

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin II (Plasmodium falciparum) | BDBM50323472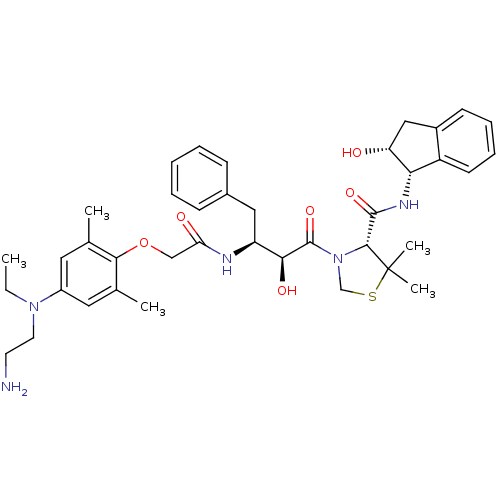 ((R)-3-((2S,3S)-3-(2-(4-((2-aminoethyl)(ethyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323469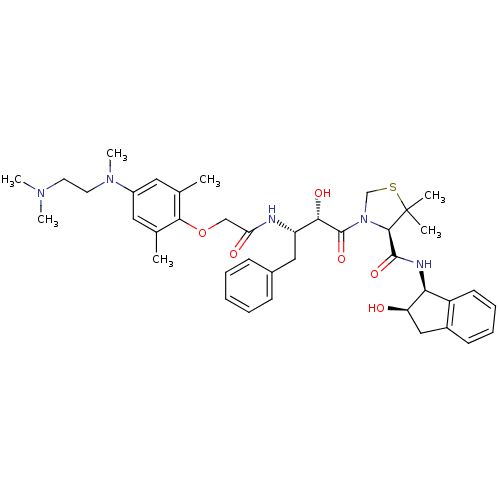 ((R)-3-((2S,3S)-3-(2-(4-((2-(dimethylamino)ethyl)(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559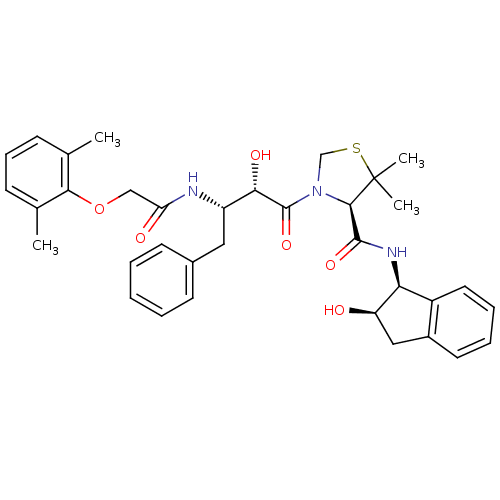 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323470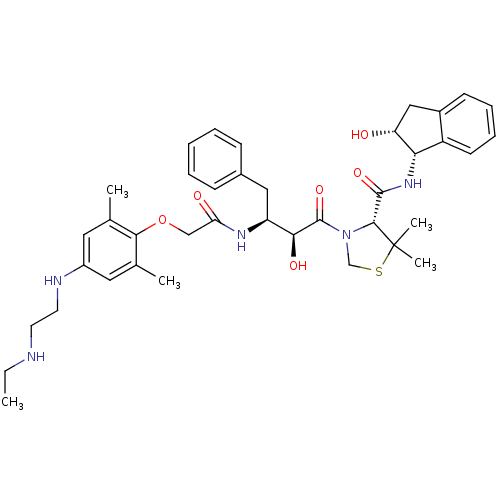 ((R)-3-((2S,3S)-3-(2-(4-(2-(ethylamino)ethylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001073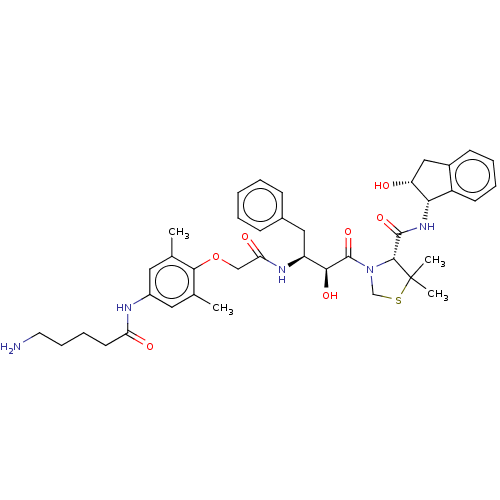 (CHEMBL3236067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001072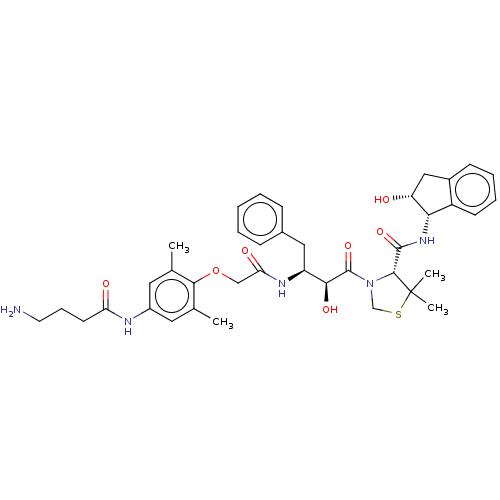 (CHEMBL3236066) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323468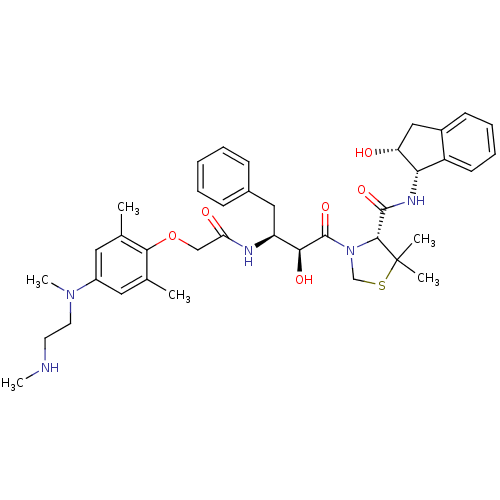 ((R)-3-((2S,3S)-3-(2-(2,6-dimethyl-4-(methyl(2-(met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001070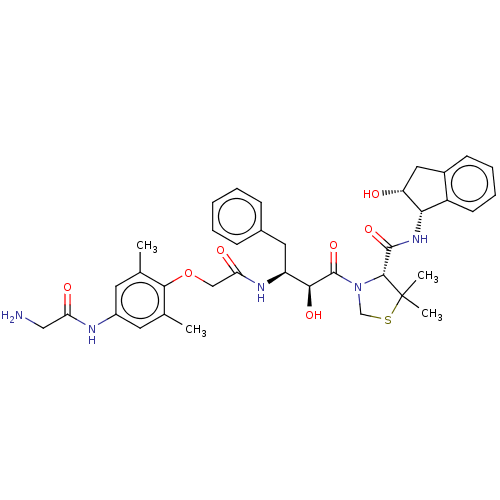 (CHEMBL3236064) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001069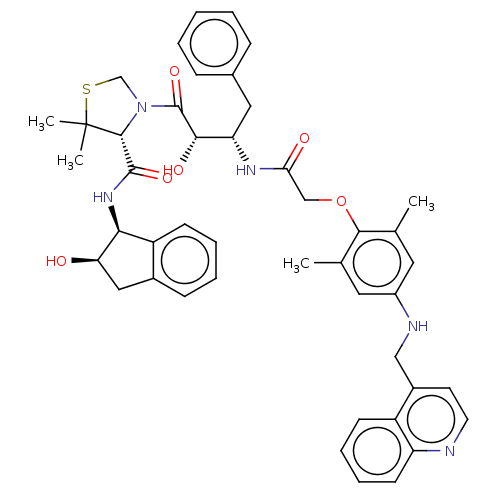 (CHEMBL3236063) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001071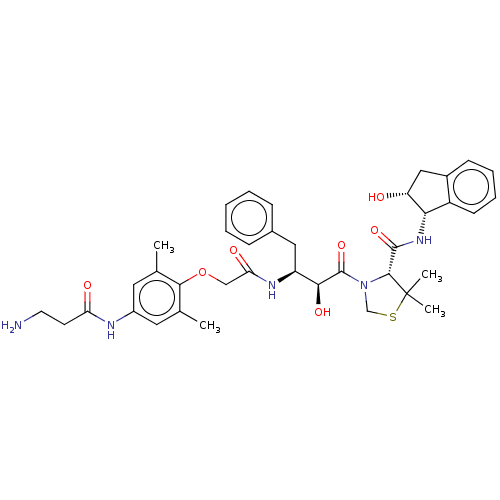 (CHEMBL3236065) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323473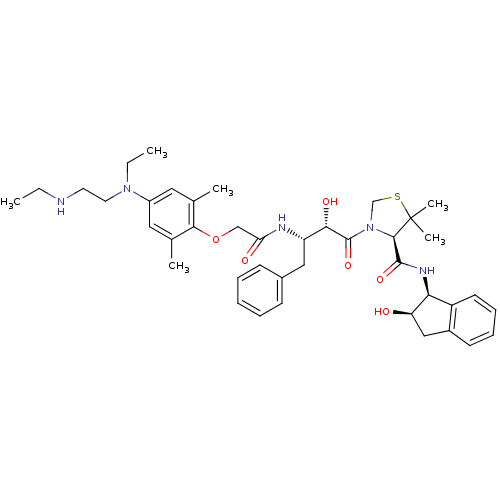 ((R)-3-((2S,3S)-3-(2-(4-(ethyl(2-(ethylamino)ethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001075 (CHEMBL3236069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001067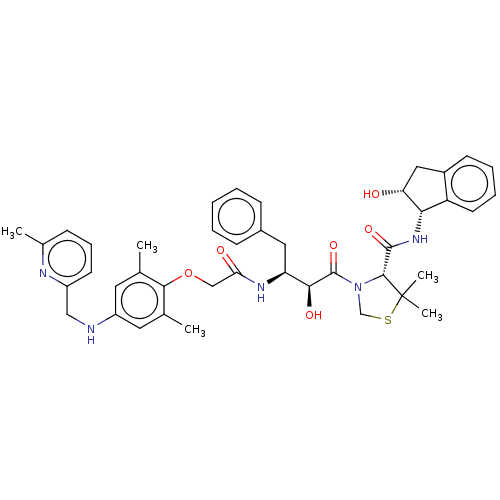 (CHEMBL3236061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001068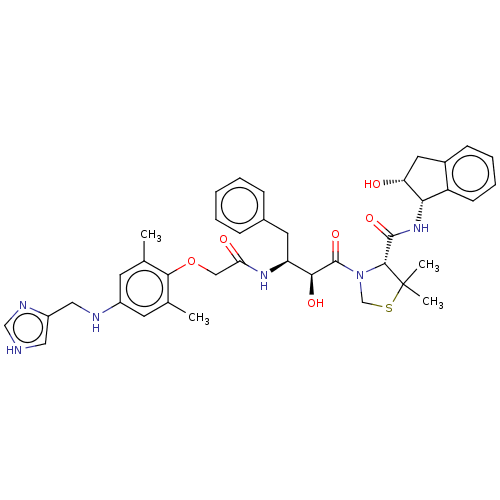 (CHEMBL3236062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323471 ((R)-3-((2S,3S)-3-(2-(4-(2-(diethylamino)ethylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001064 (CHEMBL3236058) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001074 (CHEMBL3236068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323464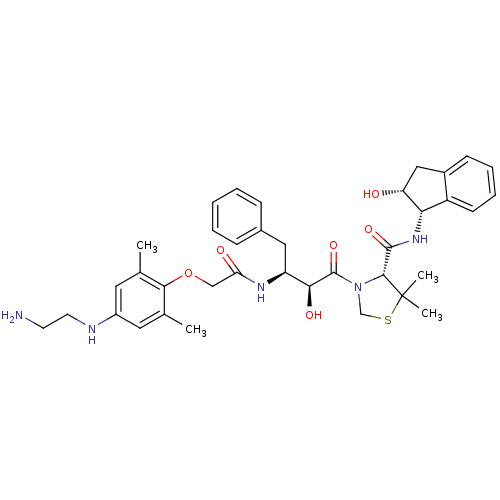 ((R)-3-((2S,3S)-3-(2-(4-(2-aminoethylamino)-2,6-dim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323467 ((R)-3-((2S,3S)-3-(2-(4-((2-aminoethyl)(methyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323465 ((R)-3-((2S,3S)-3-(2-(2,6-dimethyl-4-(2-(methylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50273749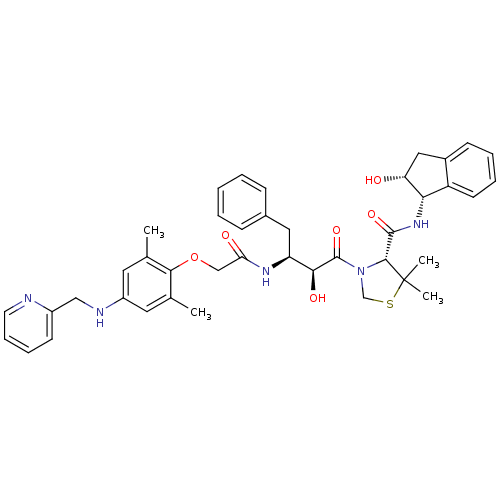 ((R)-3-((2S,3S)-3-(2-(2,6-dimethyl-4-(pyridin-2-ylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323474 ((R)-3-((2S,3S)-3-(2-(4-((2-(diethylamino)ethyl)(et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001065 (CHEMBL3236059) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323466 ((R)-3-((2S,3S)-3-(2-(4-(2-(dimethylamino)ethylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 20: 4836-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.099 BindingDB Entry DOI: 10.7270/Q2F18ZXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50001066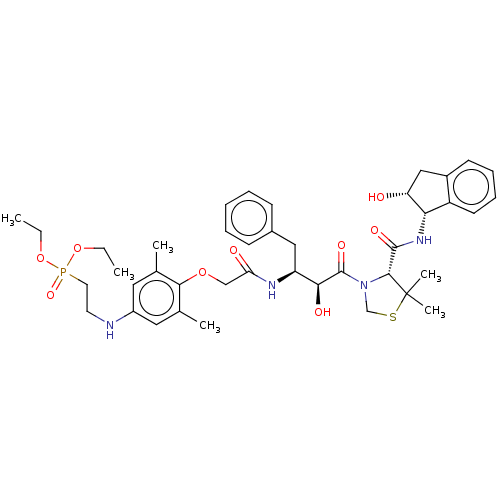 (CHEMBL3236060) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Bioorg Med Chem Lett 24: 1698-701 (2014) Article DOI: 10.1016/j.bmcl.2014.02.051 BindingDB Entry DOI: 10.7270/Q27S7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132916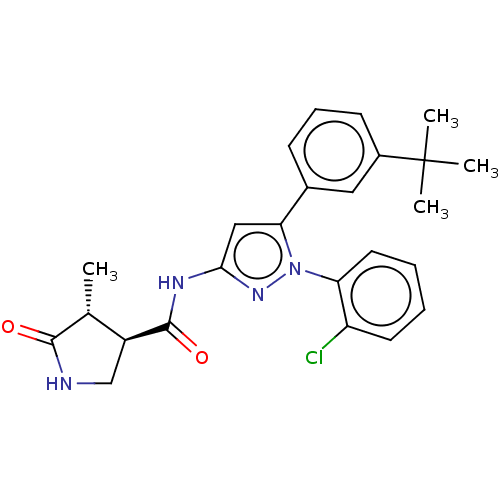 (US8846746, 194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132862 (US8846746, 132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493926 (US10988462, Example 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109863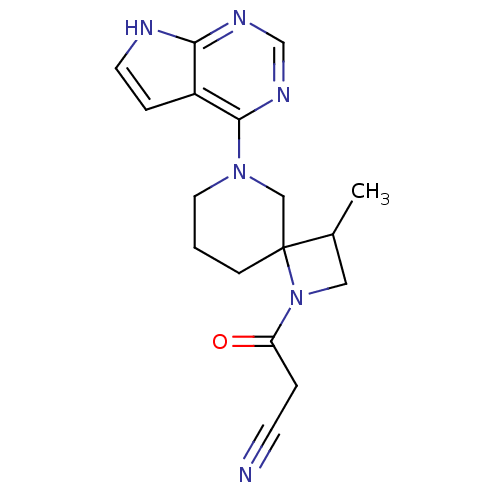 (US8609647, 1 | US8609647, 15 | US8609647, 2 | US86...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132870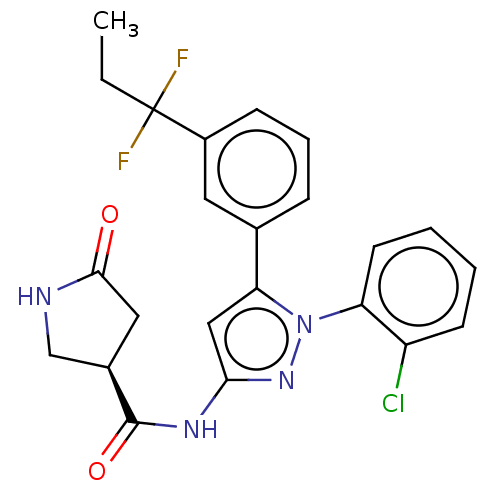 (US8846746, 140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM133145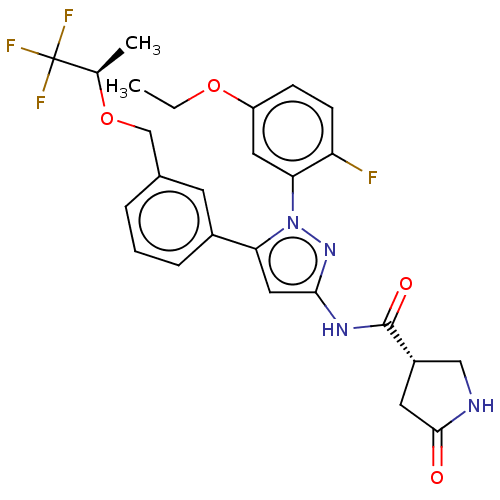 (US8846746, 463) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132882 (US8846746, 154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50167609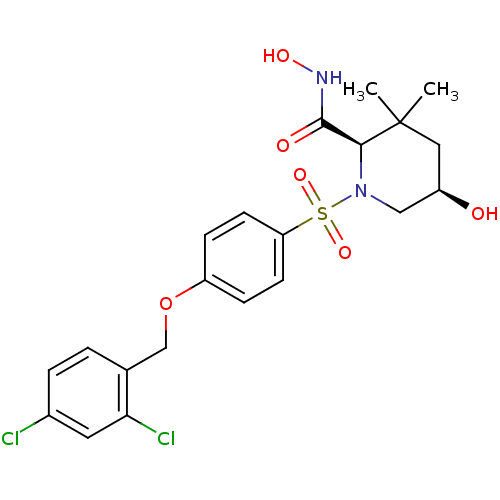 ((2R,5R)-1-[4-(2,4-Dichloro-benzyloxy)-benzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant aggrecanase 1 after 150 mins by fluorescence plate reader | J Med Chem 54: 2839-63 (2011) Article DOI: 10.1021/jm101609j BindingDB Entry DOI: 10.7270/Q2N87B3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50341824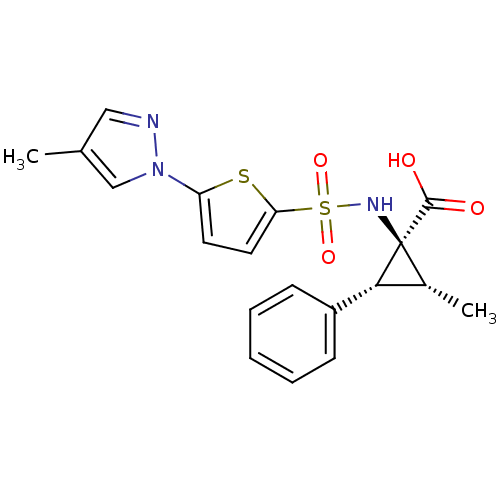 ((1S,2R,3R)-2-Methyl-1-[5-(4-methylpyrazol-1-yl)thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP14 after 60 mins by fluorescence plate reader | J Med Chem 54: 2839-63 (2011) Article DOI: 10.1021/jm101609j BindingDB Entry DOI: 10.7270/Q2N87B3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493894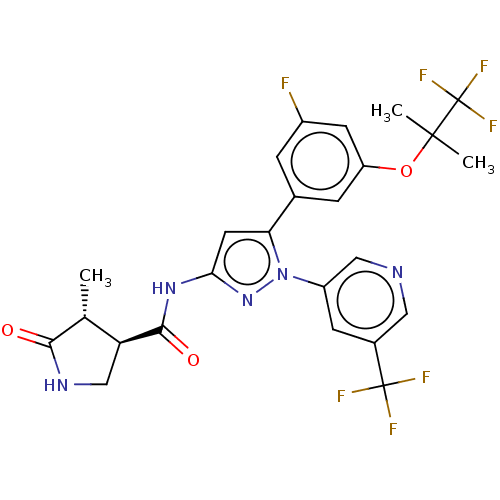 (US10988462, Example 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK2 (880 to end residues) expressed in baculovirus infected Sf21 cells using TK-substrate-biotin as substra... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132856 (US8846746, 126) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493906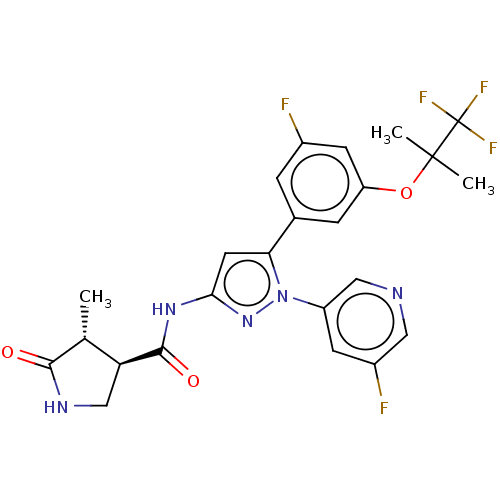 (US10988462, Example 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493905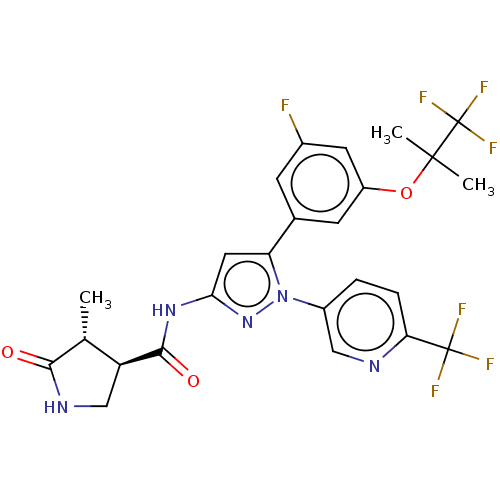 (US10988462, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50341816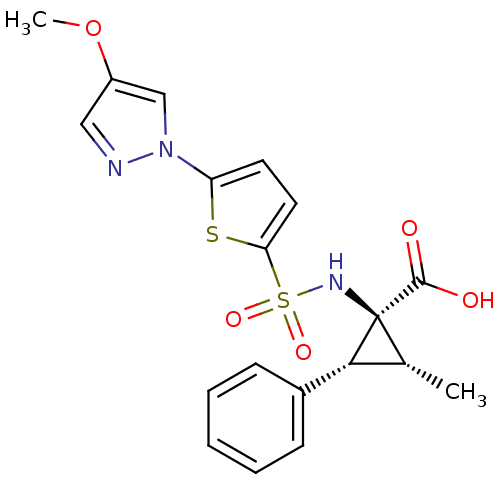 ((1S,2R,3R)-1-[5-(4-Methoxypyrazol-1-yl)thiophene-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP14 after 60 mins by fluorescence plate reader | J Med Chem 54: 2839-63 (2011) Article DOI: 10.1021/jm101609j BindingDB Entry DOI: 10.7270/Q2N87B3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132860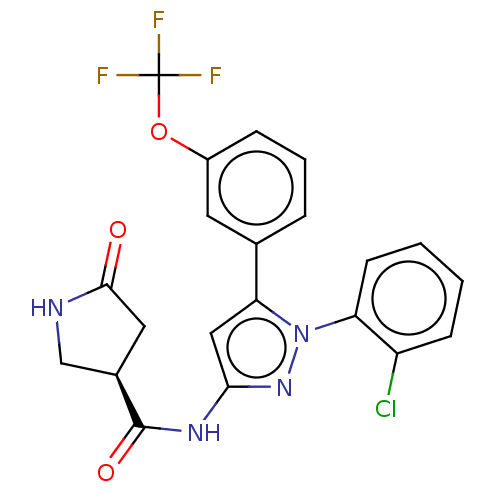 (US8846746, 130) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493901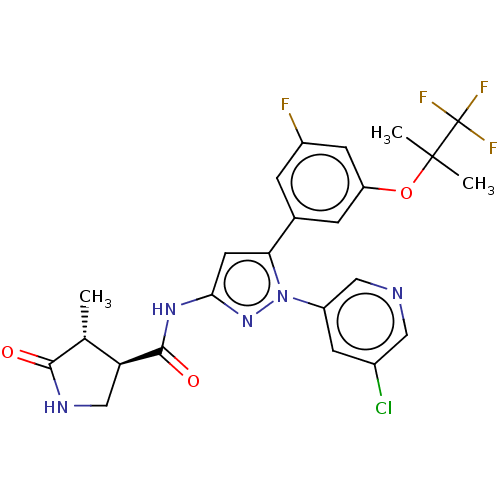 (US10988462, Example 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132929 (US8846746, 208) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132915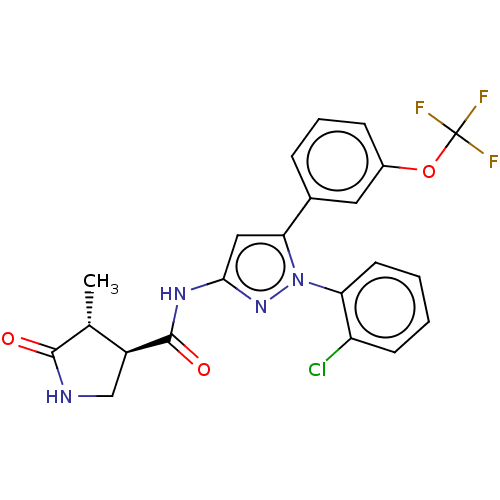 (US8846746, 193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132874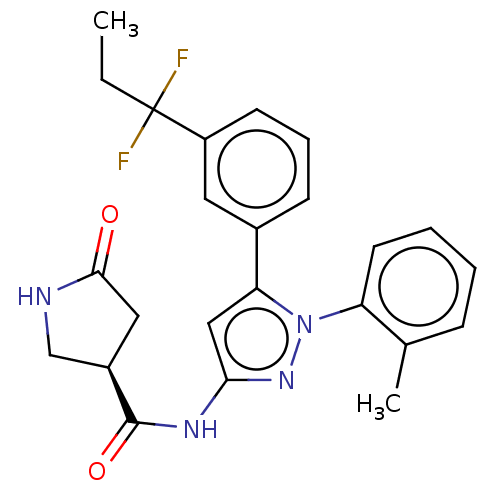 (US8846746, 144) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM133143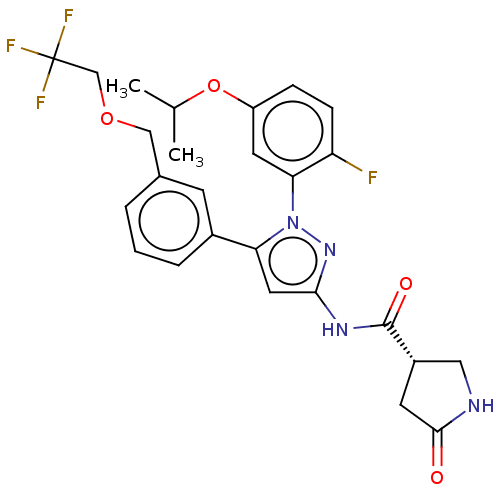 (US8846746, 461) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50167609 ((2R,5R)-1-[4-(2,4-Dichloro-benzyloxy)-benzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant aggrecanase 2 after 150 mins by fluorescence plate reader | J Med Chem 54: 2839-63 (2011) Article DOI: 10.1021/jm101609j BindingDB Entry DOI: 10.7270/Q2N87B3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM132924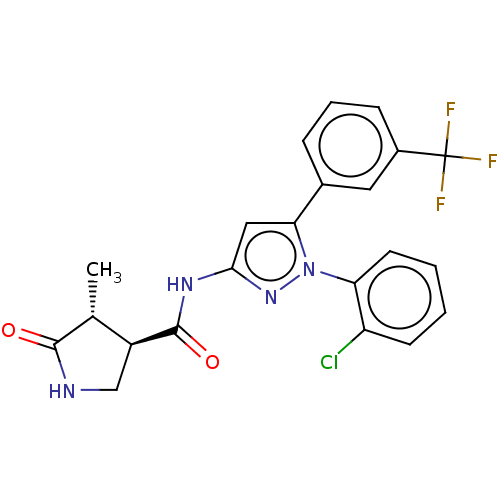 (US8846746, 203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | 37 |
Japan Tobacco Inc. US Patent | Assay Description The stable cell line expressing human SGLT1 was seeded at 5x104 cells/well on BioCoat Poly-D-Lysine 96 well plate with Lid (Becton Dickinson and Comp... | US Patent US8846746 (2014) BindingDB Entry DOI: 10.7270/Q27M06MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288232 (2-[2-(carboxymethoxy)-4-(2-{4H,5H,6H,7H-thieno[2,3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vitronectin binding to GPIIb/IIIIa Vitronectin receptor | Bioorg Med Chem Lett 6: 2601-2606 (1996) Article DOI: 10.1016/0960-894X(96)00476-3 BindingDB Entry DOI: 10.7270/Q28C9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1402 total ) | Next | Last >> |