

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM19737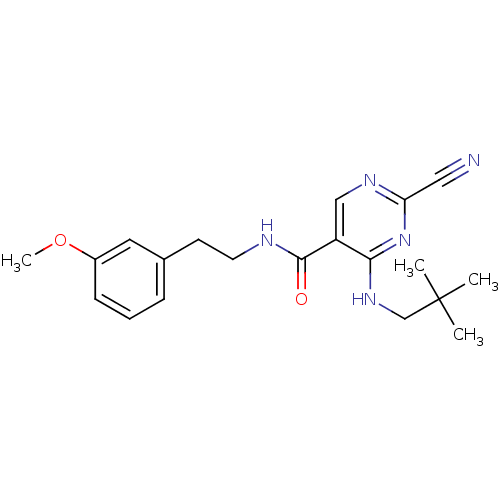 (2-Cyano-pyrimidine, 17b | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19736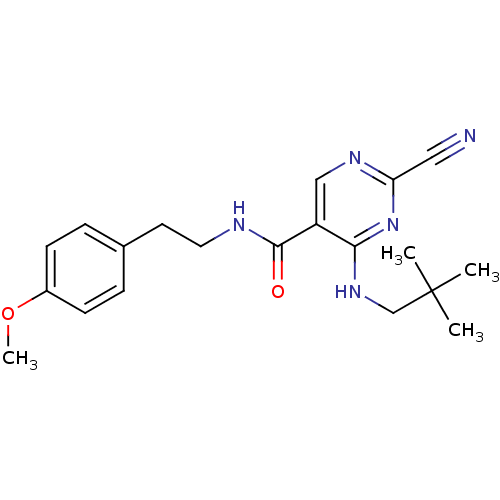 (2-Cyano-pyrimidine, 17a | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19744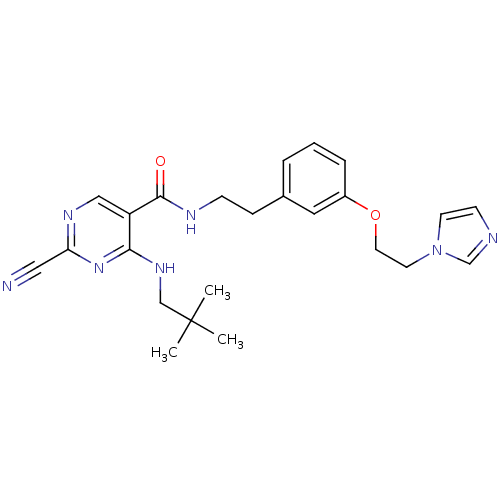 (2-Cyano-pyrimidine, 17i | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19743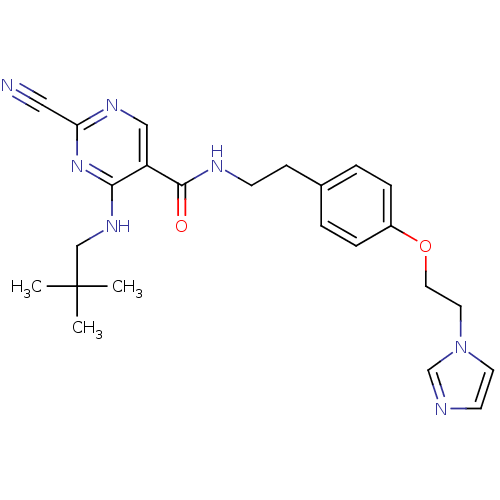 (2-Cyano-pyrimidine, 17h | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19733 (2-Cyano-pyrimidine, 16c | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19731 (2-Cyano-pyrimidine, 16a | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19740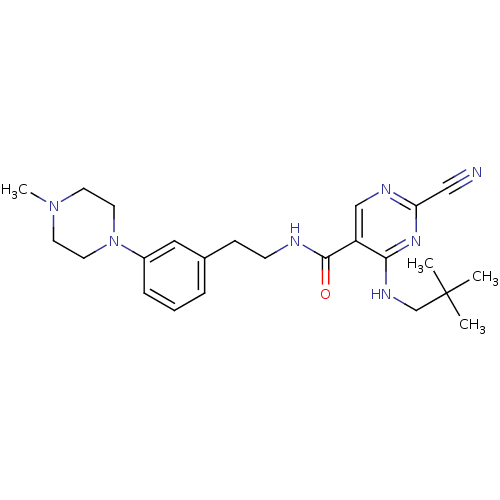 (2-Cyano-pyrimidine, 17e | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19741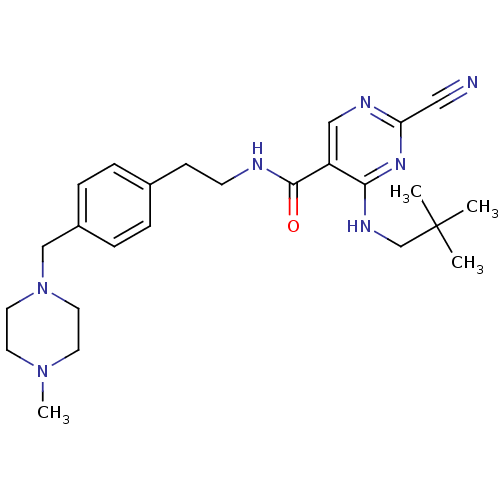 (2-Cyano-pyrimidine, 17f | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19742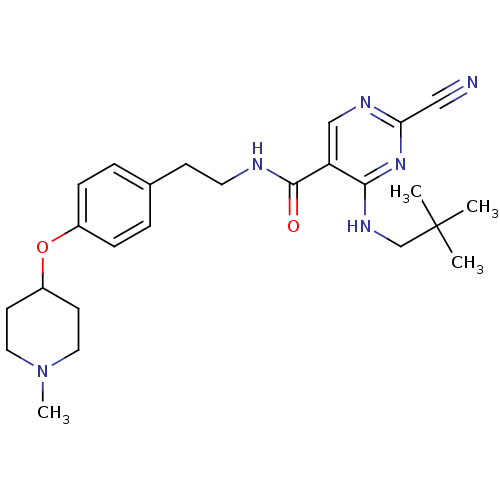 (2-Cyano-pyrimidine, 17g | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19732 (2-Cyano-pyrimidine, 16b | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19739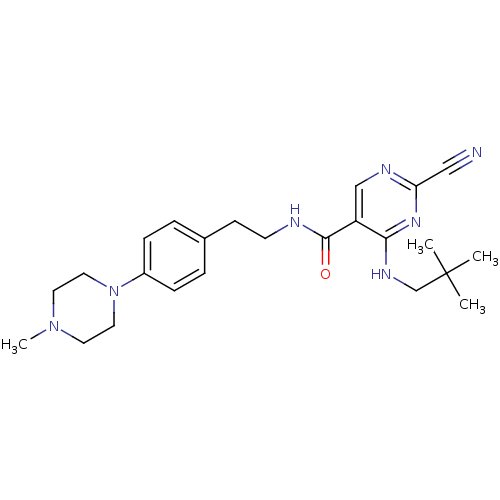 (2-Cyano-pyrimidine, 17d | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19745 (2-Cyano-pyrimidine, 17j | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19735 (2-Cyano-pyrimidine, 16e | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19731 (2-Cyano-pyrimidine, 16a | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19732 (2-Cyano-pyrimidine, 16b | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19733 (2-Cyano-pyrimidine, 16c | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19738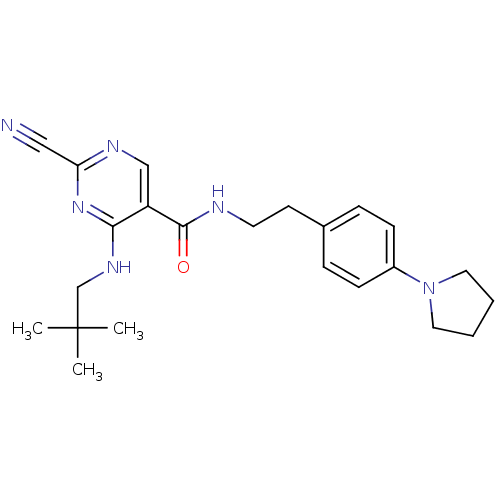 (2-Cyano-pyrimidine, 17c | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19733 (2-Cyano-pyrimidine, 16c | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19741 (2-Cyano-pyrimidine, 17f | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19736 (2-Cyano-pyrimidine, 17a | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308093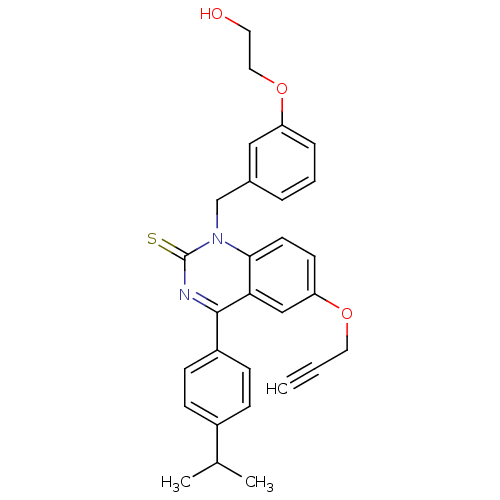 (1-[3-(2-Hydroxy-ethoxy)-benzyl]-4-(4-isopropyl-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19735 (2-Cyano-pyrimidine, 16e | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19735 (2-Cyano-pyrimidine, 16e | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19744 (2-Cyano-pyrimidine, 17i | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19743 (2-Cyano-pyrimidine, 17h | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19745 (2-Cyano-pyrimidine, 17j | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19744 (2-Cyano-pyrimidine, 17i | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19732 (2-Cyano-pyrimidine, 16b | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19734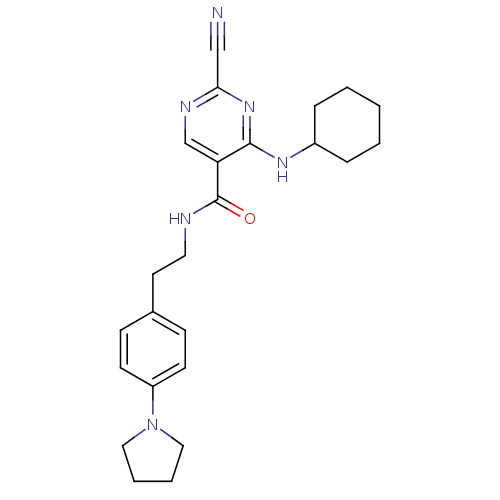 (2-Cyano-pyrimidine, 16d | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19740 (2-Cyano-pyrimidine, 17e | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19737 (2-Cyano-pyrimidine, 17b | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19743 (2-Cyano-pyrimidine, 17h | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097971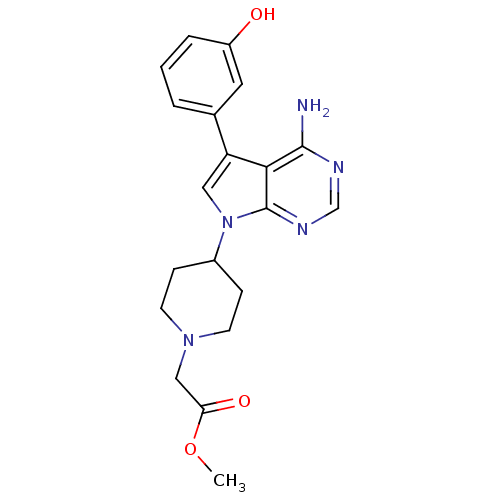 (CHEMBL262276 | methyl 2-(4-(4-amino-5-(3-hydroxyph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of v-Abl receptor by tyrosine kinase enzyme assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19765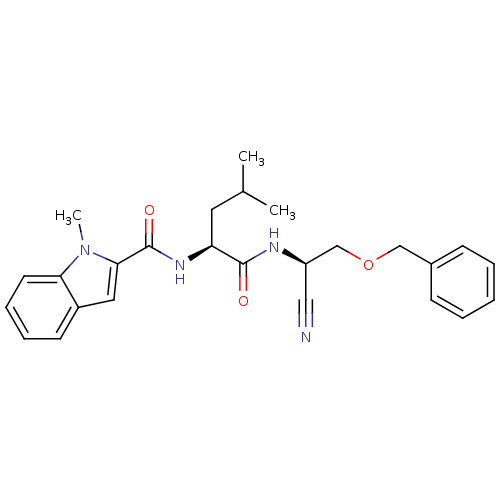 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19764 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-(1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19762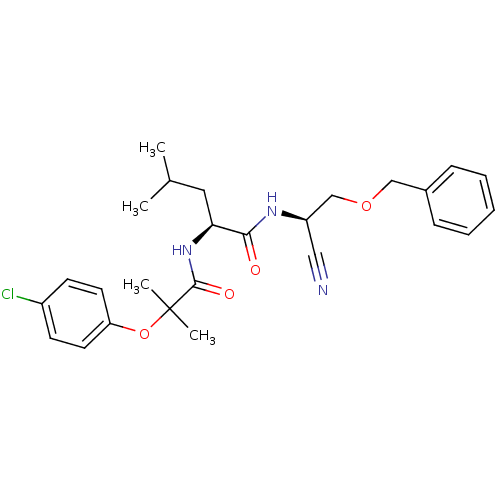 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223939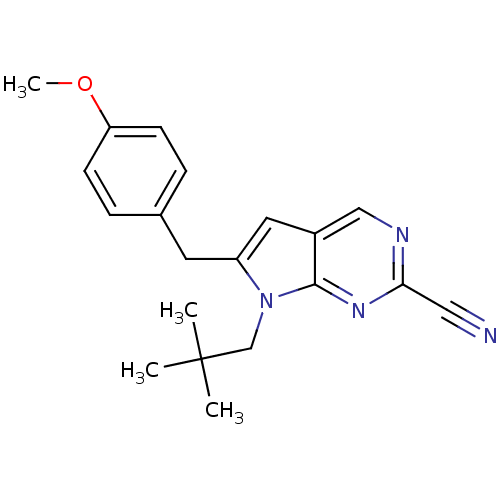 (6-(4-methoxybenzyl)-7-neopentyl-7H-pyrrolo[2,3-d]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223914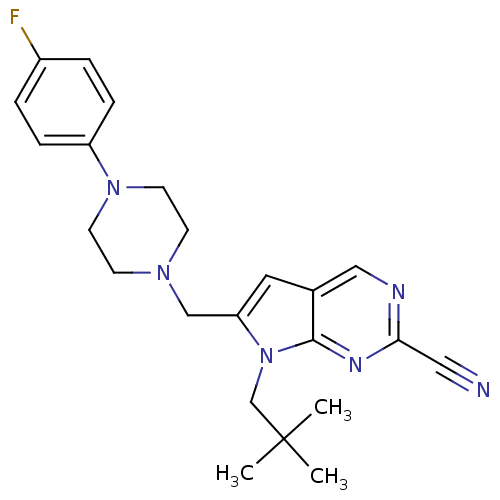 (6-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-7-neo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097967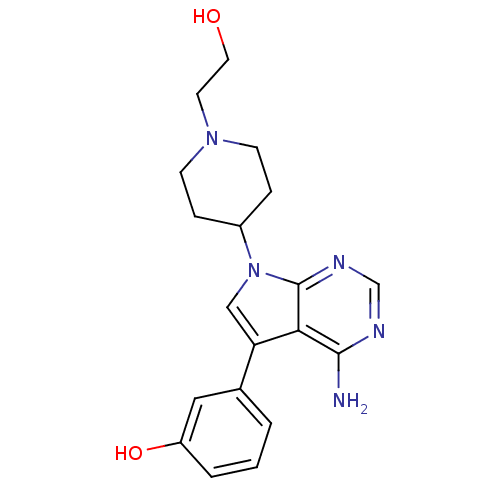 (3-(4-amino-7-(1-(2-hydroxyethyl)piperidin-4-yl)-7H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097979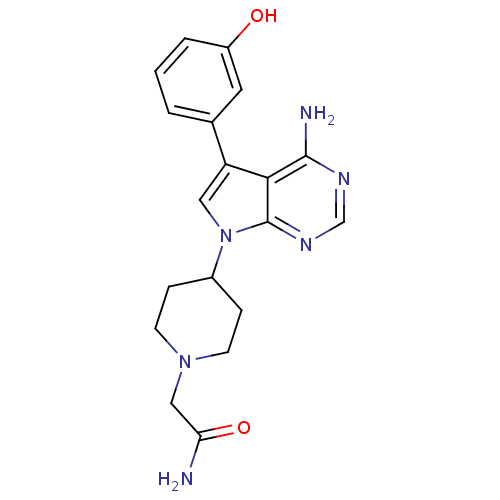 (2-(4-(4-amino-5-(3-hydroxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50097956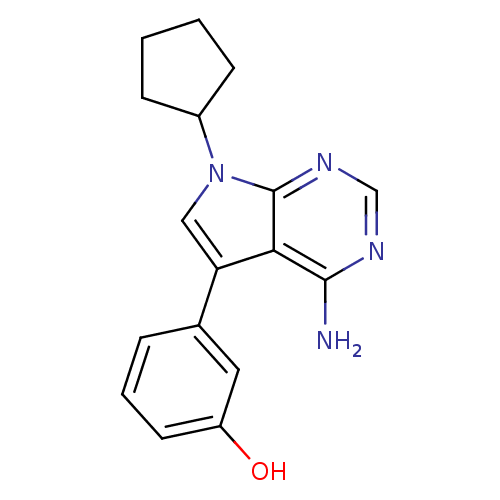 (3-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase activity | Bioorg Med Chem Lett 11: 849-52 (2001) BindingDB Entry DOI: 10.7270/Q2FF3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19731 (2-Cyano-pyrimidine, 16a | 2-cyano-4-(cyclohexylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223921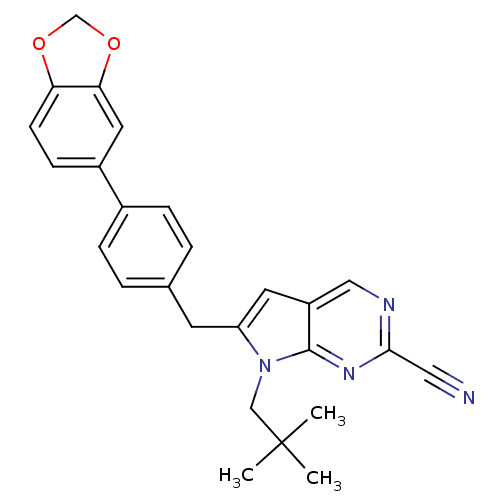 (6-(4-(benzo[d][1,3]dioxol-5-yl)benzyl)-7-neopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223935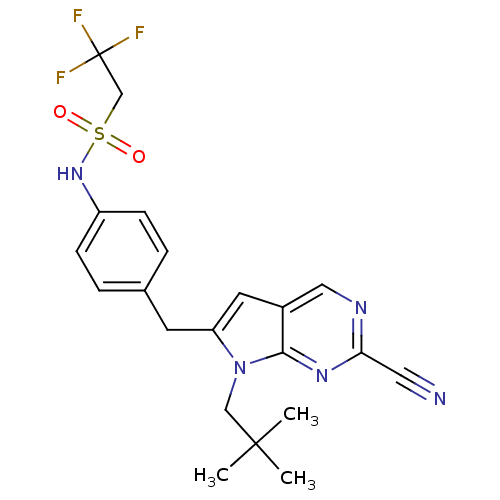 (CHEMBL399842 | N-(4-((2-cyano-7-neopentyl-7H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223915 (6-benzyl-7-neopentyl-7H-pyrrolo[2,3-d]pyrimidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19737 (2-Cyano-pyrimidine, 17b | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308090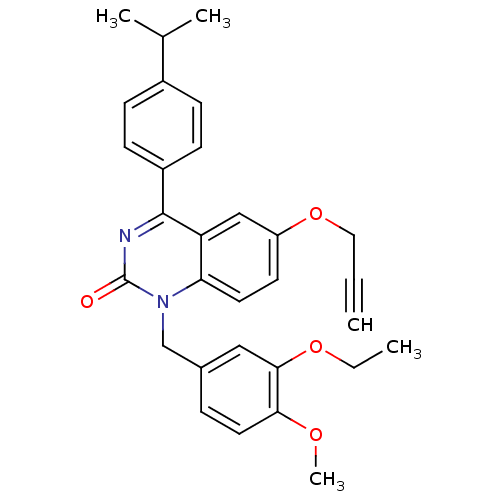 (1-(3-Ethoxy-4-methoxy-benzyl)-4-(4-isopropyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50223925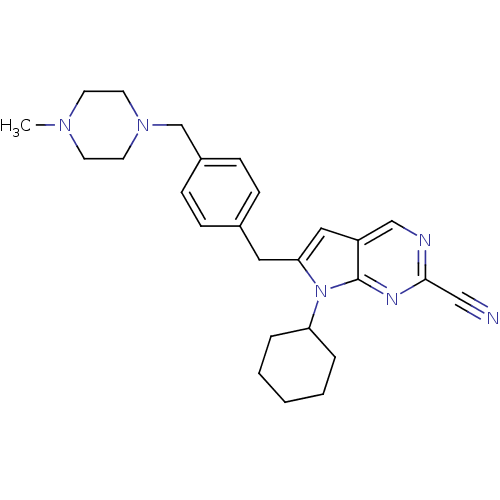 (6-(4-((4-methylpiperazin-1-yl)methyl)benzyl)-7-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of cathepsin K by fluorescence assay | Bioorg Med Chem Lett 17: 6096-100 (2007) Article DOI: 10.1016/j.bmcl.2007.09.047 BindingDB Entry DOI: 10.7270/Q2G160K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308081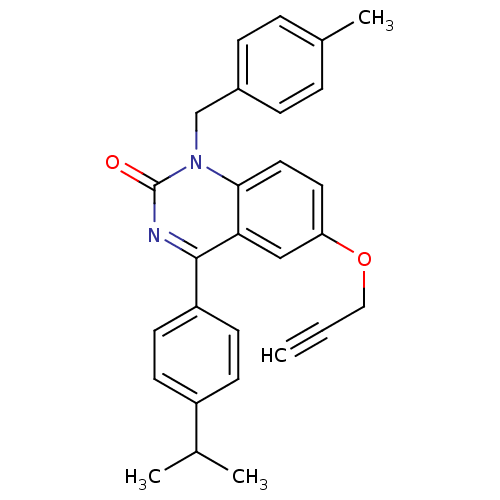 (4-(4-Isopropyl-phenyl)-1-(4-methyl-benzyl)-6-propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19736 (2-Cyano-pyrimidine, 17a | 2-cyano-4-[(2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 50: 591-4 (2007) Article DOI: 10.1021/jm0613525 BindingDB Entry DOI: 10.7270/Q22805X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 578 total ) | Next | Last >> |