

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335574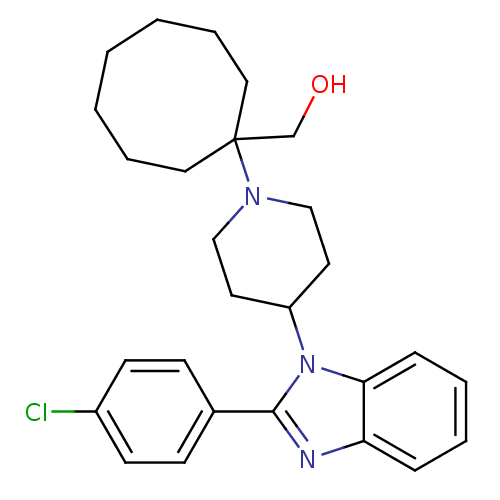 ((1-{4-[2-(4-Chlorophenyl)-1H-benzimidazol-1-yl]pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178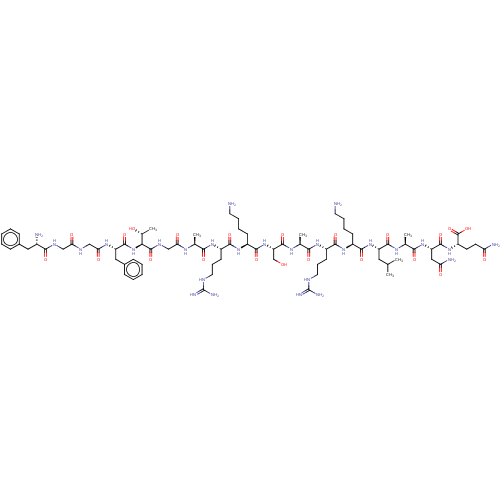 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335575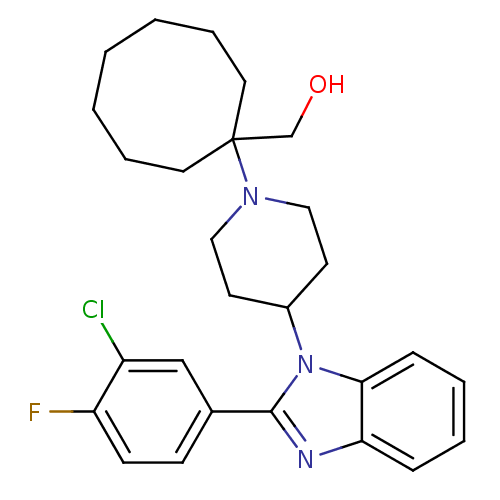 ((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335568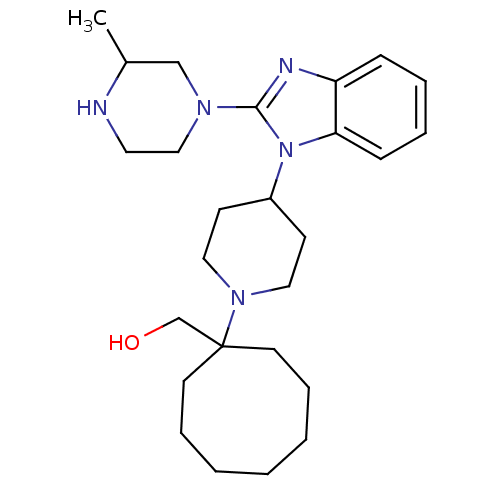 ((1-{4-[2-(3-Methylpiperazin-1-yl)-1H-benzimidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278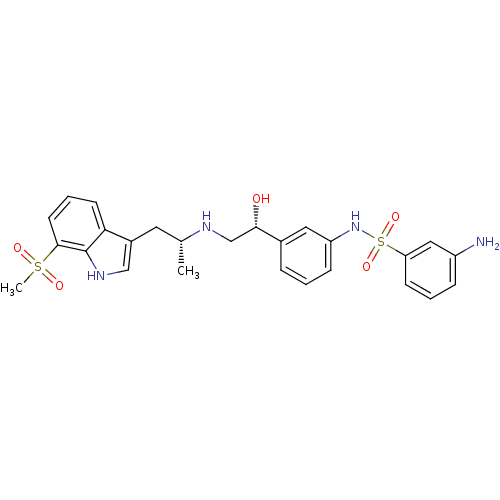 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335570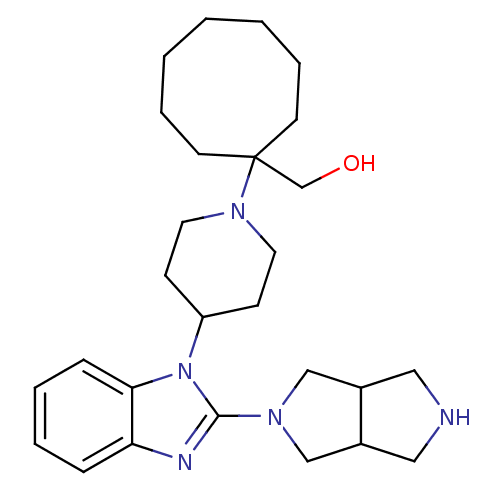 (CHEMBL1650844 | {1-[4-(2-{Hexahydropyrrolo[3,4-c]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267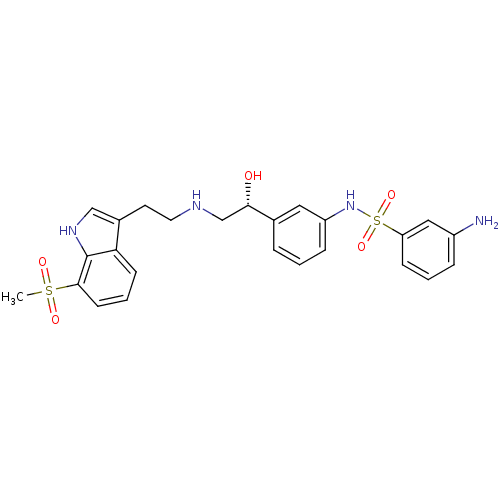 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220612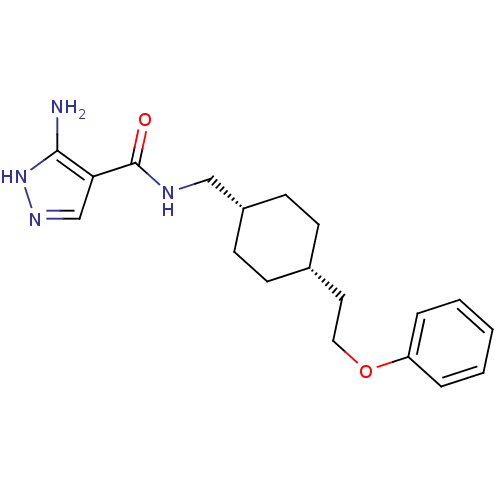 (3-amino-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring cAMP accumulation in CHO cells expressing cloned human Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220606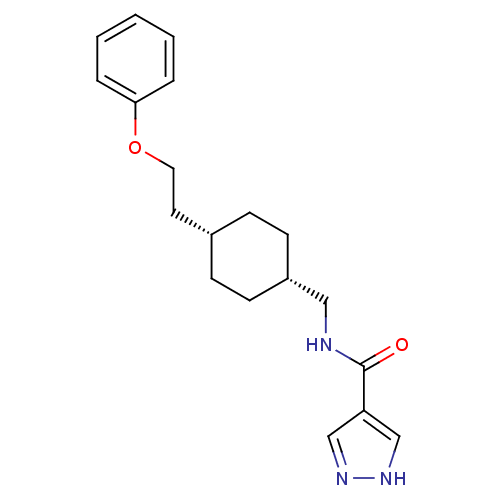 (CHEMBL249093 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335576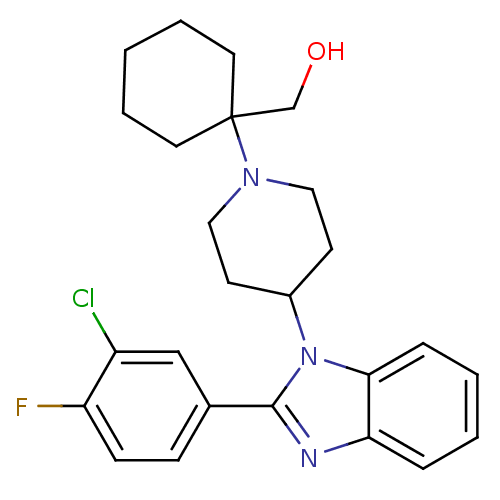 ((1-{4-[2-(3-Chloro-4-fluorophenyl)-1H-benzimidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335567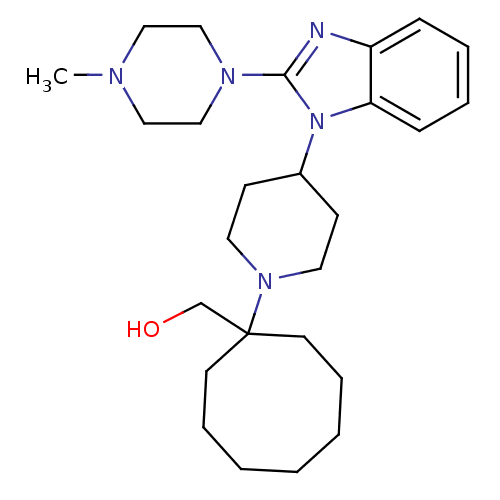 ((1-{4-[2-(4-Methylpiperazin-1-yl)-1H-benzimidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335569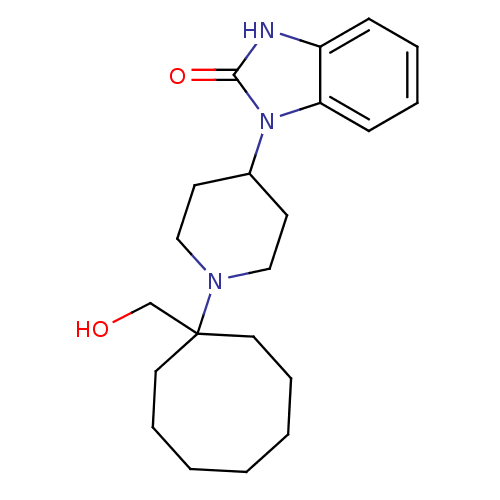 (1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335573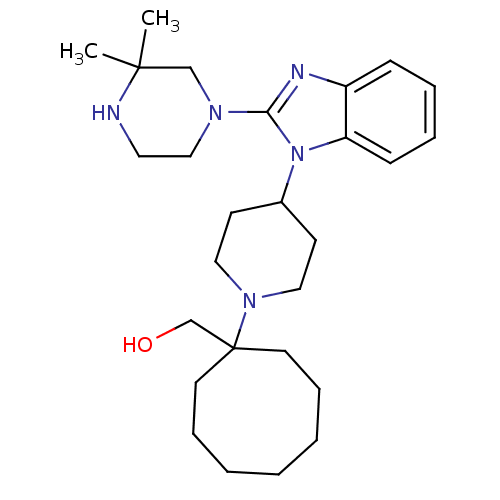 ((1-{4-[2-(3,3-Dimethylpiperazin-1-yl)-1H-benzimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220592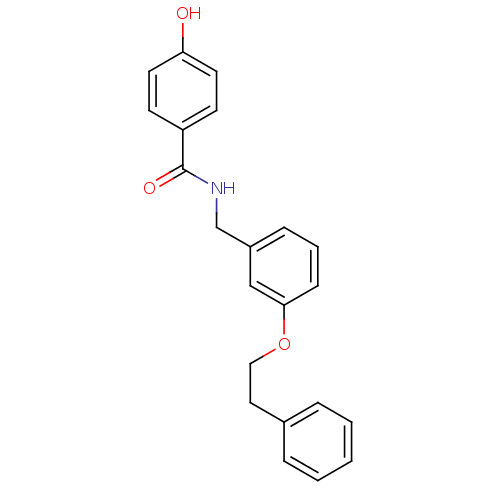 (CHEMBL248875 | N-(3-phenethoxybenzyl)-4-hydroxyben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220611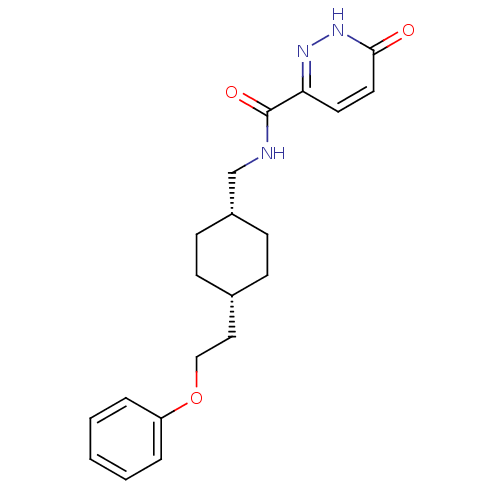 (6-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220608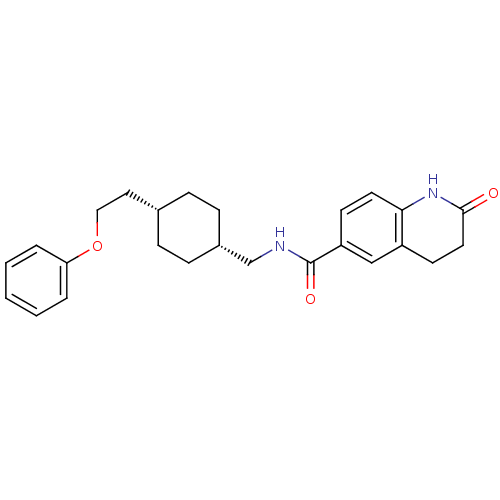 (2-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335571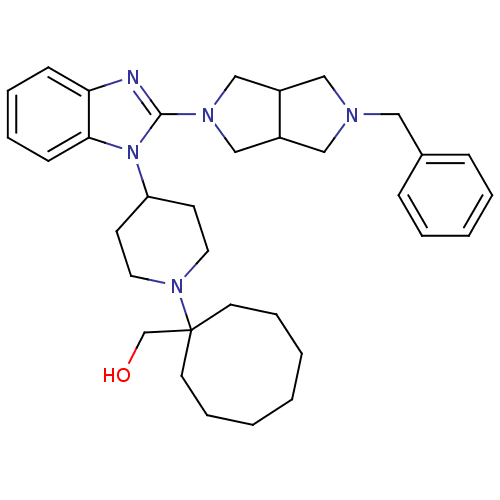 (CHEMBL1650845 | {1-[4-(2-{5-Benzylhexahydropyrrolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220602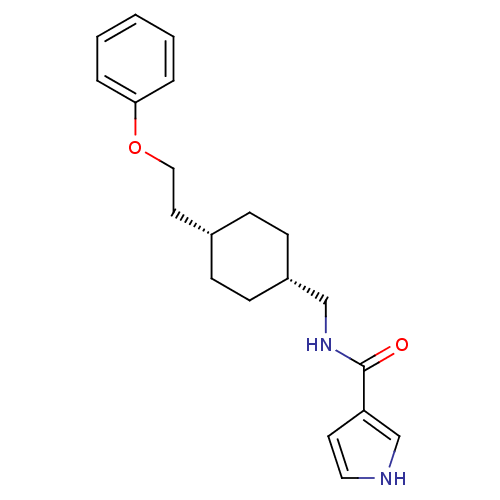 (CHEMBL249307 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220603 (4-hydroxy-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220595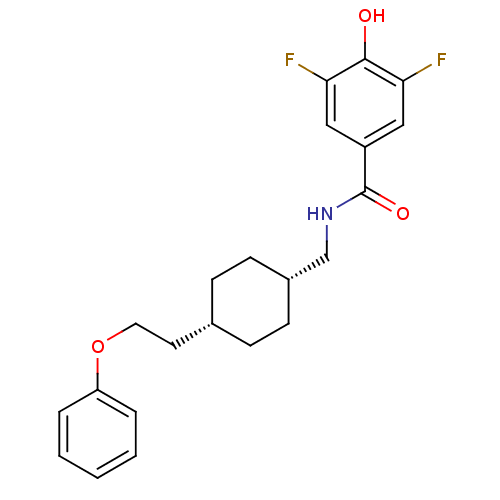 (3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(2-phenoxyeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220591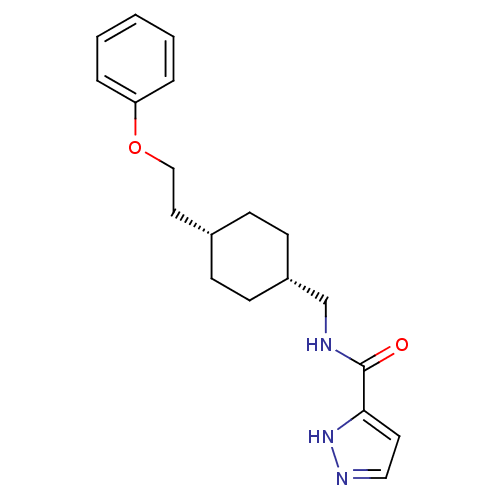 (CHEMBL249909 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220598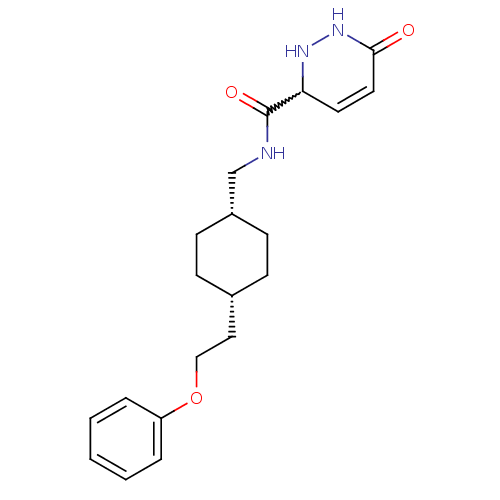 (6-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220599 (3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(phenoxymethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250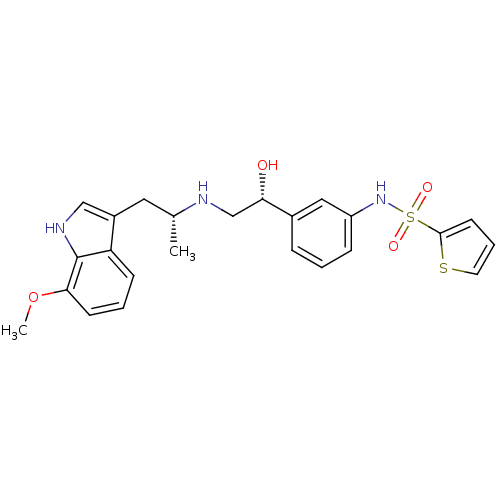 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220610 (CHEMBL250108 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220601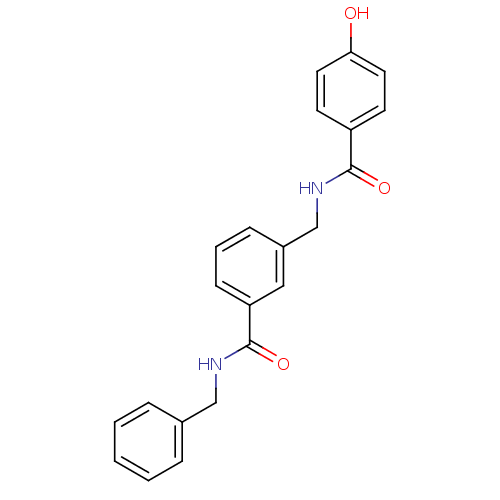 (CHEMBL400917 | N-benzyl-3-{[(4-hydroxybenzoyl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220597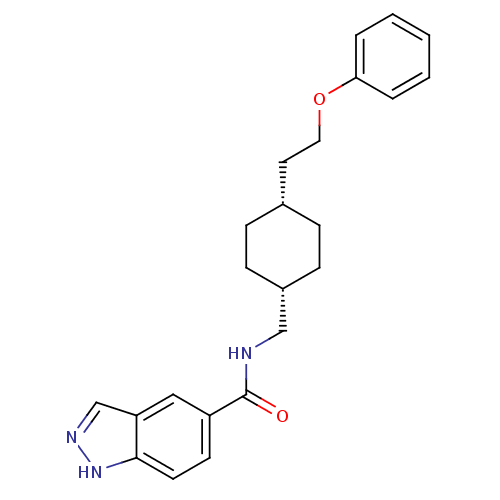 (CHEMBL398356 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50335569 (1-{1-[1-(Hydroxymethyl)cyclooctyl]piperidin-4-yl}-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOP receptor expressed in CHO-K1 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257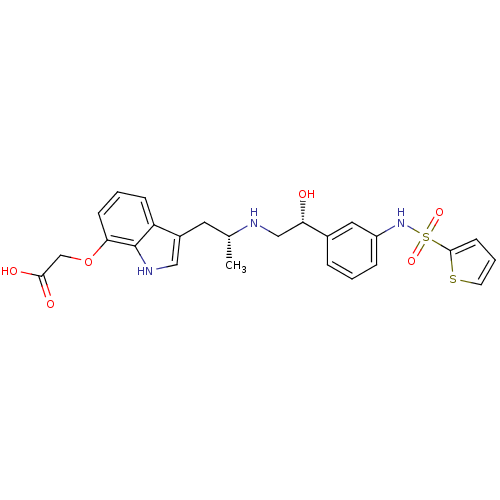 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220607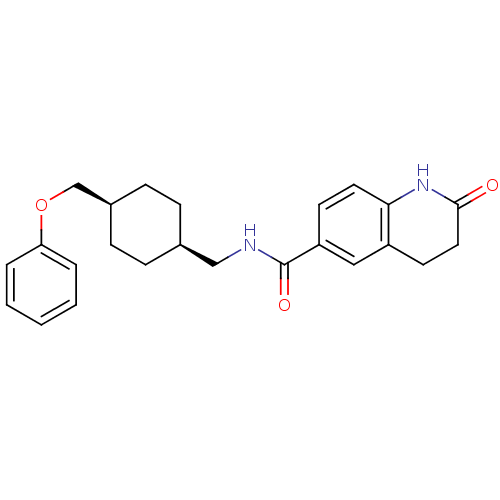 (2-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220590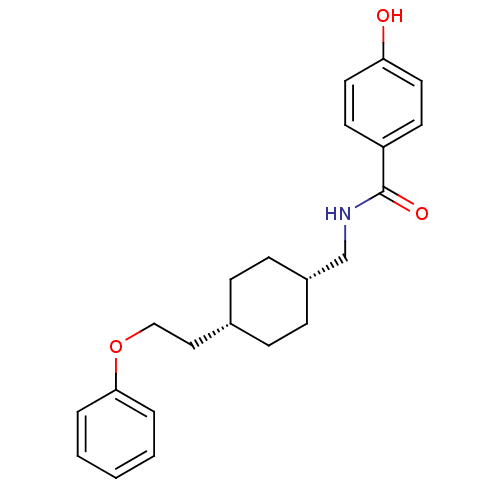 (4-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252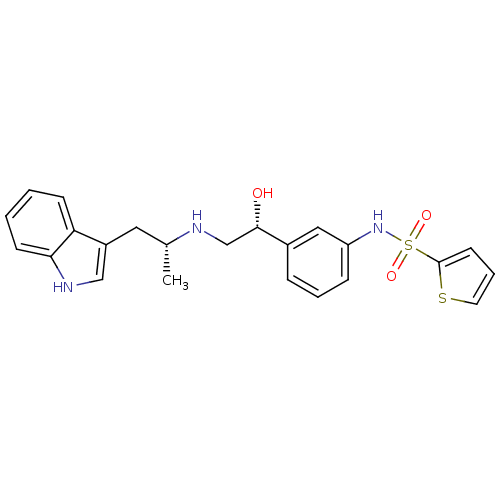 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270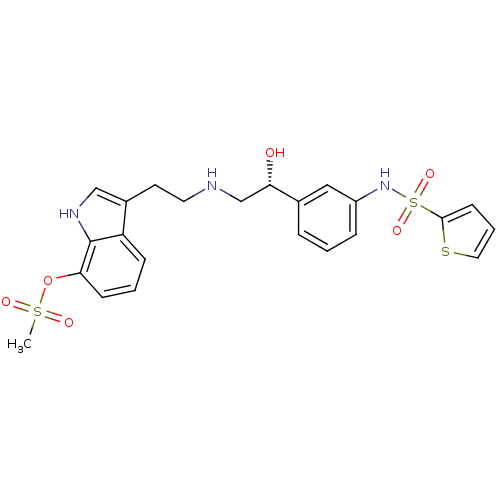 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50335571 (CHEMBL1650845 | {1-[4-(2-{5-Benzylhexahydropyrrolo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]enadoline from human KOP receptor expressed in HEK-293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50335572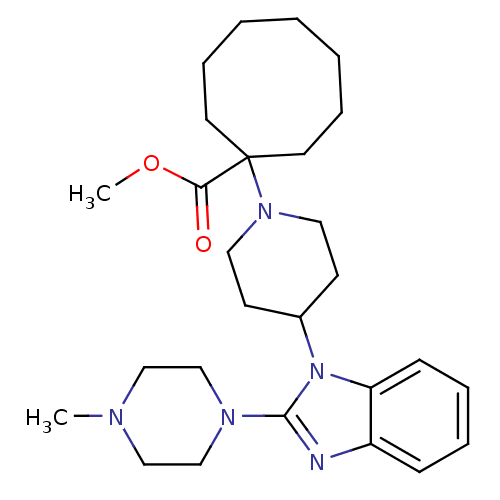 (CHEMBL1650846 | Methyl 1-{4-[2-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells after 45 mins | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220600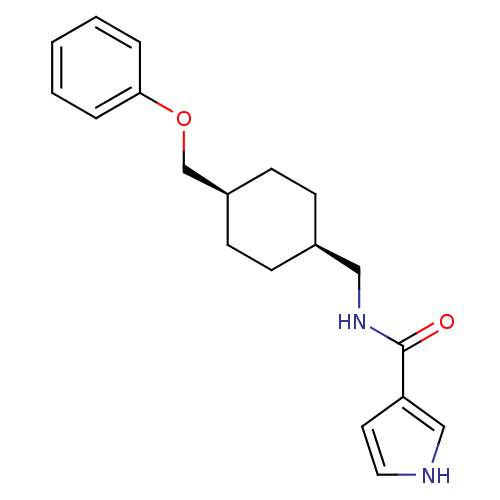 (CHEMBL398357 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220605 (6-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 342 total ) | Next | Last >> |