 Found 1349 hits with Last Name = 'mitchell' and Initial = 'dj'
Found 1349 hits with Last Name = 'mitchell' and Initial = 'dj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
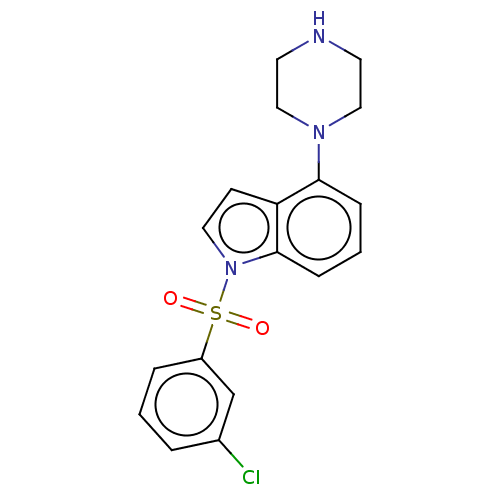
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
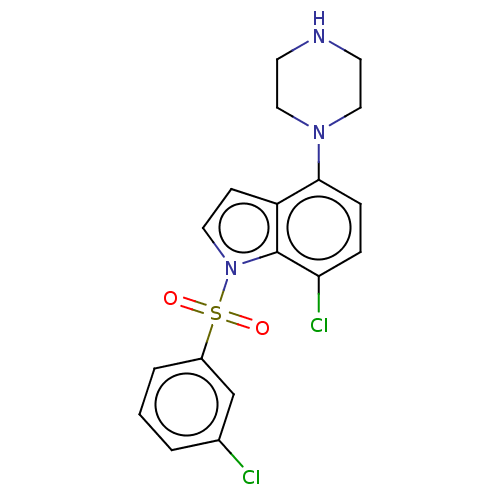
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
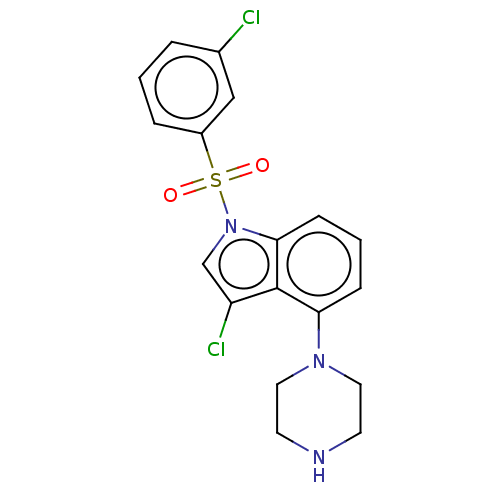
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475477
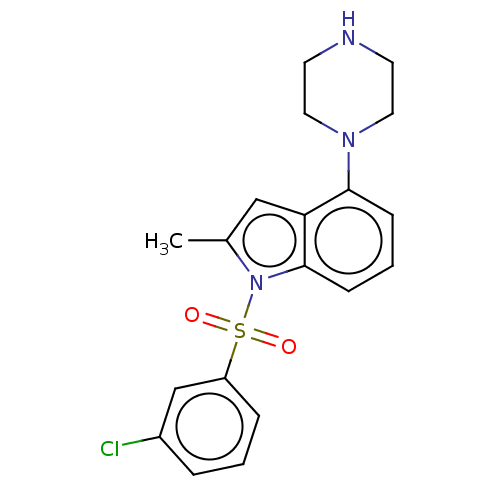
(CHEMBL372929)Show SMILES Cc1cc2c(cccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-12-17-18(22-10-8-21-9-11-22)6-3-7-19(17)23(14)26(24,25)16-5-2-4-15(20)13-16/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475475
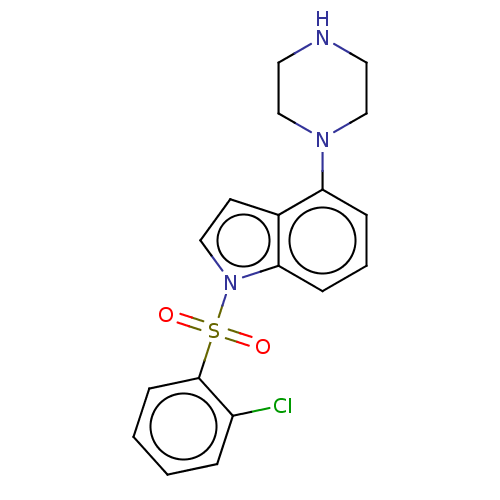
(CHEMBL372513)Show InChI InChI=1S/C18H18ClN3O2S/c19-15-4-1-2-7-18(15)25(23,24)22-11-8-14-16(5-3-6-17(14)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475463
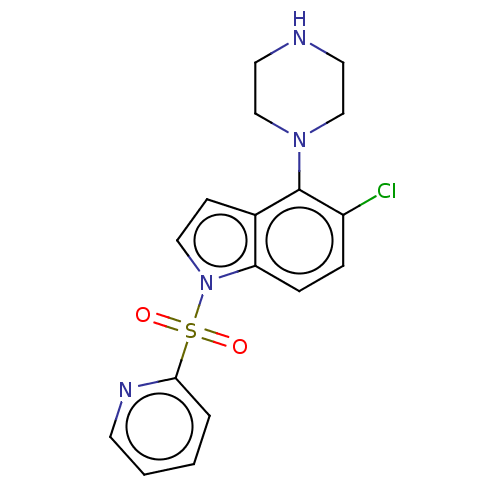
(CHEMBL194915)Show InChI InChI=1S/C17H17ClN4O2S/c18-14-4-5-15-13(17(14)21-11-8-19-9-12-21)6-10-22(15)25(23,24)16-3-1-2-7-20-16/h1-7,10,19H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044607
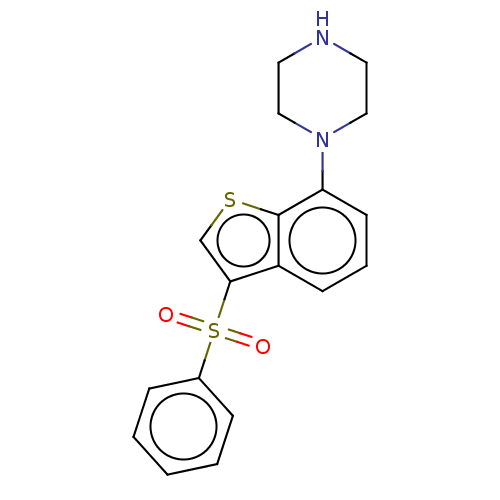
(CHEMBL372537)Show InChI InChI=1S/C18H18N2O2S2/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475473
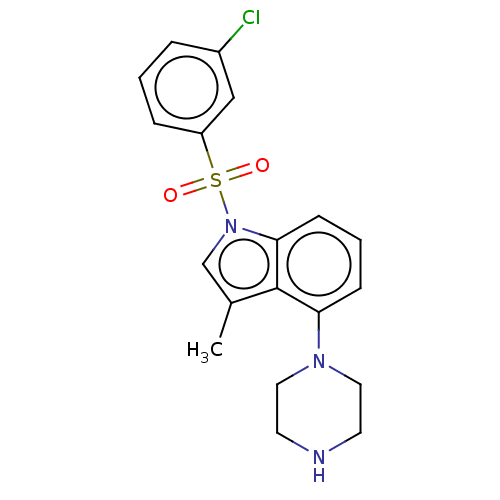
(CHEMBL194039)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-13-23(26(24,25)16-5-2-4-15(20)12-16)18-7-3-6-17(19(14)18)22-10-8-21-9-11-22/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475479
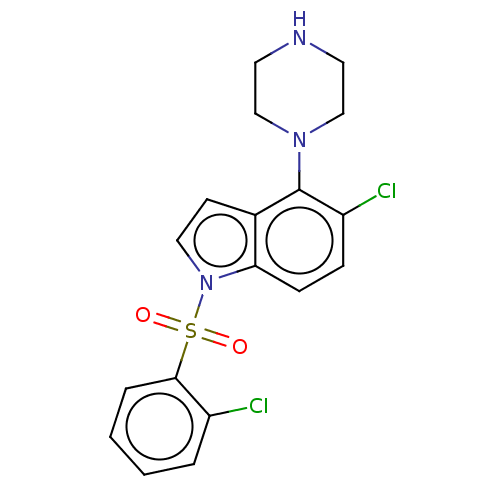
(CHEMBL371176)Show SMILES Clc1ccccc1S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-14-3-1-2-4-17(14)26(24,25)23-10-7-13-16(23)6-5-15(20)18(13)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475470

(CHEMBL370209)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,17-6-1-2-8-19-17)21-11-7-14-15(4-3-5-16(14)21)20-12-9-18-10-13-20/h1-8,11,18H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475464
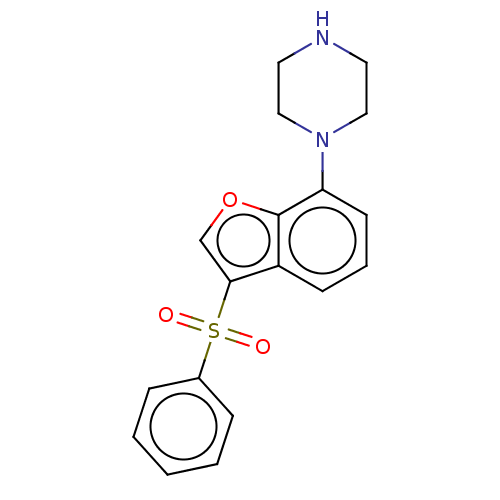
(CHEMBL197574)Show InChI InChI=1S/C18H18N2O3S/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
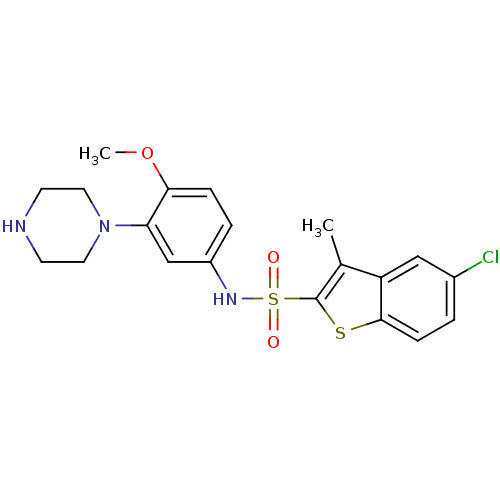
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475466
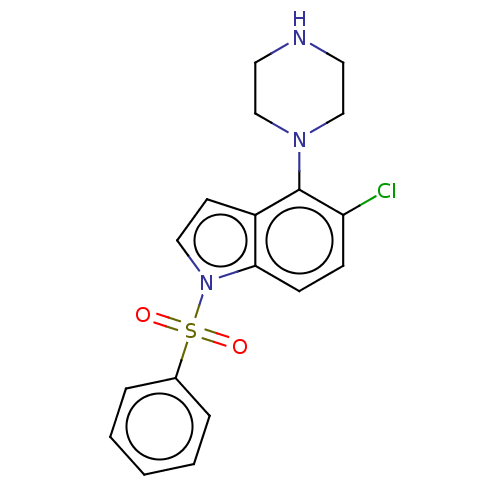
(CHEMBL193665)Show InChI InChI=1S/C18H18ClN3O2S/c19-16-6-7-17-15(18(16)21-12-9-20-10-13-21)8-11-22(17)25(23,24)14-4-2-1-3-5-14/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475471
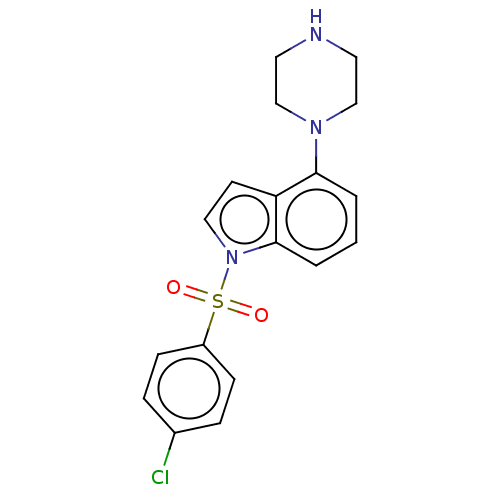
(CHEMBL371876)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-4-6-15(7-5-14)25(23,24)22-11-8-16-17(2-1-3-18(16)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475481

(CHEMBL197297)Show SMILES Cn1cc(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-22-13-18(26(24,25)15-5-2-4-14(20)12-15)16-6-3-7-17(19(16)22)23-10-8-21-9-11-23/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475478
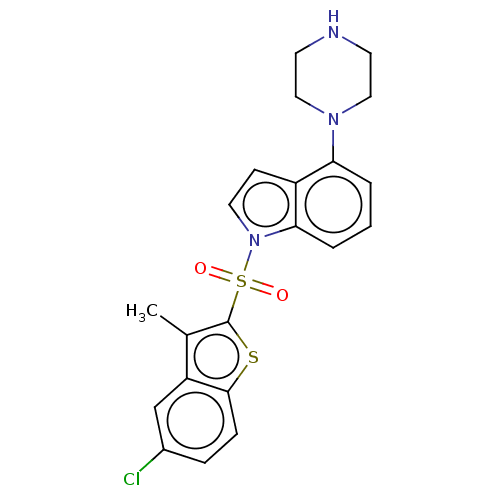
(CHEMBL196644)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2S2/c1-14-17-13-15(22)5-6-20(17)28-21(14)29(26,27)25-10-7-16-18(3-2-4-19(16)25)24-11-8-23-9-12-24/h2-7,10,13,23H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475482

(CHEMBL193379)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(ccc12)C#N Show InChI InChI=1S/C19H17ClN4O2S/c20-15-2-1-3-16(12-15)27(25,26)24-9-6-17-18(24)5-4-14(13-21)19(17)23-10-7-22-8-11-23/h1-6,9,12,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475472

(CHEMBL372287)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-1-3-14(4-2-13)26(24,25)23-10-7-15-17(23)6-5-16(20)18(15)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of human caudate |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475476
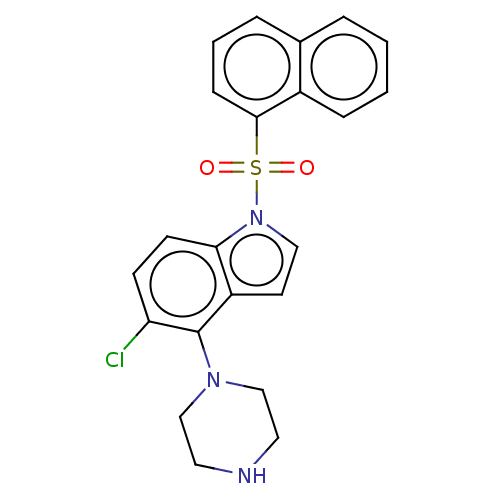
(CHEMBL196103)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H20ClN3O2S/c23-19-8-9-20-18(22(19)25-14-11-24-12-15-25)10-13-26(20)29(27,28)21-7-3-5-16-4-1-2-6-17(16)21/h1-10,13,24H,11-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475474
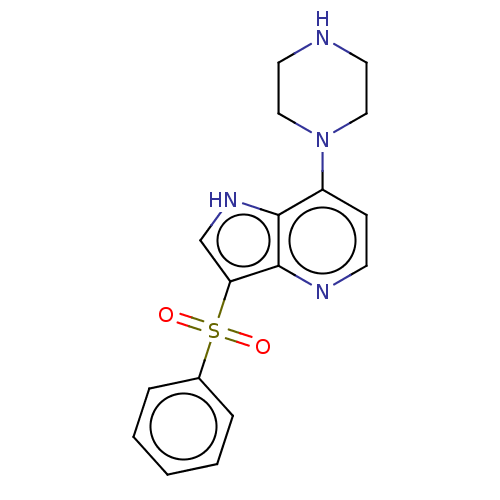
(CHEMBL426640)Show SMILES O=S(=O)(c1c[nH]c2c(ccnc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(6-7-19-17(15)16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of rat striatum |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50098311
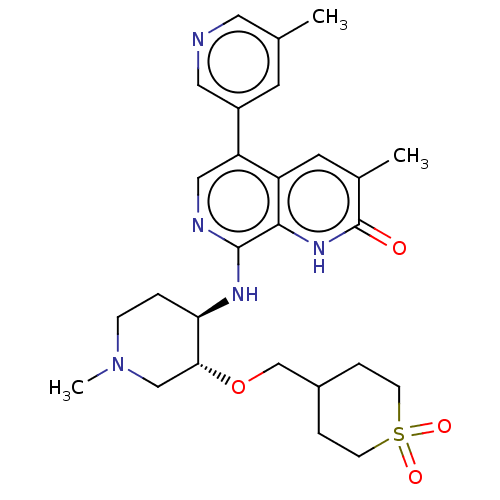
(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to ATAD2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to ATAD2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475469

(CHEMBL196524)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H20ClN3O2S/c23-20-7-8-21-19(22(20)25-13-10-24-11-14-25)9-12-26(21)29(27,28)18-6-5-16-3-1-2-4-17(16)15-18/h1-9,12,15,24H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475468
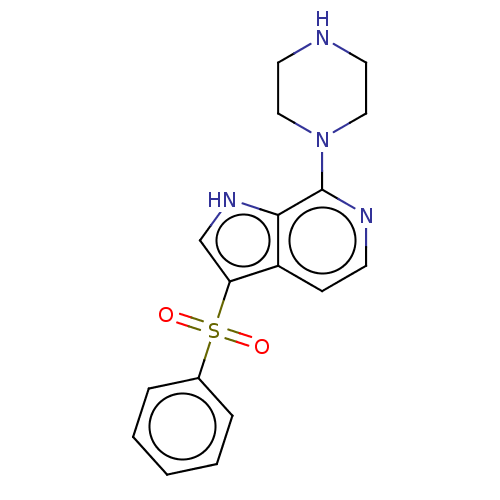
(CHEMBL366248)Show SMILES O=S(=O)(c1c[nH]c2c(nccc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(15)6-7-19-17(16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2B
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to ATAD2B (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to TAF1 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to TAF1L (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 1
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRPF2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Chromatin remodeling regulator CECR2
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to CECR2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain and PHD finger-containing protein 3
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRPF3 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Peregrin
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRPF1 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRD4 BD1 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM50148603
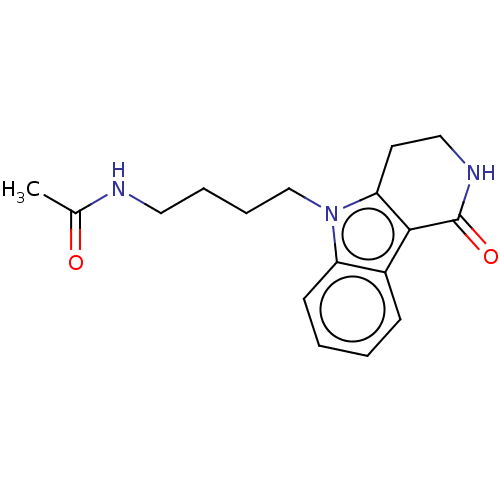
(CHEMBL3770724)Show InChI InChI=1S/C17H21N3O2/c1-12(21)18-9-4-5-11-20-14-7-3-2-6-13(14)16-15(20)8-10-19-17(16)22/h2-3,6-7H,4-5,8-11H2,1H3,(H,18,21)(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
Binding affinity to BRD2 BD1 (unknown origin) by fluorescence anisotropy competition binding assay |
J Med Chem 63: 9020-9044 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00566
BindingDB Entry DOI: 10.7270/Q2348Q0N |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50148603

(CHEMBL3770724)Show InChI InChI=1S/C17H21N3O2/c1-12(21)18-9-4-5-11-20-14-7-3-2-6-13(14)16-15(20)8-10-19-17(16)22/h2-3,6-7H,4-5,8-11H2,1H3,(H,18,21)(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
Binding affinity to BRD3 BD1 (unknown origin) by fluorescence anisotropy competition binding assay |
J Med Chem 63: 9020-9044 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00566
BindingDB Entry DOI: 10.7270/Q2348Q0N |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50148603

(CHEMBL3770724)Show InChI InChI=1S/C17H21N3O2/c1-12(21)18-9-4-5-11-20-14-7-3-2-6-13(14)16-15(20)8-10-19-17(16)22/h2-3,6-7H,4-5,8-11H2,1H3,(H,18,21)(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
Binding affinity to BRD4 BD1 (unknown origin) by fluorescence anisotropy competition binding assay |
J Med Chem 63: 9020-9044 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00566
BindingDB Entry DOI: 10.7270/Q2348Q0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peregrin
(Homo sapiens (Human)) | BDBM50189403
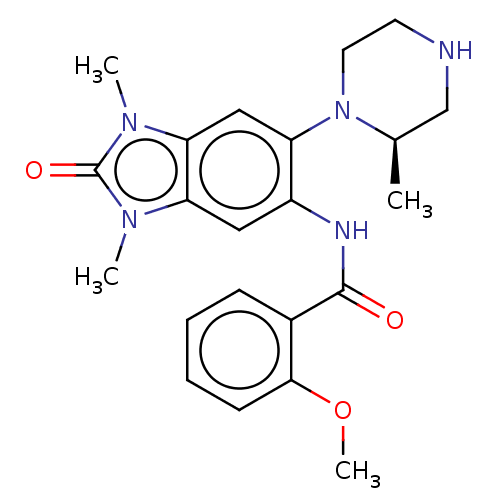
(CHEMBL3828191)Show SMILES COc1ccccc1C(=O)Nc1cc2n(C)c(=O)n(C)c2cc1N1CCNC[C@H]1C |r| Show InChI InChI=1S/C22H27N5O3/c1-14-13-23-9-10-27(14)17-12-19-18(25(2)22(29)26(19)3)11-16(17)24-21(28)15-7-5-6-8-20(15)30-4/h5-8,11-12,14,23H,9-10,13H2,1-4H3,(H,24,28)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length BRPF1 in human HUT78 cell nuclear/chromatin extract after 45 mins by chemoproteomic competition binding assay |
ACS Med Chem Lett 7: 552-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00092
BindingDB Entry DOI: 10.7270/Q2S184G2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50149651
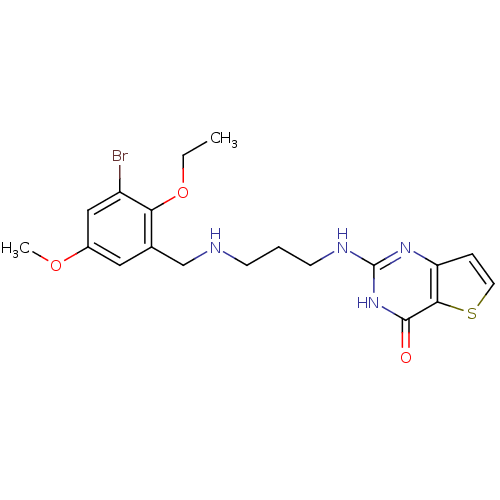
(2-[3-(3-Bromo-2-ethoxy-5-methoxy-benzylamino)-prop...)Show SMILES CCOc1c(Br)cc(OC)cc1CNCCCNc1nc2ccsc2c(=O)[nH]1 Show InChI InChI=1S/C19H23BrN4O3S/c1-3-27-16-12(9-13(26-2)10-14(16)20)11-21-6-4-7-22-19-23-15-5-8-28-17(15)18(25)24-19/h5,8-10,21H,3-4,6-7,11H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50149623
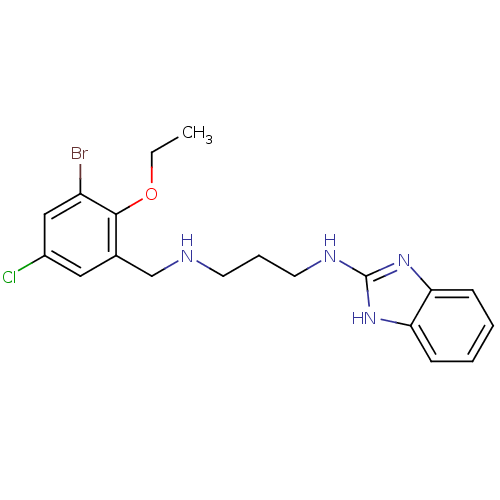
(CHEMBL424799 | N-(1H-Benzoimidazol-2-yl)-N''-(3-br...)Show InChI InChI=1S/C19H22BrClN4O/c1-2-26-18-13(10-14(21)11-15(18)20)12-22-8-5-9-23-19-24-16-6-3-4-7-17(16)25-19/h3-4,6-7,10-11,22H,2,5,8-9,12H2,1H3,(H2,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50149650

(2-[3-(3,5-Dibromo-benzylamino)-propylamino]-1H-thi...)Show InChI InChI=1S/C16H16Br2N4OS/c17-11-6-10(7-12(18)8-11)9-19-3-1-4-20-16-21-13-2-5-24-14(13)15(23)22-16/h2,5-8,19H,1,3-4,9H2,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50149646

(5-[3-(3,5-Dibromo-benzylamino)-propylamino]-4H-thi...)Show InChI InChI=1S/C17H17Br2N3OS/c18-12-6-11(7-13(19)8-12)10-20-3-1-4-21-16-9-15(23)17-14(22-16)2-5-24-17/h2,5-9,20H,1,3-4,10H2,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50149649

(2-[3-(3,5-Dibromo-2-ethoxy-benzylamino)-propylamin...)Show SMILES CCOc1c(Br)cc(Br)cc1CNCCCNc1nc2ccsc2c(=O)[nH]1 Show InChI InChI=1S/C18H20Br2N4O2S/c1-2-26-15-11(8-12(19)9-13(15)20)10-21-5-3-6-22-18-23-14-4-7-27-16(14)17(25)24-18/h4,7-9,21H,2-3,5-6,10H2,1H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50149630

(CHEMBL182960 | N-(3,5-Dibromo-benzyl)-N''-(1H-imid...)Show InChI InChI=1S/C16H17Br2N5/c17-12-7-11(8-13(18)9-12)10-19-4-2-6-21-16-22-14-3-1-5-20-15(14)23-16/h1,3,5,7-9,19H,2,4,6,10H2,(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |
Peregrin
(Homo sapiens (Human)) | BDBM50189392
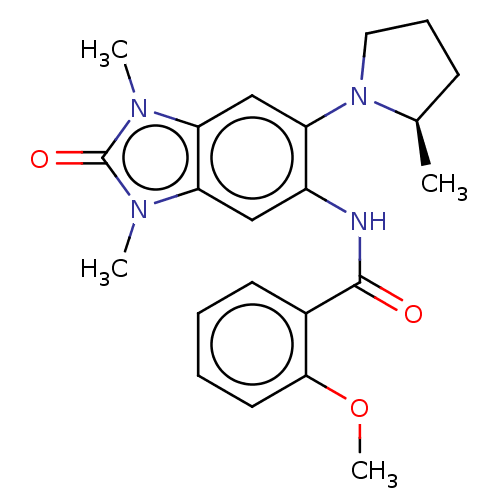
(CHEMBL3828311)Show SMILES COc1ccccc1C(=O)Nc1cc2n(C)c(=O)n(C)c2cc1N1CCC[C@H]1C |r| Show InChI InChI=1S/C22H26N4O3/c1-14-8-7-11-26(14)17-13-19-18(24(2)22(28)25(19)3)12-16(17)23-21(27)15-9-5-6-10-20(15)29-4/h5-6,9-10,12-14H,7-8,11H2,1-4H3,(H,23,27)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of synthetic fluorescent ligand binding to recombinant truncated 6H-Flag-TEV-BRPF1 (622 to 738 residues) (unknown origin) expressed in Esc... |
ACS Med Chem Lett 7: 552-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00092
BindingDB Entry DOI: 10.7270/Q2S184G2 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50149643
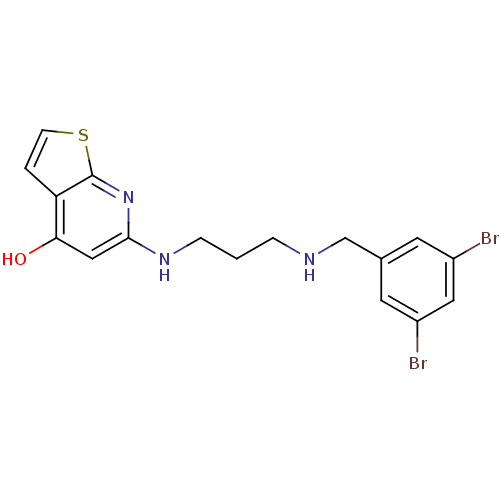
(6-[3-(3,5-Dibromo-benzylamino)-propylamino]-7H-thi...)Show InChI InChI=1S/C17H17Br2N3OS/c18-12-6-11(7-13(19)8-12)10-20-3-1-4-21-16-9-15(23)14-2-5-24-17(14)22-16/h2,5-9,20H,1,3-4,10H2,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Staphylococcus aureus (strain MW2)) | BDBM50409933
(CHEMBL2096779)Show SMILES Brc1cc(Br)c2NCC[C@@H](NCCCNc3nc4ncccc4[nH]3)c2c1 |r| Show InChI InChI=1S/C18H20Br2N6/c19-11-9-12-14(4-8-22-16(12)13(20)10-11)21-6-2-7-24-18-25-15-3-1-5-23-17(15)26-18/h1,3,5,9-10,14,21-22H,2,4,6-8H2,(H2,23,24,25,26)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Staphylococcus aureus methionyl tRNA synthetase |
Bioorg Med Chem Lett 14: 3937-41 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.070
BindingDB Entry DOI: 10.7270/Q2QC02Z9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data