

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549700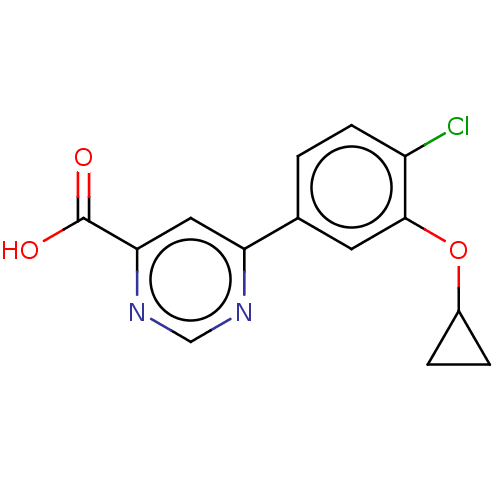 (CHEMBL4740126) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KMO (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549707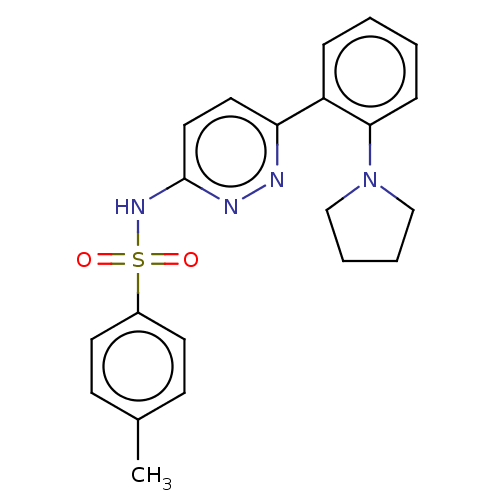 (CHEMBL4781856) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549704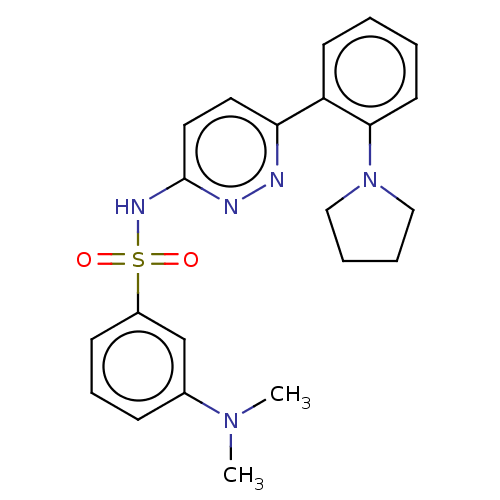 (CHEMBL4764931) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549705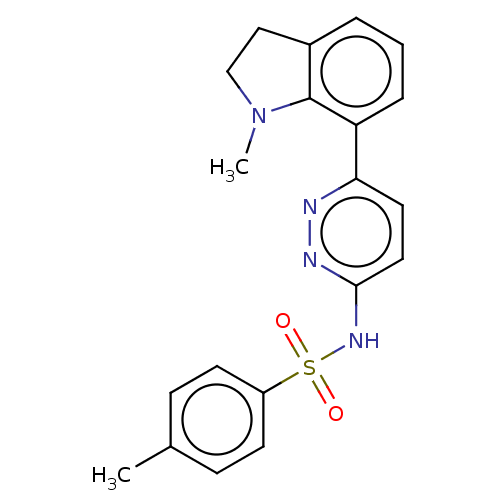 (CHEMBL4756311) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230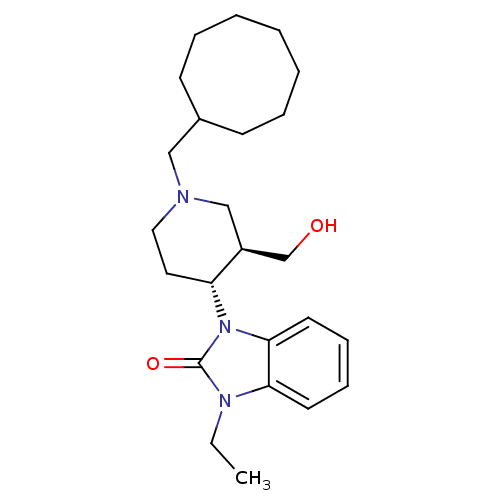 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50266003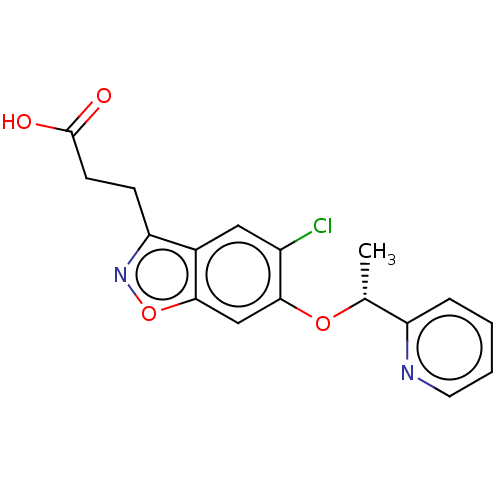 (CHEMBL4091152) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KMO (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549706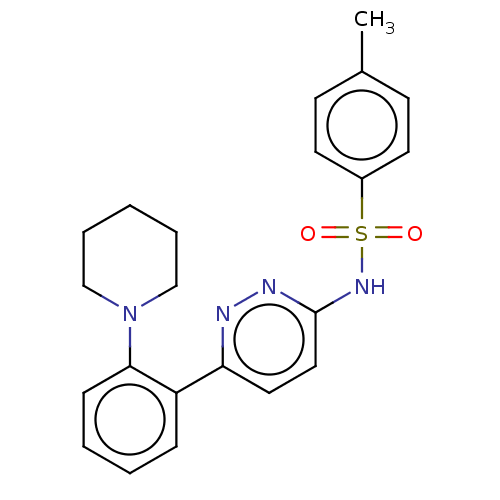 (CHEMBL4744083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549704 (CHEMBL4764931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549706 (CHEMBL4744083) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549714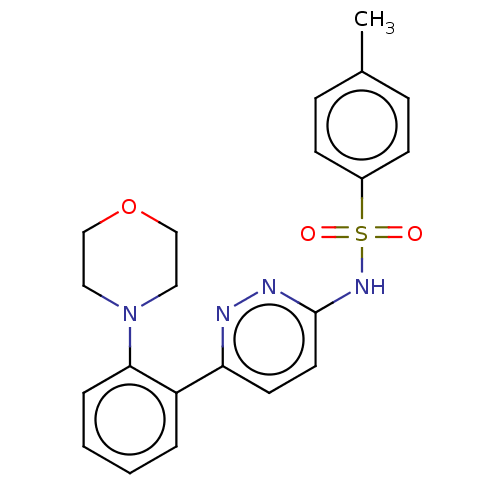 (CHEMBL4762572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549707 (CHEMBL4781856) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549700 (CHEMBL4740126) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549715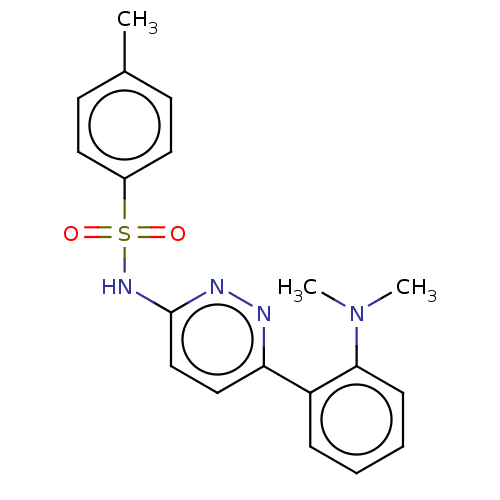 (CHEMBL4760680) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083227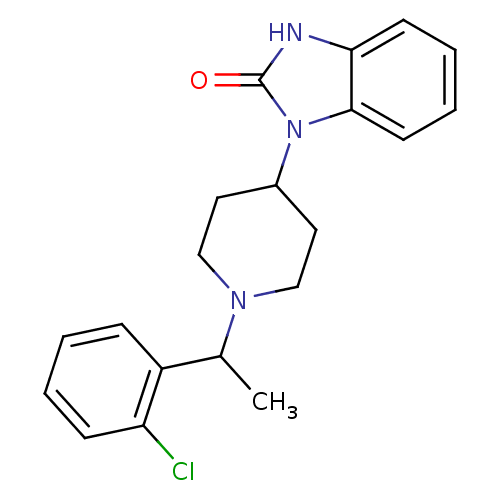 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083232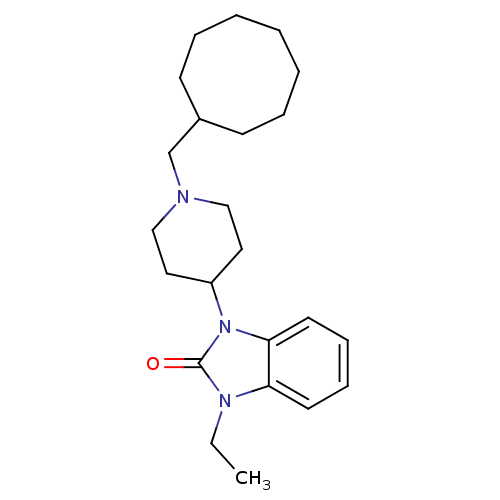 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549711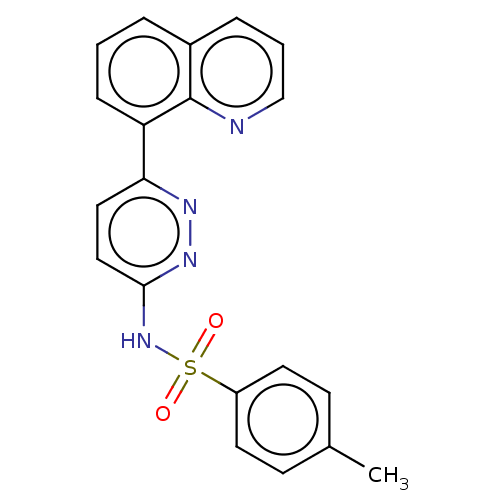 (CHEMBL4764672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549700 (CHEMBL4740126) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]U-69593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549705 (CHEMBL4756311) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549708 (CHEMBL4776854) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549712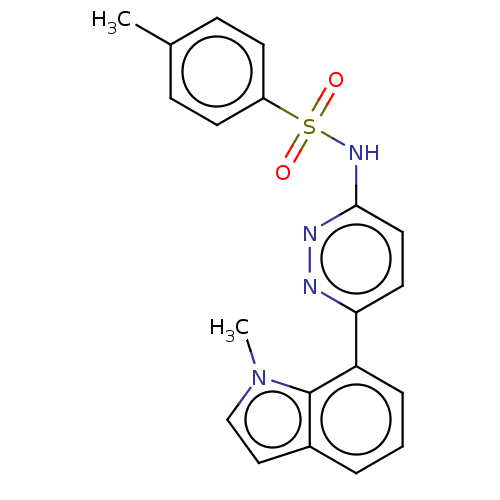 (CHEMBL4790147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549719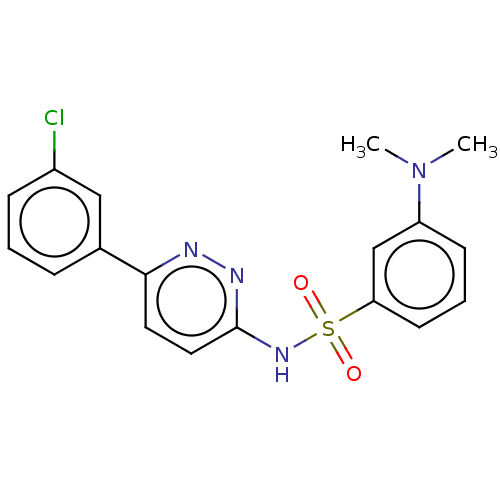 (CHEMBL4762842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]-U-69,593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549701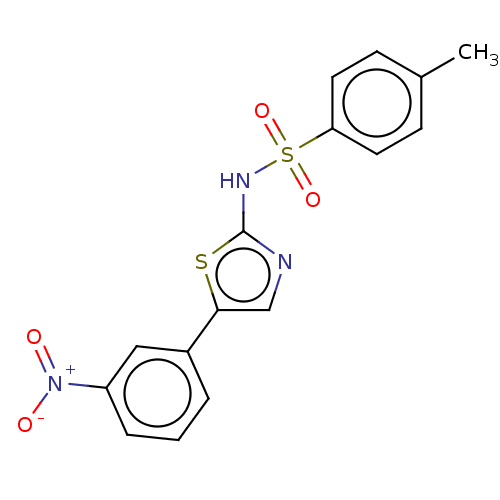 (CHEMBL4745247) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549709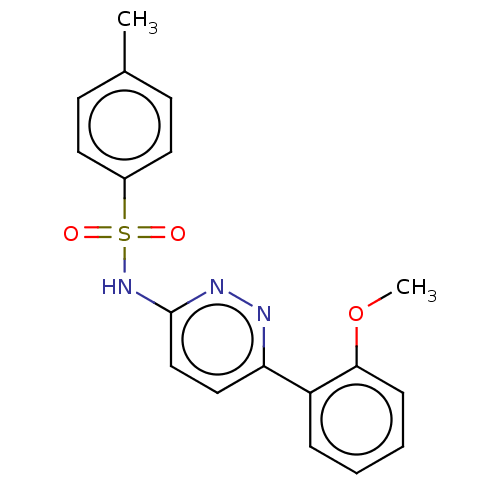 (CHEMBL4743950) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549701 (CHEMBL4745247) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KMO (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50126147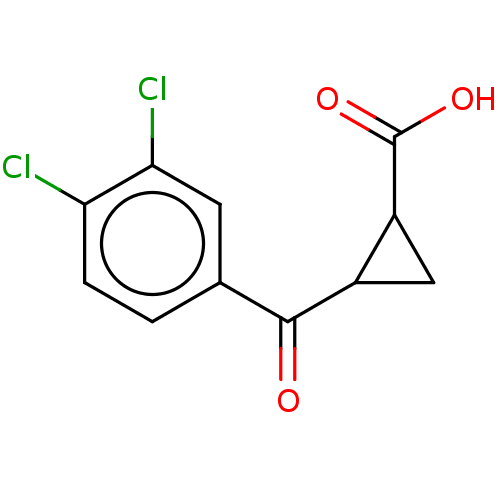 (CHEMBL3629571) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KMO (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor mu 1 by displacing diprenorphine | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549710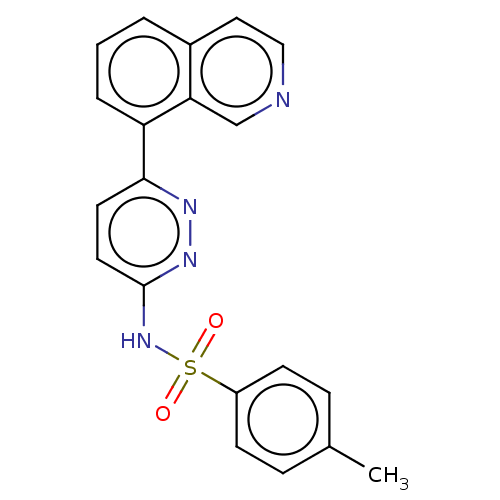 (CHEMBL4745015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549701 (CHEMBL4745247) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549716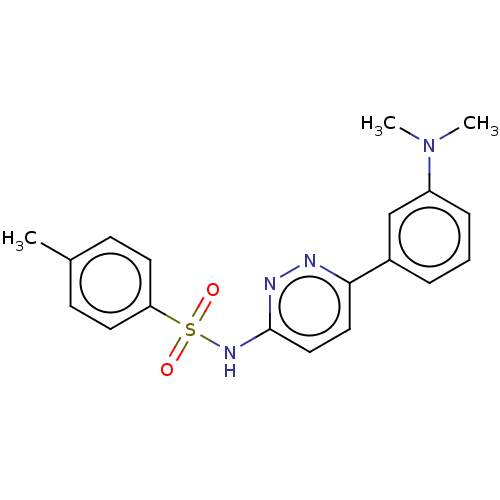 (CHEMBL4749459) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549713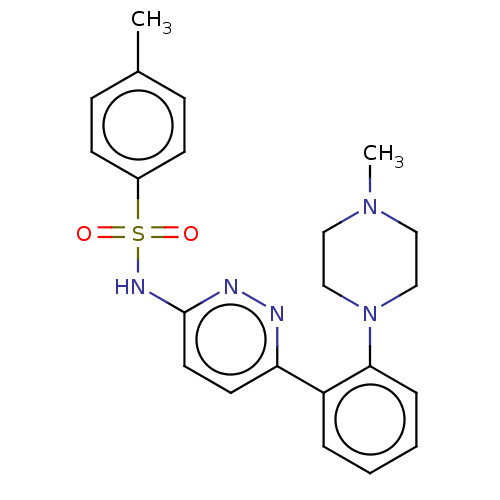 (CHEMBL4790669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549721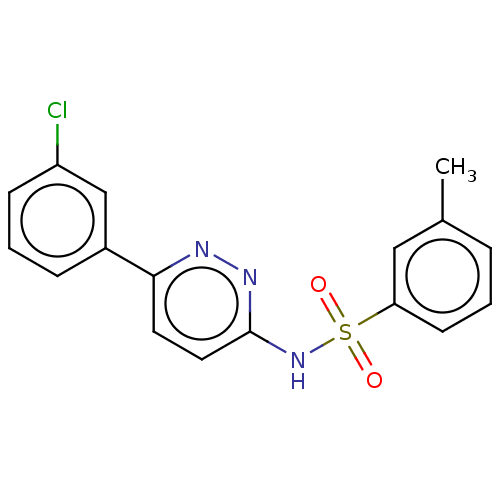 (CHEMBL4791546) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137562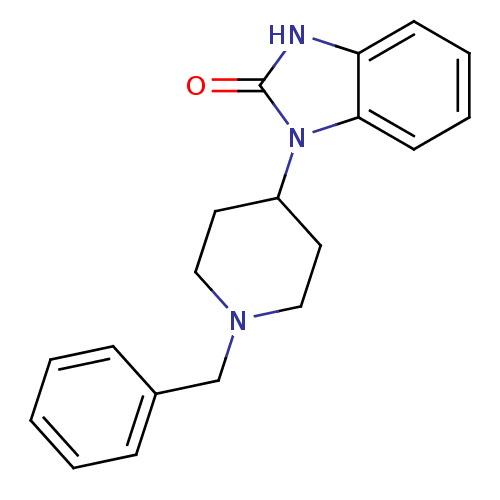 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]-U-69,593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549718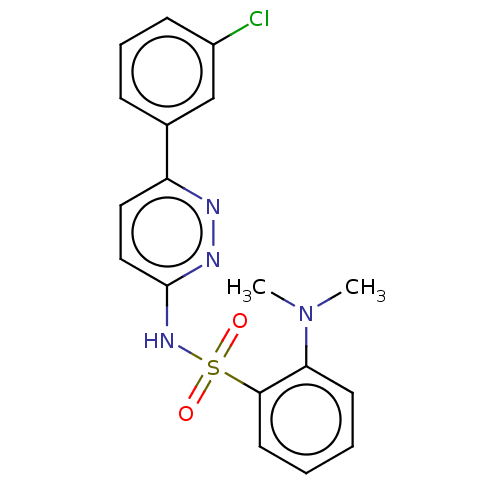 (CHEMBL4784999) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137562 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549720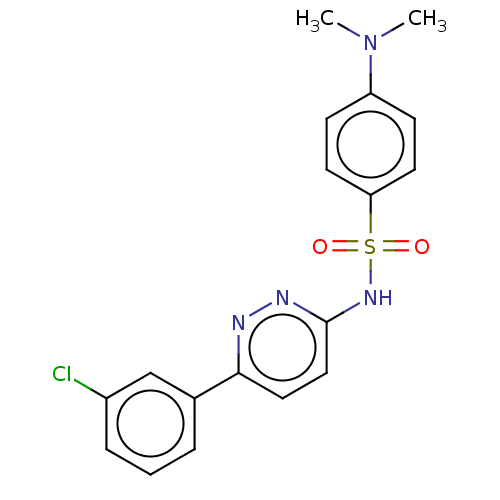 (CHEMBL4763938) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]U-69593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50549717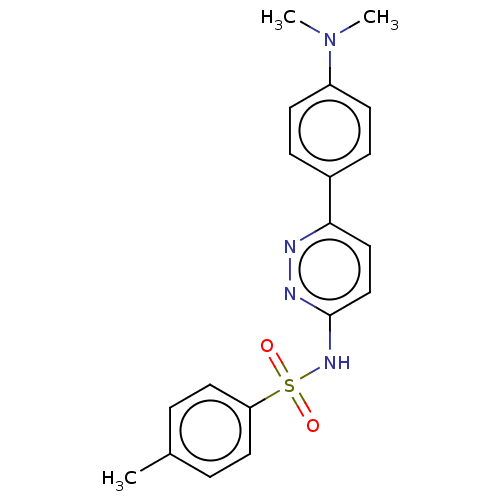 (CHEMBL4751196) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127753 BindingDB Entry DOI: 10.7270/Q2XK8K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-diprenorphine binding to human Opioid receptor mu 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083229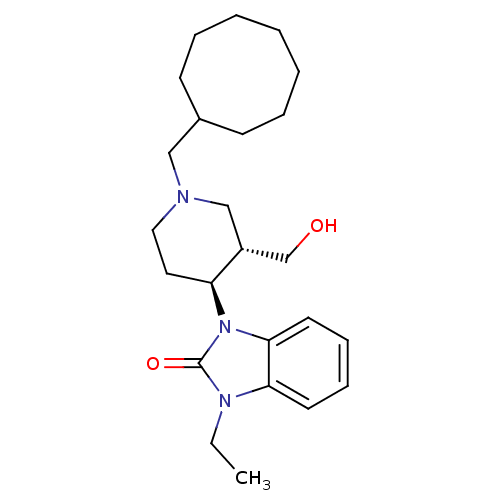 (1-((3S,4S)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-diprenorphine binding to human Opioid receptor mu 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]-U-69,593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137562 (1-(1-Benzyl-piperidin-4-yl)-1,3-dihydro-benzoimida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor mu 1 by displacing diprenorphine | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083227 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor delta 1 by displacing radioligand [3H][D-Ala2,D-Leu5]enk... | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor mu 1 by displacing diprenorphine | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 68 total ) | Next | Last >> |