

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204291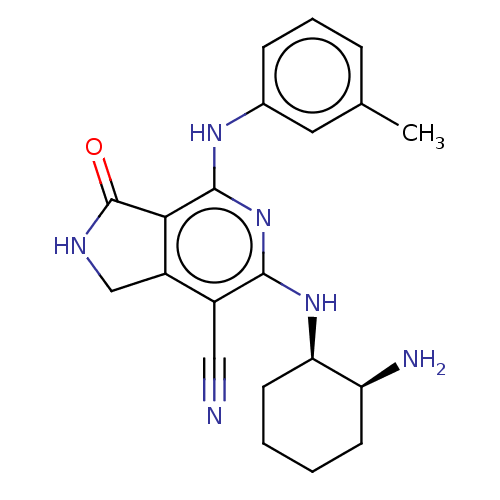 (CHEMBL3953104 | US20230295171, Example 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204296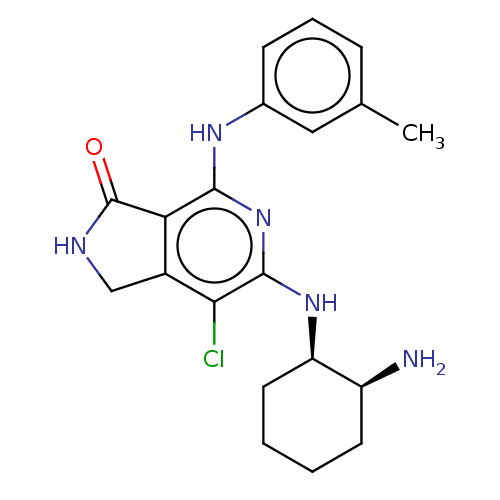 (CHEMBL3941633 | US20230295171, Example 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM620222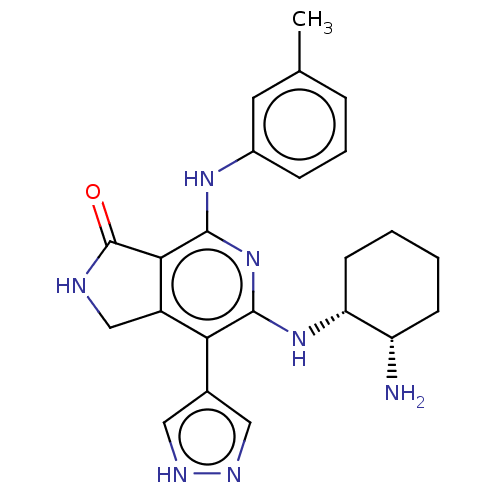 (BDBM50204292 | US20230295171, Example 82) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204293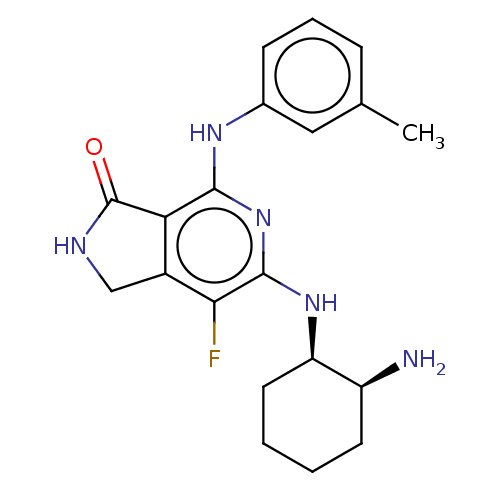 (CHEMBL3983415 | US20230295171, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413341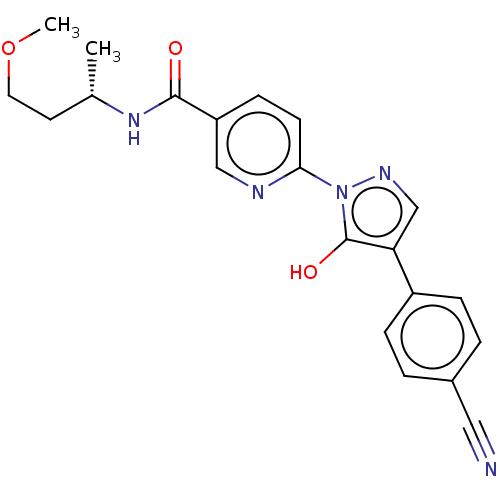 ((S)-6-(4-(4-cyanophenyl)-5-hydroxy-1H-pyrazol-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204290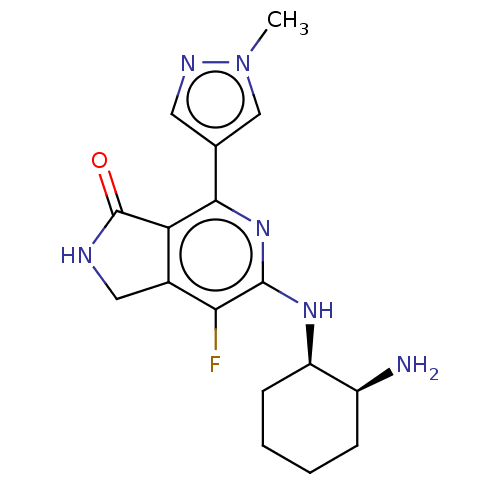 (CHEMBL3979920 | US11077111, Compound IIIa | US2023...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length N-terminal GST-tagged SYK cytoplasmic domain expressed in baculovirus expression system by Z'-LYTE assay | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413007 (6-(4-(4-cyano-2-methylphenyl)-5-hydroxy-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413146 (2-fluoro-4-(5-hydroxy-1-(5-(morpholine-4-carbonyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204295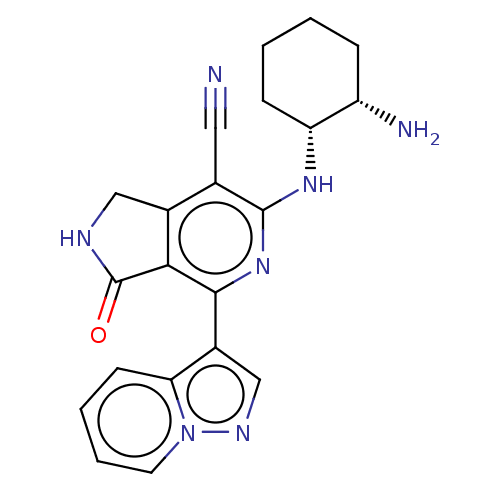 (CHEMBL3944381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413152 (2-fluoro-4-(5-hydroxy-1-(5-(4-methyl-4,7-diazaspir...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413246 (4-(5-hydroxy-1-(5-(4-propylpiperazine-1-carbonyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413353 ((S)-6-(4-(4-cyano-2-methylphenyl)-5-hydroxy-1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413134 (2-fluoro-4-(5-hydroxy-1-(5-(4-methylpiperazine-1-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413193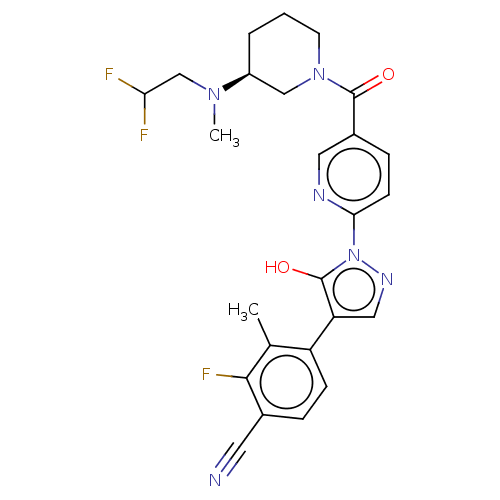 ((S)-4-(1-(5-(3-((2,2-difluoroethyl)(methyl)amino)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413139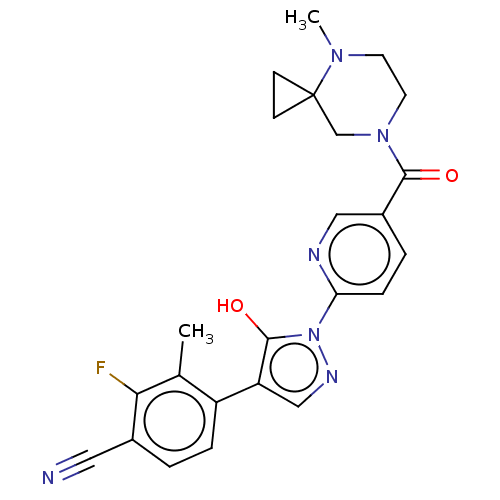 (2-fluoro-4-(5-hydroxy-1-(5-(4-methyl-4,7-diazaspir...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413210 ((R)-4-(1-(5-(3-((2,2-difluoroethyl)(methyl)amino)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein 2 (Homo sapiens (Human)) | BDBM50167916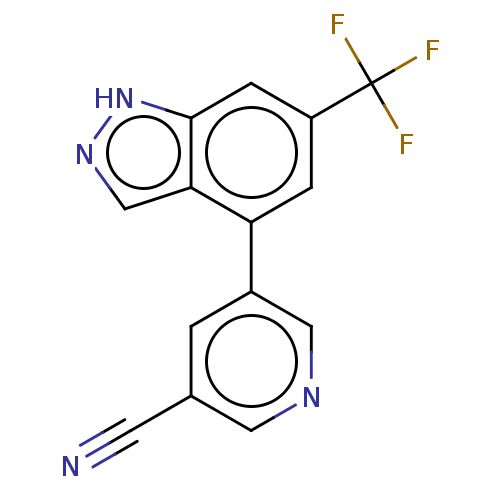 (CHEMBL3798615) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Reversal inhibition of full length recombinant human MetAP2 expressed in baculovirus infected insect Sf9 cells using Met-AMC as substrate measured fo... | Bioorg Med Chem Lett 26: 2774-2778 (2016) Article DOI: 10.1016/j.bmcl.2016.04.073 BindingDB Entry DOI: 10.7270/Q2DF6T4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413140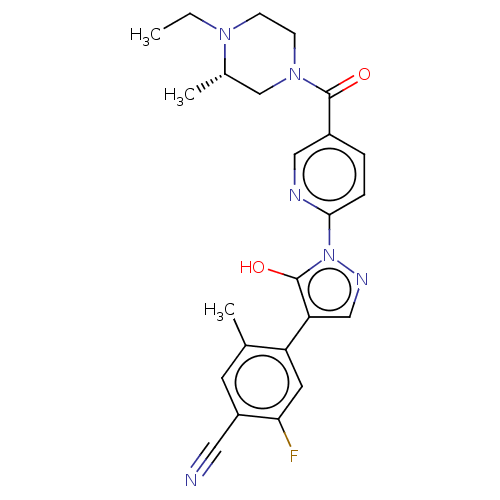 ((S)-4-(1-(5-(4-ethyl-3-methylpiperazine-1-carbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413063 (4-(1-(5-(4-(2,2-difluoroethyl)piperazine-1-carbony...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204288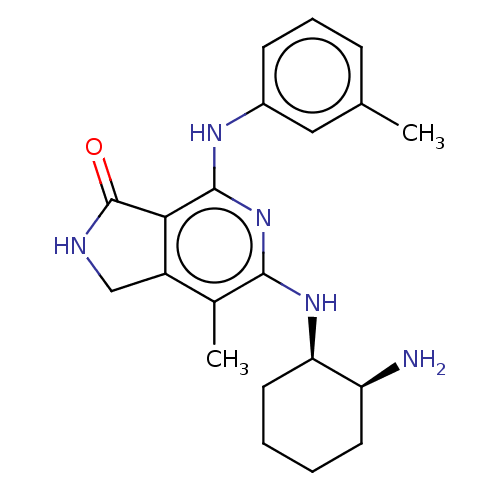 (CHEMBL3925430 | US20230295171, Example 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413069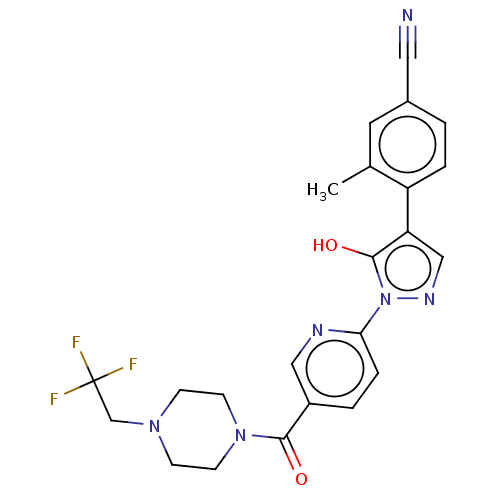 (4-(5-hydroxy-1-(5-(4-(2,2,2-trifluoroethyl)piperaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413199 ((R)-2-fluoro-4-(5-hydroxy-1-(5-(octahydropyrrolo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413169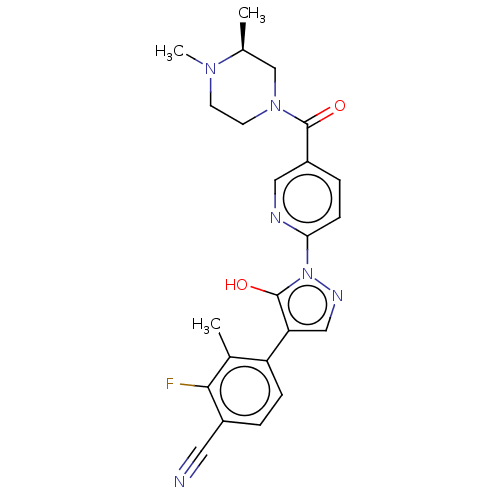 ((S)-4-(1-(5-(3,4-dimethylpiperazine-1-carbonyl)pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413158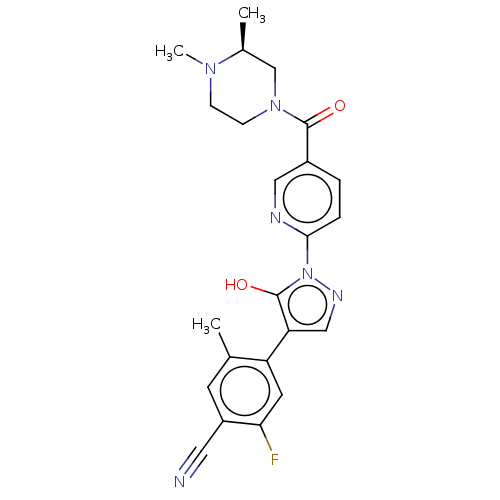 ((S)-4-(1-(5-(3,4-dimethylpiperazine-1-carbonyl)pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50204290 (CHEMBL3979920 | US11077111, Compound IIIa | US2023...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California, Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal 6His-tagged SYK (356 to 635 residues) expressed in Sf9 insect cells using 5-carboxyfluorescein(FAM)-EEPLYWSFPAKKK-NH2 ... | Bioorg Med Chem Lett 26: 5947-5950 (2016) Article DOI: 10.1016/j.bmcl.2016.10.087 BindingDB Entry DOI: 10.7270/Q29888Z2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413194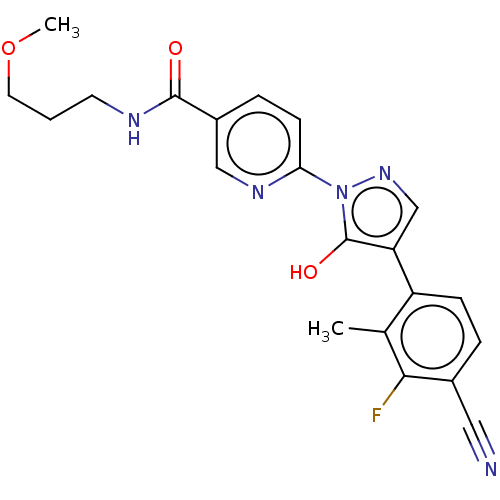 (6-(4-(4-cyano-3-fluoro-2-methylphenyl)-5-hydroxy-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413340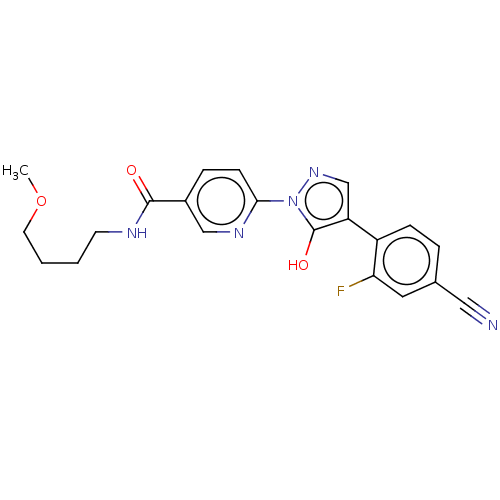 (6-(4-(4-cyano-2-fluorophenyl)-5-hydroxy-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413128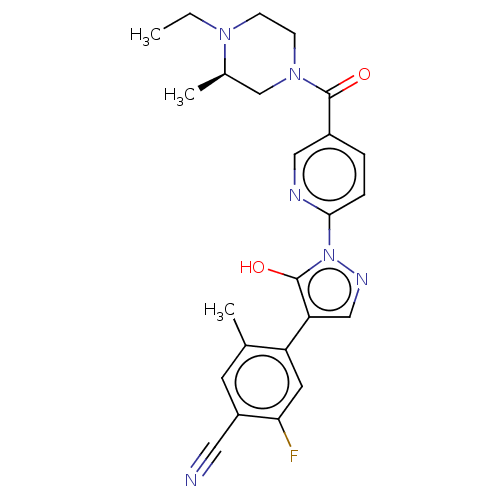 ((R)-4-(1-(5-(4-ethyl-3-methylpiperazine-1-carbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413200 (4-(5-hydroxy-1-(5-(octahydropyrrolo[1,2-a]pyrazine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413188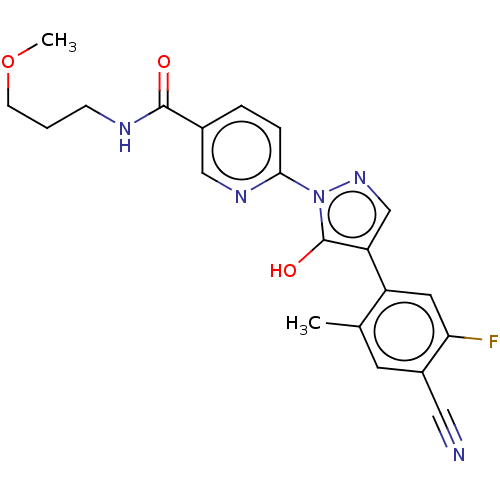 (6-(4-(4-cyano-5-fluoro-2-methylphenyl)-5-hydroxy-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413239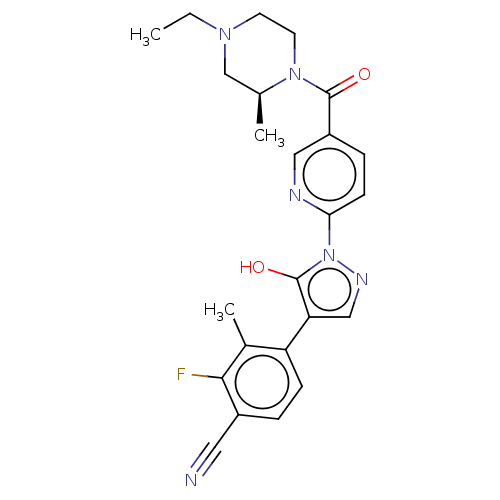 ((S)-4-(1-(5-(4-ethyl-2-methylpiperazine-1-carbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413187 ((S)-2-fluoro-4-(5-hydroxy-1-(5-(3-(methyl(2,2,2-tr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413157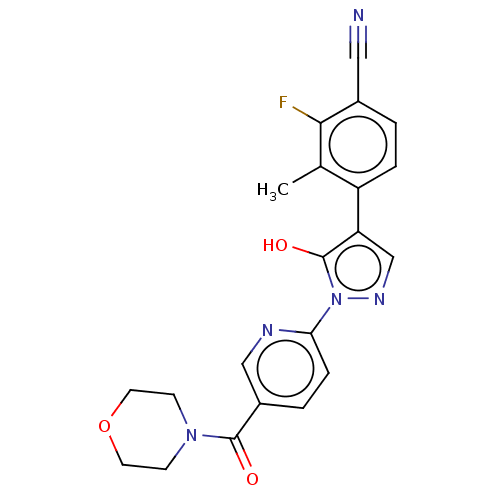 (2-fluoro-4-(5-hydroxy-1-(5-(morpholine-4-carbonyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM412995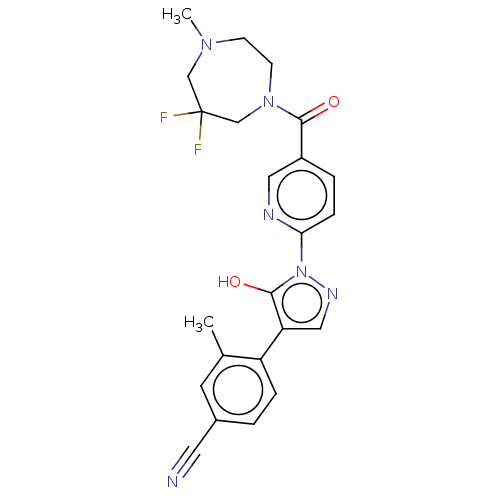 (4-(1-(5-(6,6-difluoro-4-methyl-1,4-diazepane-1-car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413335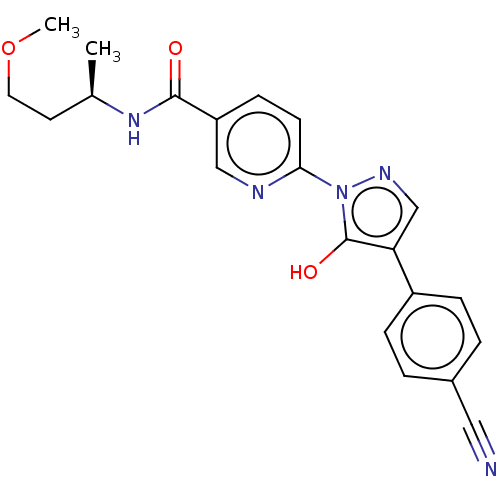 ((R)-6-(4-(4-cyanophenyl)-5-hydroxy-1H-pyrazol-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413347 ((R)-6-(4-(4-cyano-2-methylphenyl)-5-hydroxy-1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413023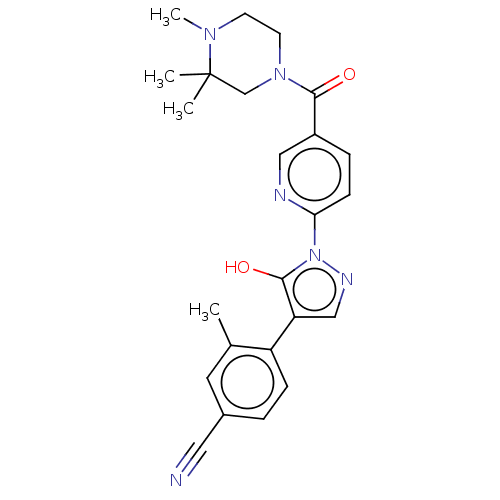 (4-(5-hydroxy-1-(5-(3,3,4-trimethylpiperazine-1-car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413145 (2-fluoro-4-(5-hydroxy-1-(5-(pyrrolidine-1-carbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413164 ((R)-4-(1-(5-(3,4-dimethylpiperazine-1-carbonyl)pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413205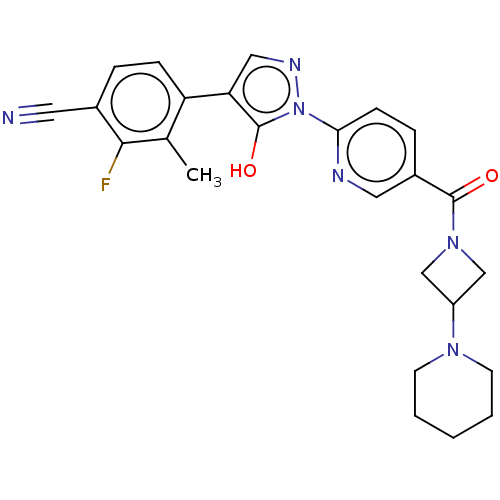 (2-fluoro-4-(5-hydroxy-1-(5-(3-(piperidin-1-yl)azet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413246 (4-(5-hydroxy-1-(5-(4-propylpiperazine-1-carbonyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413011 (6-(4-(4-cyano-2-methylphenyl)-5-hydroxy-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413121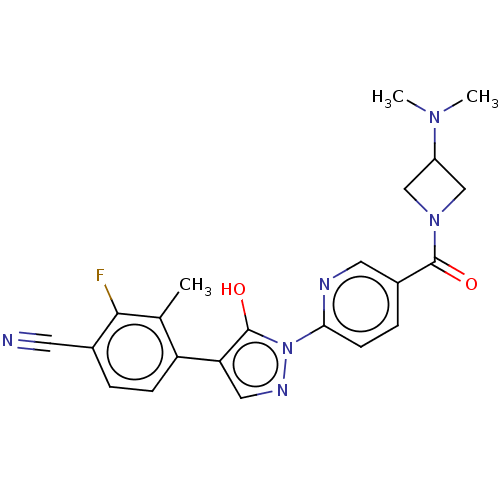 (4-(1-(5-(3-(dimethylamino)azetidine-1-carbonyl)pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413104 (6-(4-(4-cyano-2-methylphenyl)-5-hydroxy-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413098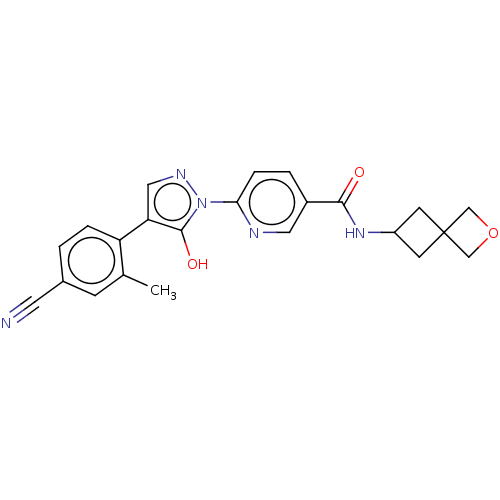 (6-(4-(4-cyano-2-methylphenyl)-5-hydroxy-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413223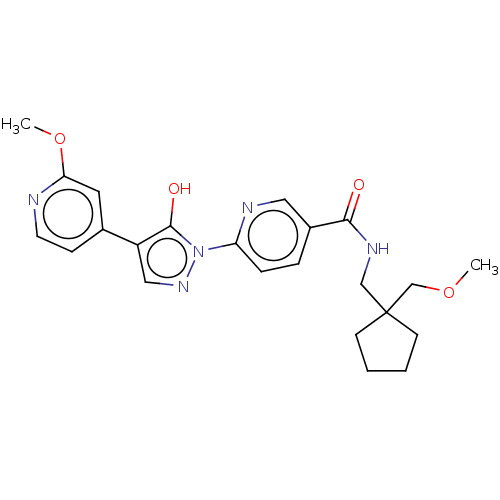 (6-(5-hydroxy-4-(2-methoxypyridin-4-yl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413221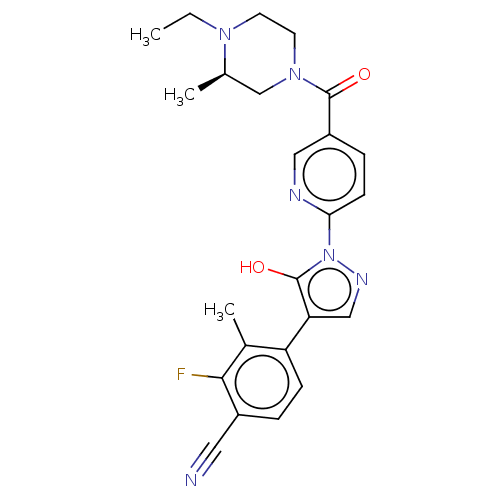 ((R)-4-(1-(5-(4-ethyl-3-methylpiperazine-1-carbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 [181-417] (Homo sapiens (Human)) | BDBM413148 (6-(4-(4-cyano-2-methylphenyl)-5-hydroxy-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The IC50 values for the PHD2 enzyme (residues 181-417) were determined by mixing increasing amounts of inhibitor with a fixed amount of enzyme (5 nM,... | US Patent US10407409 (2019) BindingDB Entry DOI: 10.7270/Q2D79DR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein 2 (Homo sapiens (Human)) | BDBM50167926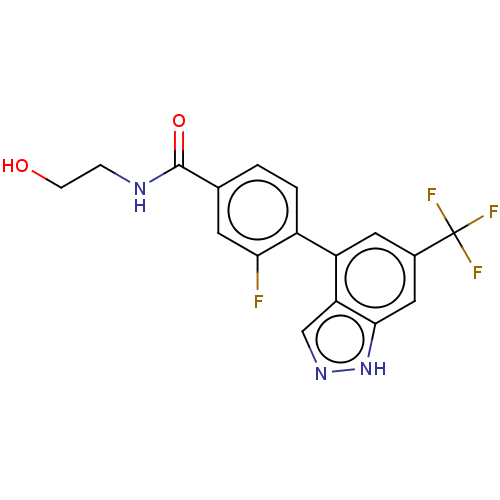 (CHEMBL3798871) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Reversal inhibition of full length recombinant human MetAP2 expressed in baculovirus infected insect Sf9 cells using Met-AMC as substrate measured fo... | Bioorg Med Chem Lett 26: 2774-2778 (2016) Article DOI: 10.1016/j.bmcl.2016.04.073 BindingDB Entry DOI: 10.7270/Q2DF6T4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein 2 (Homo sapiens (Human)) | BDBM50167915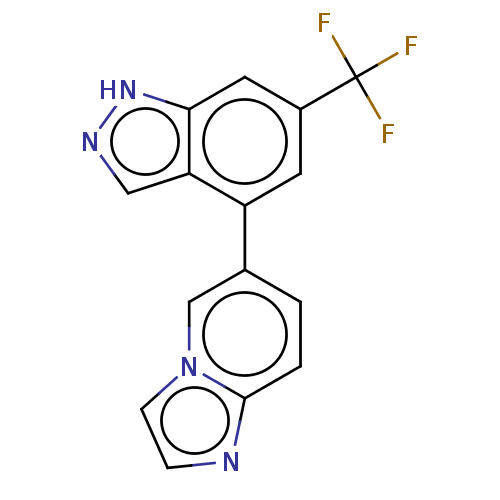 (CHEMBL3798727) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Reversal inhibition of full length recombinant human MetAP2 expressed in baculovirus infected insect Sf9 cells using Met-AMC as substrate measured fo... | Bioorg Med Chem Lett 26: 2774-2778 (2016) Article DOI: 10.1016/j.bmcl.2016.04.073 BindingDB Entry DOI: 10.7270/Q2DF6T4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 645 total ) | Next | Last >> |