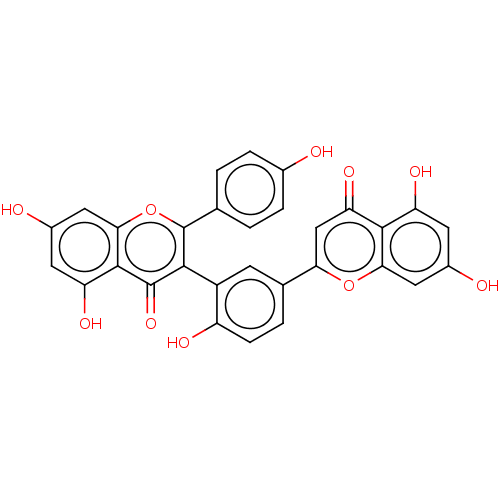 (taiwaniaflavone) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50259862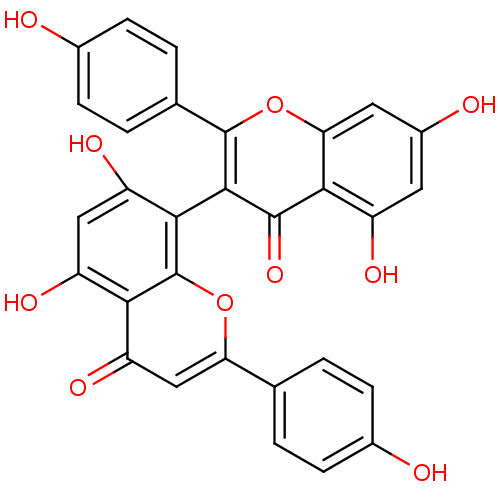 (13,II8-biapigenin | 3,8''-biapigenin | CHEMBL51525...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092529 (CHEMBL3586197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092533 (CHEMBL3586201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50323212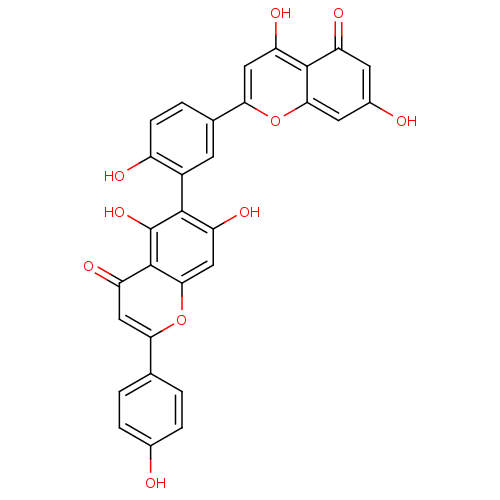 (6-[5-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50129952 (2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092532 (CHEMBL3586200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50060921 (CHEMBL3394770) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis | J Nat Prod 78: 34-42 (2015) Article DOI: 10.1021/np5005856 BindingDB Entry DOI: 10.7270/Q2TX3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226153 (Selaginellin U (2)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 9.70E+3 | -29.8 | 1.38E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50060920 (CHEMBL3394771) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis | J Nat Prod 78: 34-42 (2015) Article DOI: 10.1021/np5005856 BindingDB Entry DOI: 10.7270/Q2TX3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093524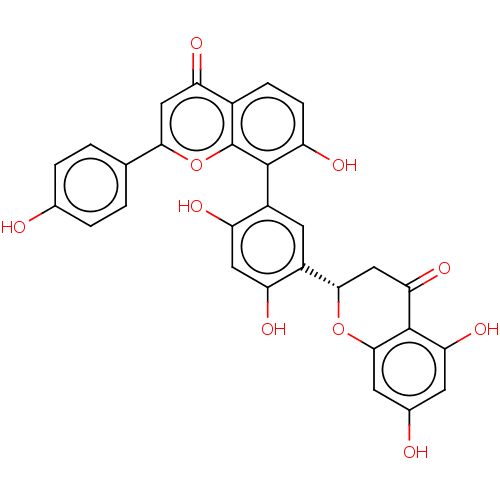 (CHEMBL3585680) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50323213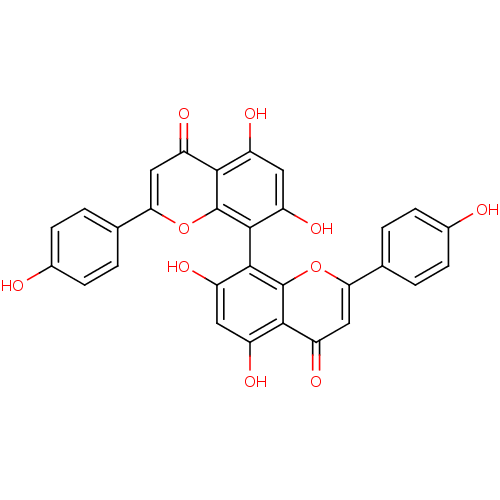 (CHEMBL1208973 | cupressuflavone) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226155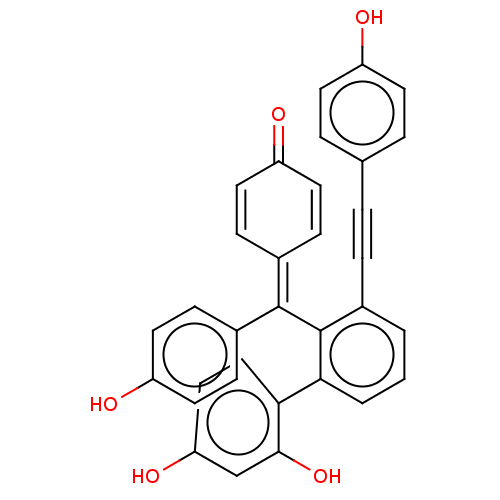 (Selaginellin W (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.11E+4 | -29.4 | 1.46E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226154 (Selaginellin V (3)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.13E+4 | -29.4 | 1.45E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226156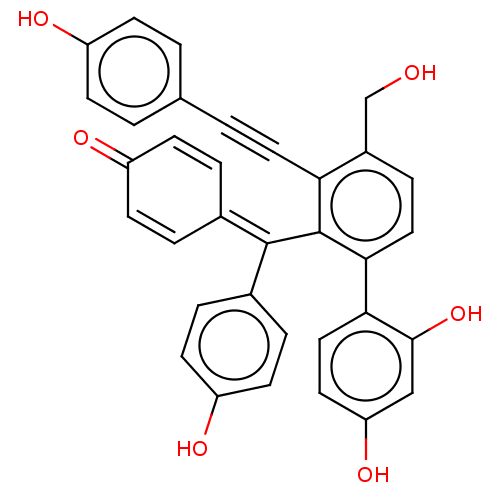 (PTP1B spring 5 (5)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 1.39E+4 | -28.8 | 1.59E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50060918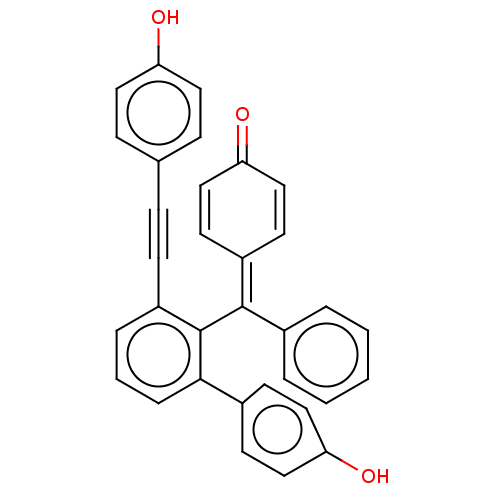 (Selaginellin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis | J Nat Prod 78: 34-42 (2015) Article DOI: 10.1021/np5005856 BindingDB Entry DOI: 10.7270/Q2TX3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093523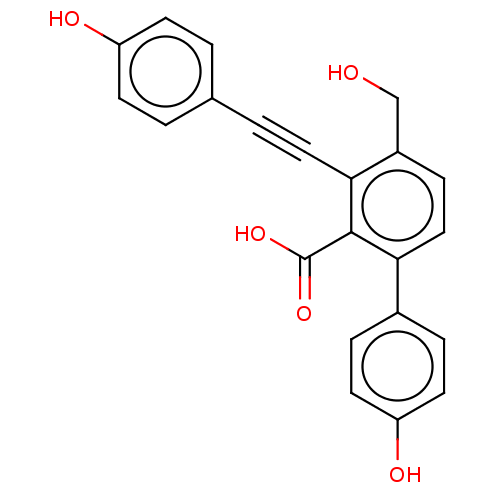 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 1.45E+4 | -28.7 | 1.32E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093523 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50060919 (CHEMBL3394772) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis | J Nat Prod 78: 34-42 (2015) Article DOI: 10.1021/np5005856 BindingDB Entry DOI: 10.7270/Q2TX3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50303006 (CHEMBL517247 | mulberrofuran D) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50203126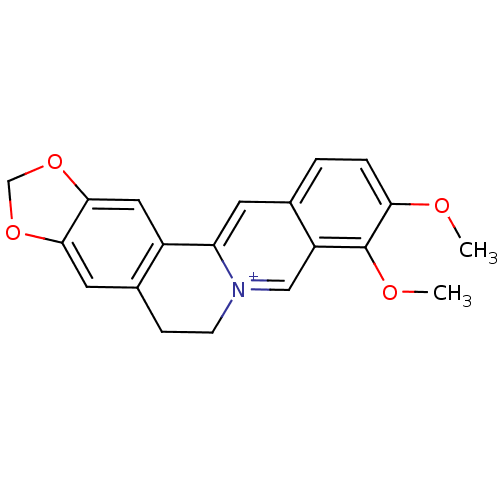 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092540 (CHEMBL3586207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092529 (CHEMBL3586197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092537 (CHEMBL3586204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168024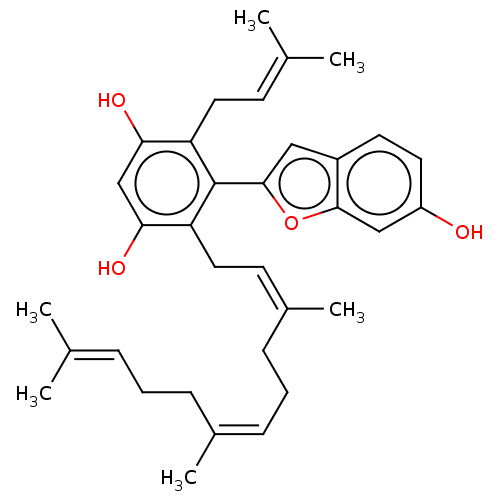 (CHEMBL3800462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092532 (CHEMBL3586200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168022 (CHEMBL3797266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168023 (CHEMBL3800628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50303004 (CHEMBL562810 | mulberrofuran W) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50060923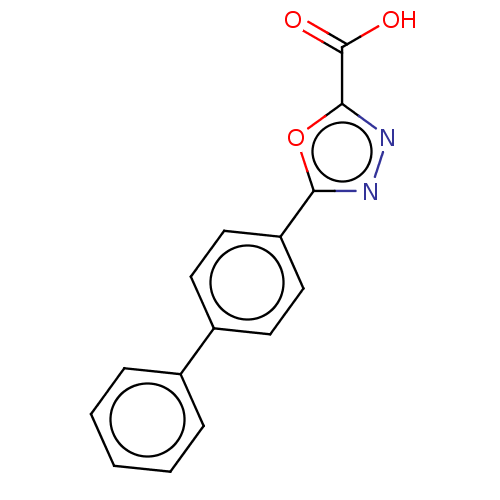 (CHEMBL3394773) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate | J Nat Prod 78: 34-42 (2015) Article DOI: 10.1021/np5005856 BindingDB Entry DOI: 10.7270/Q2TX3H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168019 (CHEMBL3798170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168021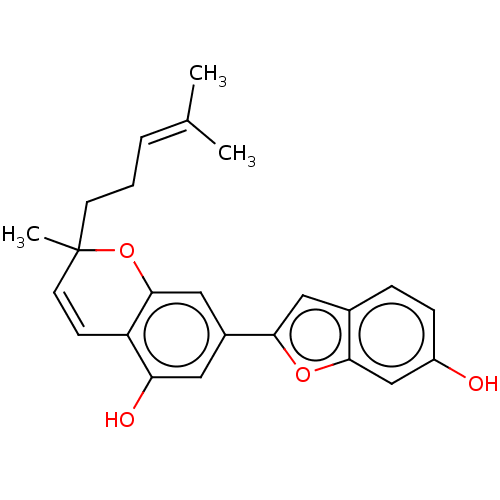 (CHEMBL3800241) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | n/a | n/a | 770 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168018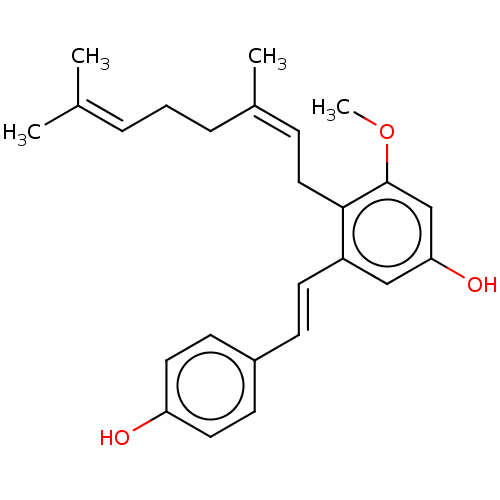 (CHEMBL3798236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092531 (CHEMBL3586199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168020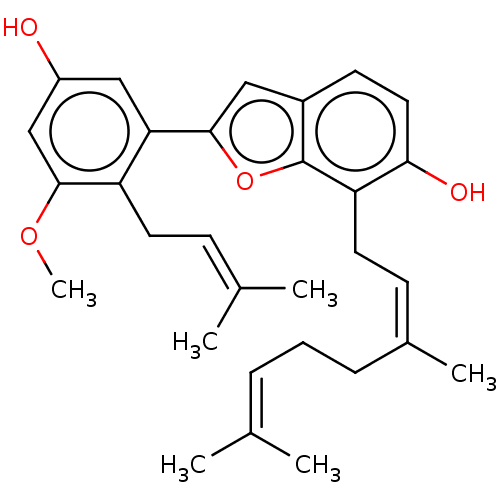 (CHEMBL3797932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092530 (CHEMBL3586198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092527 (CHEMBL3586196) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |