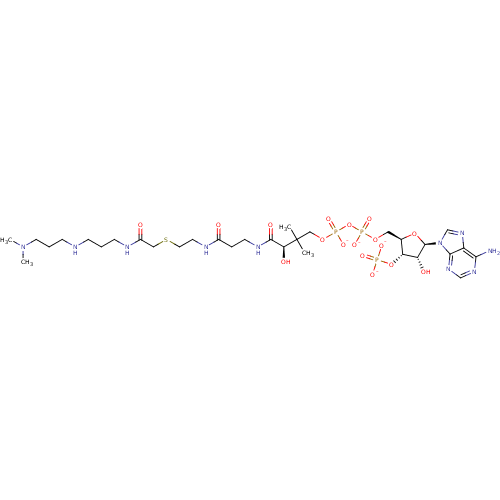Found 467 hits with Last Name = 'mohan' and Initial = 's'
Found 467 hits with Last Name = 'mohan' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuraminidase
(Influenza A virus) | BDBM50330326
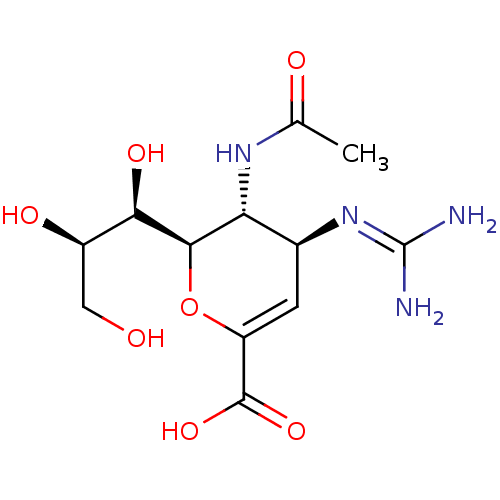
((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50309856
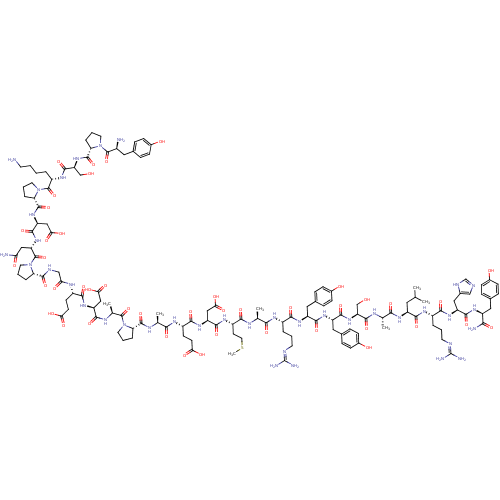
(CHEMBL604165)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r,wU:115.119,108.110,102.107,86.87,70.76,42.47,25.25,8.12,128.133,144.148,168.174,179.185,198.204,wD:93.101,78.84,63.63,50.56,37.38,30.29,16.21,4.4,133.137,156.161,174.181,187.193,208.215,(-7.15,2.46,;-5.84,1.71,;-5.84,.16,;-4.52,-.61,;-4.52,-2.17,;-3.17,-2.95,;-1.85,-2.19,;-1.85,-.63,;-.5,-2.97,;-.5,-4.49,;-1.85,-5.26,;-3.2,-4.49,;-1.85,-6.79,;.81,-2.2,;2.17,-2.98,;2.17,-4.51,;3.49,-2.22,;3.49,-.67,;2.16,.09,;2.16,1.65,;3.51,2.43,;.85,2.41,;4.83,-3,;6.15,-2.24,;6.15,-.69,;7.5,-3.01,;7.5,-4.55,;8.83,-2.25,;10.16,-3.02,;10.16,-4.56,;11.49,-2.25,;11.42,-.71,;13.08,-.39,;13.89,-1.85,;12.75,-3.09,;12.94,-4.62,;11.71,-5.54,;14.36,-5.22,;15.59,-4.29,;14.54,-6.75,;15.96,-7.35,;17.19,-6.42,;16.15,-8.88,;14.92,-9.8,;13.5,-9.21,;13.31,-7.67,;12.27,-10.13,;17.57,-9.48,;17.75,-11.01,;16.52,-11.93,;19.17,-11.61,;20.4,-10.69,;20.22,-9.16,;21.44,-8.23,;22.86,-8.83,;21.26,-6.7,;19.36,-13.14,;20.78,-13.74,;22.01,-12.82,;20.96,-15.27,;22.39,-15.87,;22.57,-17.4,;21.34,-18.33,;23.95,-18.08,;25.2,-17.14,;26.4,-18.33,;25.65,-19.83,;23.98,-19.58,;22.82,-20.6,;21.36,-20.1,;23.12,-22.11,;21.96,-23.12,;20.5,-22.63,;19.34,-23.64,;20.2,-21.12,;24.58,-22.61,;24.88,-24.12,;23.72,-25.13,;26.34,-24.61,;27.49,-23.6,;27.19,-22.08,;25.73,-21.59,;28.35,-21.07,;26.63,-26.12,;28.09,-26.62,;29.25,-25.6,;28.39,-28.13,;27.17,-29.09,;28.02,-30.56,;29.65,-30.21,;29.84,-28.53,;31.14,-27.64,;31.14,-26.14,;32.51,-28.42,;33.81,-27.67,;33.81,-26.09,;35.11,-25.34,;36.4,-26.09,;36.4,-27.59,;32.51,-29.93,;33.88,-30.71,;35.18,-29.96,;33.88,-32.22,;32.52,-33.01,;32.52,-34.58,;35.2,-32.99,;36.54,-32.22,;36.54,-30.67,;37.86,-32.99,;37.82,-34.51,;39.48,-34.82,;40.27,-33.34,;39.12,-32.12,;39.29,-30.59,;38.07,-29.64,;40.71,-30,;40.91,-28.47,;41.94,-30.93,;43.36,-30.35,;43.56,-28.83,;44.98,-28.25,;46.2,-29.19,;47.63,-28.61,;45.99,-30.72,;44.58,-31.29,;-5.84,-2.93,;-5.84,-4.45,;-7.19,-2.15,;-8.5,-2.91,;-8.5,-4.43,;-9.85,-2.13,;-9.85,-.57,;-11.18,-2.89,;-12.53,-2.11,;-12.53,-.55,;-13.85,.21,;-13.85,1.77,;-12.5,2.54,;-12.5,4.06,;-13.82,4.83,;-11.14,4.84,;-13.85,-2.87,;-13.85,-4.39,;-15.2,-2.09,;-16.52,-2.85,;-16.52,-4.38,;-17.87,-5.15,;-17.89,-6.68,;-19.23,-7.43,;-20.56,-6.65,;-21.88,-7.42,;-20.54,-5.11,;-19.2,-4.36,;-17.87,-2.07,;-17.87,-.51,;-19.18,-2.83,;-20.53,-2.06,;-20.53,-.49,;-21.85,.27,;-21.84,1.82,;-23.15,2.6,;-24.49,1.84,;-25.82,2.61,;-24.5,.3,;-23.17,-.48,;-21.85,-2.82,;-21.85,-4.33,;-23.2,-2.04,;-24.51,-2.8,;-24.51,-4.32,;-23.2,-5.08,;-25.86,-2.02,;-25.86,-.46,;-27.19,-2.77,;-28.54,-2,;-28.54,-.44,;-29.86,-2.76,;-29.86,-4.28,;-31.21,-1.98,;-32.52,-2.74,;-32.52,-4.26,;-33.87,-5.04,;-33.87,-6.56,;-35.22,-4.26,;-33.87,-1.96,;-33.87,-.4,;-35.19,-2.72,;-36.54,-1.94,;-36.54,-.38,;-37.86,.38,;-37.86,1.94,;-36.5,2.71,;-36.5,4.24,;-37.82,5,;-35.15,5.01,;-37.86,-2.7,;-37.86,-4.22,;-39.21,-1.92,;-40.52,-2.68,;-40.52,-4.2,;-41.88,-4.98,;-42.06,-6.5,;-43.56,-6.8,;-44.32,-5.46,;-43.29,-4.33,;-41.88,-1.9,;-41.88,-.34,;-43.2,-2.66,;-44.55,-1.89,;-44.55,-.33,;-45.85,.44,;-45.83,2,;-47.17,2.77,;-48.5,2.02,;-49.83,2.78,;-48.51,.47,;-47.18,-.3,;-45.85,-2.64,;-47.2,-1.86,;-45.85,-4.17,)| Show InChI InChI=1S/C137H198N38O44S/c1-67(2)52-89(120(204)158-83(18-11-46-148-137(144)145)116(200)165-92(57-76-62-146-66-150-76)123(207)161-88(110(141)194)54-73-25-33-78(179)34-26-73)162-113(197)69(4)152-126(210)97(64-176)170-122(206)91(56-75-29-37-80(181)38-30-75)164-121(205)90(55-74-27-35-79(180)36-28-74)163-115(199)82(17-10-45-147-136(142)143)156-111(195)68(3)151-114(198)86(43-51-220-7)159-124(208)94(60-108(190)191)167-118(202)85(40-42-106(186)187)157-112(196)70(5)153-129(213)100-20-13-47-172(100)132(216)71(6)154-119(203)93(59-107(188)189)166-117(201)84(39-41-105(184)185)155-104(183)63-149-128(212)99-19-12-49-174(99)135(219)96(58-103(140)182)169-125(209)95(61-109(192)193)168-130(214)102-22-15-50-175(102)134(218)87(16-8-9-44-138)160-127(211)98(65-177)171-131(215)101-21-14-48-173(101)133(217)81(139)53-72-23-31-77(178)32-24-72/h23-38,62,66-71,81-102,176-181H,8-22,39-61,63-65,138-139H2,1-7H3,(H2,140,182)(H2,141,194)(H,146,150)(H,149,212)(H,151,198)(H,152,210)(H,153,213)(H,154,203)(H,155,183)(H,156,195)(H,157,196)(H,158,204)(H,159,208)(H,160,211)(H,161,207)(H,162,197)(H,163,199)(H,164,205)(H,165,200)(H,166,201)(H,167,202)(H,168,214)(H,169,209)(H,170,206)(H,171,215)(H,184,185)(H,186,187)(H,188,189)(H,190,191)(H,192,193)(H4,142,143,147)(H4,144,145,148)/t68-,69-,70-,71-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pancreatic polypeptide from human neuropeptide Y4 receptor in human HEK293 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343682

(((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-ylox...)Show SMILES [#6]-[#6]-[#6](-[#6]-[#6])-[#8]-[#6@@H]-1-[#6]-[#6](=[#6]-[#6@H](\[#7]=[#6](/[#7])-[#7])-[#6@H]-1-[#7]-[#6](-[#6])=O)-[#6](-[#8])=O |r,c:8| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h6,10-13H,4-5,7H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193475
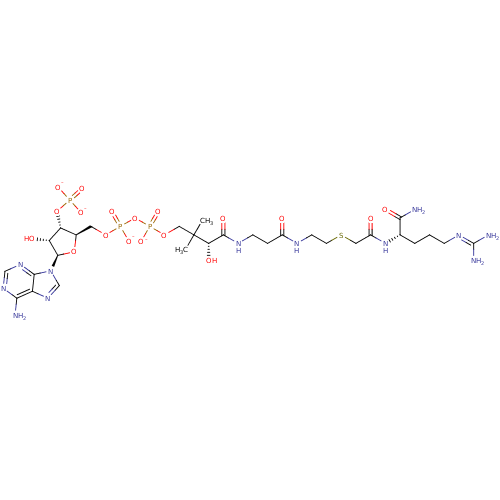
((3R)-3-{[2-({2-[({[(1S)-1-carbamoyl-4-[(diaminomet...)Show SMILES [#6]C([#6])([#6]-[#8]P([#8-])(=O)[#8]P([#8-])(=O)[#8]-[#6]-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8]P([#8-])([#8-])=O)-n1cnc2c(-[#7])ncnc12)[#6@@H](-[#8])-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C29H51N12O18P3S/c1-29(2,22(45)26(47)35-7-5-17(42)34-8-9-63-11-18(43)40-15(24(31)46)4-3-6-36-28(32)33)12-56-62(53,54)59-61(51,52)55-10-16-21(58-60(48,49)50)20(44)27(57-16)41-14-39-19-23(30)37-13-38-25(19)41/h13-16,20-22,27,44-45H,3-12H2,1-2H3,(H2,31,46)(H,34,42)(H,35,47)(H,40,43)(H,51,52)(H,53,54)(H2,30,37,38)(H4,32,33,36)(H2,48,49,50)/p-4/t15-,16+,20+,21+,22-,27+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343681
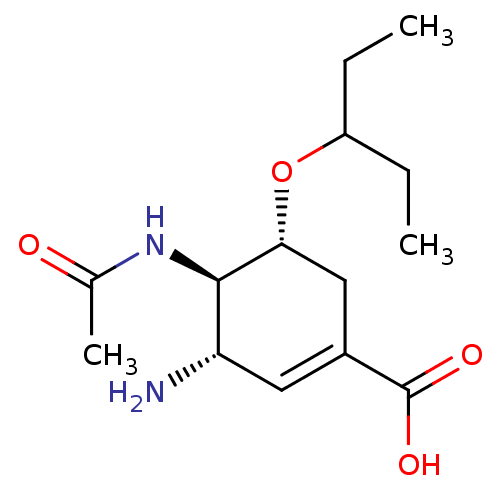
((3S,4R,5R)-4-Acetamido-3-amino-5-(1-ethylpropoxy)c...)Show SMILES CCC(CC)O[C@@H]1CC(=C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:8| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h6,10-13H,4-5,7,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193477
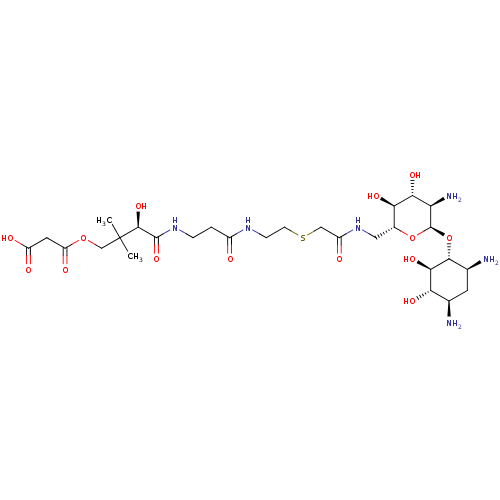
(3-((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1...)Show SMILES CC(C)(COC(=O)CC(O)=O)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C28H50N6O14S/c1-28(2,11-46-18(39)8-17(37)38)25(44)26(45)33-4-3-15(35)32-5-6-49-10-16(36)34-9-14-21(41)22(42)19(31)27(47-14)48-24-13(30)7-12(29)20(40)23(24)43/h12-14,19-25,27,40-44H,3-11,29-31H2,1-2H3,(H,32,35)(H,33,45)(H,34,36)(H,37,38)/t12-,13+,14-,19-,20+,21-,22-,23-,24-,25+,27-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309856

(CHEMBL604165)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r,wU:115.119,108.110,102.107,86.87,70.76,42.47,25.25,8.12,128.133,144.148,168.174,179.185,198.204,wD:93.101,78.84,63.63,50.56,37.38,30.29,16.21,4.4,133.137,156.161,174.181,187.193,208.215,(-7.15,2.46,;-5.84,1.71,;-5.84,.16,;-4.52,-.61,;-4.52,-2.17,;-3.17,-2.95,;-1.85,-2.19,;-1.85,-.63,;-.5,-2.97,;-.5,-4.49,;-1.85,-5.26,;-3.2,-4.49,;-1.85,-6.79,;.81,-2.2,;2.17,-2.98,;2.17,-4.51,;3.49,-2.22,;3.49,-.67,;2.16,.09,;2.16,1.65,;3.51,2.43,;.85,2.41,;4.83,-3,;6.15,-2.24,;6.15,-.69,;7.5,-3.01,;7.5,-4.55,;8.83,-2.25,;10.16,-3.02,;10.16,-4.56,;11.49,-2.25,;11.42,-.71,;13.08,-.39,;13.89,-1.85,;12.75,-3.09,;12.94,-4.62,;11.71,-5.54,;14.36,-5.22,;15.59,-4.29,;14.54,-6.75,;15.96,-7.35,;17.19,-6.42,;16.15,-8.88,;14.92,-9.8,;13.5,-9.21,;13.31,-7.67,;12.27,-10.13,;17.57,-9.48,;17.75,-11.01,;16.52,-11.93,;19.17,-11.61,;20.4,-10.69,;20.22,-9.16,;21.44,-8.23,;22.86,-8.83,;21.26,-6.7,;19.36,-13.14,;20.78,-13.74,;22.01,-12.82,;20.96,-15.27,;22.39,-15.87,;22.57,-17.4,;21.34,-18.33,;23.95,-18.08,;25.2,-17.14,;26.4,-18.33,;25.65,-19.83,;23.98,-19.58,;22.82,-20.6,;21.36,-20.1,;23.12,-22.11,;21.96,-23.12,;20.5,-22.63,;19.34,-23.64,;20.2,-21.12,;24.58,-22.61,;24.88,-24.12,;23.72,-25.13,;26.34,-24.61,;27.49,-23.6,;27.19,-22.08,;25.73,-21.59,;28.35,-21.07,;26.63,-26.12,;28.09,-26.62,;29.25,-25.6,;28.39,-28.13,;27.17,-29.09,;28.02,-30.56,;29.65,-30.21,;29.84,-28.53,;31.14,-27.64,;31.14,-26.14,;32.51,-28.42,;33.81,-27.67,;33.81,-26.09,;35.11,-25.34,;36.4,-26.09,;36.4,-27.59,;32.51,-29.93,;33.88,-30.71,;35.18,-29.96,;33.88,-32.22,;32.52,-33.01,;32.52,-34.58,;35.2,-32.99,;36.54,-32.22,;36.54,-30.67,;37.86,-32.99,;37.82,-34.51,;39.48,-34.82,;40.27,-33.34,;39.12,-32.12,;39.29,-30.59,;38.07,-29.64,;40.71,-30,;40.91,-28.47,;41.94,-30.93,;43.36,-30.35,;43.56,-28.83,;44.98,-28.25,;46.2,-29.19,;47.63,-28.61,;45.99,-30.72,;44.58,-31.29,;-5.84,-2.93,;-5.84,-4.45,;-7.19,-2.15,;-8.5,-2.91,;-8.5,-4.43,;-9.85,-2.13,;-9.85,-.57,;-11.18,-2.89,;-12.53,-2.11,;-12.53,-.55,;-13.85,.21,;-13.85,1.77,;-12.5,2.54,;-12.5,4.06,;-13.82,4.83,;-11.14,4.84,;-13.85,-2.87,;-13.85,-4.39,;-15.2,-2.09,;-16.52,-2.85,;-16.52,-4.38,;-17.87,-5.15,;-17.89,-6.68,;-19.23,-7.43,;-20.56,-6.65,;-21.88,-7.42,;-20.54,-5.11,;-19.2,-4.36,;-17.87,-2.07,;-17.87,-.51,;-19.18,-2.83,;-20.53,-2.06,;-20.53,-.49,;-21.85,.27,;-21.84,1.82,;-23.15,2.6,;-24.49,1.84,;-25.82,2.61,;-24.5,.3,;-23.17,-.48,;-21.85,-2.82,;-21.85,-4.33,;-23.2,-2.04,;-24.51,-2.8,;-24.51,-4.32,;-23.2,-5.08,;-25.86,-2.02,;-25.86,-.46,;-27.19,-2.77,;-28.54,-2,;-28.54,-.44,;-29.86,-2.76,;-29.86,-4.28,;-31.21,-1.98,;-32.52,-2.74,;-32.52,-4.26,;-33.87,-5.04,;-33.87,-6.56,;-35.22,-4.26,;-33.87,-1.96,;-33.87,-.4,;-35.19,-2.72,;-36.54,-1.94,;-36.54,-.38,;-37.86,.38,;-37.86,1.94,;-36.5,2.71,;-36.5,4.24,;-37.82,5,;-35.15,5.01,;-37.86,-2.7,;-37.86,-4.22,;-39.21,-1.92,;-40.52,-2.68,;-40.52,-4.2,;-41.88,-4.98,;-42.06,-6.5,;-43.56,-6.8,;-44.32,-5.46,;-43.29,-4.33,;-41.88,-1.9,;-41.88,-.34,;-43.2,-2.66,;-44.55,-1.89,;-44.55,-.33,;-45.85,.44,;-45.83,2,;-47.17,2.77,;-48.5,2.02,;-49.83,2.78,;-48.51,.47,;-47.18,-.3,;-45.85,-2.64,;-47.2,-1.86,;-45.85,-4.17,)| Show InChI InChI=1S/C137H198N38O44S/c1-67(2)52-89(120(204)158-83(18-11-46-148-137(144)145)116(200)165-92(57-76-62-146-66-150-76)123(207)161-88(110(141)194)54-73-25-33-78(179)34-26-73)162-113(197)69(4)152-126(210)97(64-176)170-122(206)91(56-75-29-37-80(181)38-30-75)164-121(205)90(55-74-27-35-79(180)36-28-74)163-115(199)82(17-10-45-147-136(142)143)156-111(195)68(3)151-114(198)86(43-51-220-7)159-124(208)94(60-108(190)191)167-118(202)85(40-42-106(186)187)157-112(196)70(5)153-129(213)100-20-13-47-172(100)132(216)71(6)154-119(203)93(59-107(188)189)166-117(201)84(39-41-105(184)185)155-104(183)63-149-128(212)99-19-12-49-174(99)135(219)96(58-103(140)182)169-125(209)95(61-109(192)193)168-130(214)102-22-15-50-175(102)134(218)87(16-8-9-44-138)160-127(211)98(65-177)171-131(215)101-21-14-48-173(101)133(217)81(139)53-72-23-31-77(178)32-24-72/h23-38,62,66-71,81-102,176-181H,8-22,39-61,63-65,138-139H2,1-7H3,(H2,140,182)(H2,141,194)(H,146,150)(H,149,212)(H,151,198)(H,152,210)(H,153,213)(H,154,203)(H,155,183)(H,156,195)(H,157,196)(H,158,204)(H,159,208)(H,160,211)(H,161,207)(H,162,197)(H,163,199)(H,164,205)(H,165,200)(H,166,201)(H,167,202)(H,168,214)(H,169,209)(H,170,206)(H,171,215)(H,184,185)(H,186,187)(H,188,189)(H,190,191)(H,192,193)(H4,142,143,147)(H4,144,145,148)/t68-,69-,70-,71-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193487
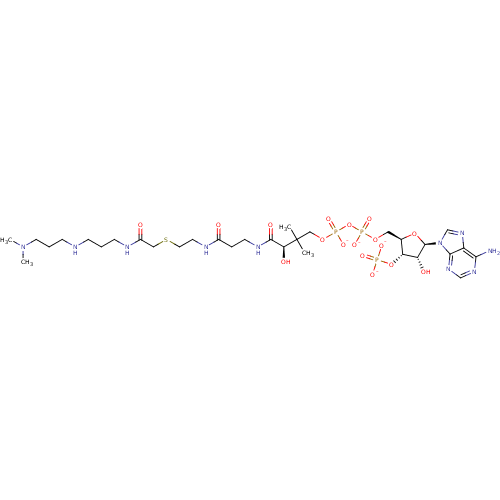
((3R)-3-[(2-{[2-({[(3-{[3-(dimethylamino)propyl]ami...)Show SMILES CN(C)CCCNCCCNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C31H57N10O17P3S/c1-31(2,26(45)29(46)36-11-7-21(42)35-12-14-62-16-22(43)34-10-5-8-33-9-6-13-40(3)4)17-55-61(52,53)58-60(50,51)54-15-20-25(57-59(47,48)49)24(44)30(56-20)41-19-39-23-27(32)37-18-38-28(23)41/h18-20,24-26,30,33,44-45H,5-17H2,1-4H3,(H,34,43)(H,35,42)(H,36,46)(H,50,51)(H,52,53)(H2,32,37,38)(H2,47,48,49)/p-4/t20-,24-,25-,26+,30-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193484
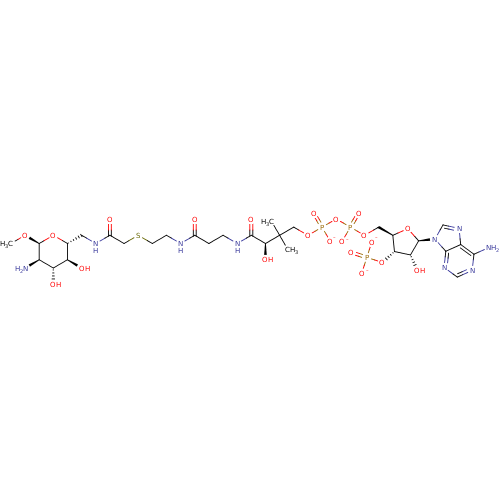
(CID44414951 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CO[C@H]1O[C@H](CNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2OP([O-])([O-])=O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H](O)[C@H]1N Show InChI InChI=1S/C30H52N9O21P3S/c1-30(2,24(45)27(46)34-5-4-16(40)33-6-7-64-10-17(41)35-8-14-20(42)21(43)18(31)29(54-3)58-14)11-56-63(52,53)60-62(50,51)55-9-15-23(59-61(47,48)49)22(44)28(57-15)39-13-38-19-25(32)36-12-37-26(19)39/h12-15,18,20-24,28-29,42-45H,4-11,31H2,1-3H3,(H,33,40)(H,34,46)(H,35,41)(H,50,51)(H,52,53)(H2,32,36,37)(H2,47,48,49)/p-4/t14-,15-,18-,20-,21-,22-,23-,24+,28-,29+/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193478
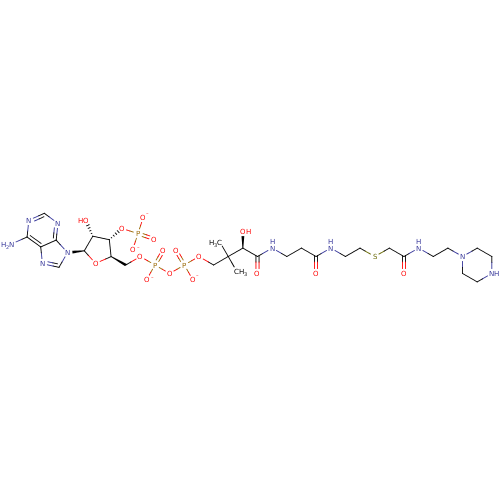
(CID44415032 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NCCN1CCNCC1 Show InChI InChI=1S/C29H51N10O17P3S/c1-29(2,24(43)27(44)34-4-3-19(40)33-8-12-60-14-20(41)32-7-11-38-9-5-31-6-10-38)15-53-59(50,51)56-58(48,49)52-13-18-23(55-57(45,46)47)22(42)28(54-18)39-17-37-21-25(30)35-16-36-26(21)39/h16-18,22-24,28,31,42-43H,3-15H2,1-2H3,(H,32,41)(H,33,40)(H,34,44)(H,48,49)(H,50,51)(H2,30,35,36)(H2,45,46,47)/p-4/t18-,22-,23-,24+,28-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309855
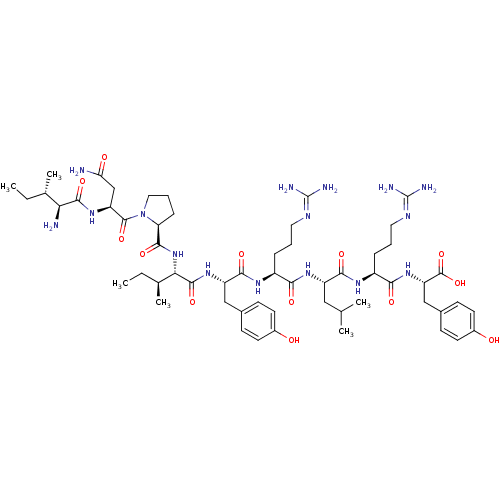
((3S,6S,9S,12S,15S,18S)-1-((S)-1-((S)-4-amino-2-((2...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H90N16O13/c1-7-31(5)45(59)52(82)70-41(29-44(58)76)54(84)73-25-11-14-43(73)51(81)72-46(32(6)8-2)53(83)69-40(27-33-15-19-35(74)20-16-33)50(80)67-37(12-9-23-64-56(60)61)47(77)68-39(26-30(3)4)49(79)66-38(13-10-24-65-57(62)63)48(78)71-42(55(85)86)28-34-17-21-36(75)22-18-34/h15-22,30-32,37-43,45-46,74-75H,7-14,23-29,59H2,1-6H3,(H2,58,76)(H,66,79)(H,67,80)(H,68,77)(H,69,83)(H,70,82)(H,71,78)(H,72,81)(H,85,86)(H4,60,61,64)(H4,62,63,65)/t31-,32-,37-,38-,39-,40-,41-,42-,43-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309855

((3S,6S,9S,12S,15S,18S)-1-((S)-1-((S)-4-amino-2-((2...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H90N16O13/c1-7-31(5)45(59)52(82)70-41(29-44(58)76)54(84)73-25-11-14-43(73)51(81)72-46(32(6)8-2)53(83)69-40(27-33-15-19-35(74)20-16-33)50(80)67-37(12-9-23-64-56(60)61)47(77)68-39(26-30(3)4)49(79)66-38(13-10-24-65-57(62)63)48(78)71-42(55(85)86)28-34-17-21-36(75)22-18-34/h15-22,30-32,37-43,45-46,74-75H,7-14,23-29,59H2,1-6H3,(H2,58,76)(H,66,79)(H,67,80)(H,68,77)(H,69,83)(H,70,82)(H,71,78)(H,72,81)(H,85,86)(H4,60,61,64)(H4,62,63,65)/t31-,32-,37-,38-,39-,40-,41-,42-,43-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50309856

(CHEMBL604165)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r,wU:115.119,108.110,102.107,86.87,70.76,42.47,25.25,8.12,128.133,144.148,168.174,179.185,198.204,wD:93.101,78.84,63.63,50.56,37.38,30.29,16.21,4.4,133.137,156.161,174.181,187.193,208.215,(-7.15,2.46,;-5.84,1.71,;-5.84,.16,;-4.52,-.61,;-4.52,-2.17,;-3.17,-2.95,;-1.85,-2.19,;-1.85,-.63,;-.5,-2.97,;-.5,-4.49,;-1.85,-5.26,;-3.2,-4.49,;-1.85,-6.79,;.81,-2.2,;2.17,-2.98,;2.17,-4.51,;3.49,-2.22,;3.49,-.67,;2.16,.09,;2.16,1.65,;3.51,2.43,;.85,2.41,;4.83,-3,;6.15,-2.24,;6.15,-.69,;7.5,-3.01,;7.5,-4.55,;8.83,-2.25,;10.16,-3.02,;10.16,-4.56,;11.49,-2.25,;11.42,-.71,;13.08,-.39,;13.89,-1.85,;12.75,-3.09,;12.94,-4.62,;11.71,-5.54,;14.36,-5.22,;15.59,-4.29,;14.54,-6.75,;15.96,-7.35,;17.19,-6.42,;16.15,-8.88,;14.92,-9.8,;13.5,-9.21,;13.31,-7.67,;12.27,-10.13,;17.57,-9.48,;17.75,-11.01,;16.52,-11.93,;19.17,-11.61,;20.4,-10.69,;20.22,-9.16,;21.44,-8.23,;22.86,-8.83,;21.26,-6.7,;19.36,-13.14,;20.78,-13.74,;22.01,-12.82,;20.96,-15.27,;22.39,-15.87,;22.57,-17.4,;21.34,-18.33,;23.95,-18.08,;25.2,-17.14,;26.4,-18.33,;25.65,-19.83,;23.98,-19.58,;22.82,-20.6,;21.36,-20.1,;23.12,-22.11,;21.96,-23.12,;20.5,-22.63,;19.34,-23.64,;20.2,-21.12,;24.58,-22.61,;24.88,-24.12,;23.72,-25.13,;26.34,-24.61,;27.49,-23.6,;27.19,-22.08,;25.73,-21.59,;28.35,-21.07,;26.63,-26.12,;28.09,-26.62,;29.25,-25.6,;28.39,-28.13,;27.17,-29.09,;28.02,-30.56,;29.65,-30.21,;29.84,-28.53,;31.14,-27.64,;31.14,-26.14,;32.51,-28.42,;33.81,-27.67,;33.81,-26.09,;35.11,-25.34,;36.4,-26.09,;36.4,-27.59,;32.51,-29.93,;33.88,-30.71,;35.18,-29.96,;33.88,-32.22,;32.52,-33.01,;32.52,-34.58,;35.2,-32.99,;36.54,-32.22,;36.54,-30.67,;37.86,-32.99,;37.82,-34.51,;39.48,-34.82,;40.27,-33.34,;39.12,-32.12,;39.29,-30.59,;38.07,-29.64,;40.71,-30,;40.91,-28.47,;41.94,-30.93,;43.36,-30.35,;43.56,-28.83,;44.98,-28.25,;46.2,-29.19,;47.63,-28.61,;45.99,-30.72,;44.58,-31.29,;-5.84,-2.93,;-5.84,-4.45,;-7.19,-2.15,;-8.5,-2.91,;-8.5,-4.43,;-9.85,-2.13,;-9.85,-.57,;-11.18,-2.89,;-12.53,-2.11,;-12.53,-.55,;-13.85,.21,;-13.85,1.77,;-12.5,2.54,;-12.5,4.06,;-13.82,4.83,;-11.14,4.84,;-13.85,-2.87,;-13.85,-4.39,;-15.2,-2.09,;-16.52,-2.85,;-16.52,-4.38,;-17.87,-5.15,;-17.89,-6.68,;-19.23,-7.43,;-20.56,-6.65,;-21.88,-7.42,;-20.54,-5.11,;-19.2,-4.36,;-17.87,-2.07,;-17.87,-.51,;-19.18,-2.83,;-20.53,-2.06,;-20.53,-.49,;-21.85,.27,;-21.84,1.82,;-23.15,2.6,;-24.49,1.84,;-25.82,2.61,;-24.5,.3,;-23.17,-.48,;-21.85,-2.82,;-21.85,-4.33,;-23.2,-2.04,;-24.51,-2.8,;-24.51,-4.32,;-23.2,-5.08,;-25.86,-2.02,;-25.86,-.46,;-27.19,-2.77,;-28.54,-2,;-28.54,-.44,;-29.86,-2.76,;-29.86,-4.28,;-31.21,-1.98,;-32.52,-2.74,;-32.52,-4.26,;-33.87,-5.04,;-33.87,-6.56,;-35.22,-4.26,;-33.87,-1.96,;-33.87,-.4,;-35.19,-2.72,;-36.54,-1.94,;-36.54,-.38,;-37.86,.38,;-37.86,1.94,;-36.5,2.71,;-36.5,4.24,;-37.82,5,;-35.15,5.01,;-37.86,-2.7,;-37.86,-4.22,;-39.21,-1.92,;-40.52,-2.68,;-40.52,-4.2,;-41.88,-4.98,;-42.06,-6.5,;-43.56,-6.8,;-44.32,-5.46,;-43.29,-4.33,;-41.88,-1.9,;-41.88,-.34,;-43.2,-2.66,;-44.55,-1.89,;-44.55,-.33,;-45.85,.44,;-45.83,2,;-47.17,2.77,;-48.5,2.02,;-49.83,2.78,;-48.51,.47,;-47.18,-.3,;-45.85,-2.64,;-47.2,-1.86,;-45.85,-4.17,)| Show InChI InChI=1S/C137H198N38O44S/c1-67(2)52-89(120(204)158-83(18-11-46-148-137(144)145)116(200)165-92(57-76-62-146-66-150-76)123(207)161-88(110(141)194)54-73-25-33-78(179)34-26-73)162-113(197)69(4)152-126(210)97(64-176)170-122(206)91(56-75-29-37-80(181)38-30-75)164-121(205)90(55-74-27-35-79(180)36-28-74)163-115(199)82(17-10-45-147-136(142)143)156-111(195)68(3)151-114(198)86(43-51-220-7)159-124(208)94(60-108(190)191)167-118(202)85(40-42-106(186)187)157-112(196)70(5)153-129(213)100-20-13-47-172(100)132(216)71(6)154-119(203)93(59-107(188)189)166-117(201)84(39-41-105(184)185)155-104(183)63-149-128(212)99-19-12-49-174(99)135(219)96(58-103(140)182)169-125(209)95(61-109(192)193)168-130(214)102-22-15-50-175(102)134(218)87(16-8-9-44-138)160-127(211)98(65-177)171-131(215)101-21-14-48-173(101)133(217)81(139)53-72-23-31-77(178)32-24-72/h23-38,62,66-71,81-102,176-181H,8-22,39-61,63-65,138-139H2,1-7H3,(H2,140,182)(H2,141,194)(H,146,150)(H,149,212)(H,151,198)(H,152,210)(H,153,213)(H,154,203)(H,155,183)(H,156,195)(H,157,196)(H,158,204)(H,159,208)(H,160,211)(H,161,207)(H,162,197)(H,163,199)(H,164,205)(H,165,200)(H,166,201)(H,167,202)(H,168,214)(H,169,209)(H,170,206)(H,171,215)(H,184,185)(H,186,187)(H,188,189)(H,190,191)(H,192,193)(H4,142,143,147)(H4,144,145,148)/t68-,69-,70-,71-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human neuropeptide Y2 receptor expressed in human SK-N-BE2 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50309855

((3S,6S,9S,12S,15S,18S)-1-((S)-1-((S)-4-amino-2-((2...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H90N16O13/c1-7-31(5)45(59)52(82)70-41(29-44(58)76)54(84)73-25-11-14-43(73)51(81)72-46(32(6)8-2)53(83)69-40(27-33-15-19-35(74)20-16-33)50(80)67-37(12-9-23-64-56(60)61)47(77)68-39(26-30(3)4)49(79)66-38(13-10-24-65-57(62)63)48(78)71-42(55(85)86)28-34-17-21-36(75)22-18-34/h15-22,30-32,37-43,45-46,74-75H,7-14,23-29,59H2,1-6H3,(H2,58,76)(H,66,79)(H,67,80)(H,68,77)(H,69,83)(H,70,82)(H,71,78)(H,72,81)(H,85,86)(H4,60,61,64)(H4,62,63,65)/t31-,32-,37-,38-,39-,40-,41-,42-,43-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pancreatic polypeptide from human neuropeptide Y4 receptor in human HEK293 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309860
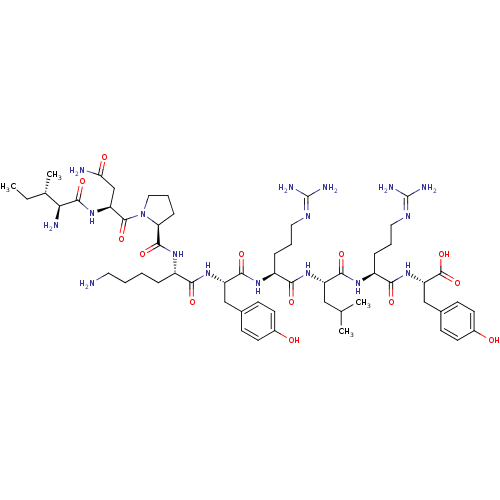
((3S,6S,9S,12S,15S,18S)-1-((S)-1-((S)-4-amino-2-((2...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H91N17O13/c1-5-32(4)46(60)53(84)72-42(30-45(59)77)54(85)74-26-10-14-44(74)52(83)69-37(11-6-7-23-58)47(78)71-41(28-33-15-19-35(75)20-16-33)51(82)68-38(12-8-24-65-56(61)62)48(79)70-40(27-31(2)3)50(81)67-39(13-9-25-66-57(63)64)49(80)73-43(55(86)87)29-34-17-21-36(76)22-18-34/h15-22,31-32,37-44,46,75-76H,5-14,23-30,58,60H2,1-4H3,(H2,59,77)(H,67,81)(H,68,82)(H,69,83)(H,70,79)(H,71,78)(H,72,84)(H,73,80)(H,86,87)(H4,61,62,65)(H4,63,64,66)/t32-,37-,38-,39-,40-,41-,42-,43-,44-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193488
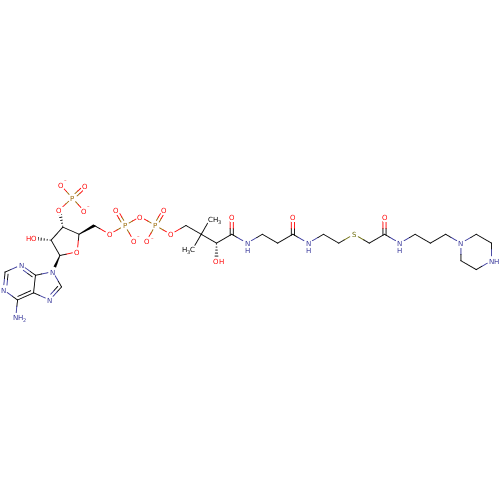
(CID44415033 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NCCCN1CCNCC1 Show InChI InChI=1S/C30H53N10O17P3S/c1-30(2,25(44)28(45)35-6-4-20(41)34-9-13-61-15-21(42)33-5-3-10-39-11-7-32-8-12-39)16-54-60(51,52)57-59(49,50)53-14-19-24(56-58(46,47)48)23(43)29(55-19)40-18-38-22-26(31)36-17-37-27(22)40/h17-19,23-25,29,32,43-44H,3-16H2,1-2H3,(H,33,42)(H,34,41)(H,35,45)(H,49,50)(H,51,52)(H2,31,36,37)(H2,46,47,48)/p-4/t19-,23-,24-,25+,29-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193474
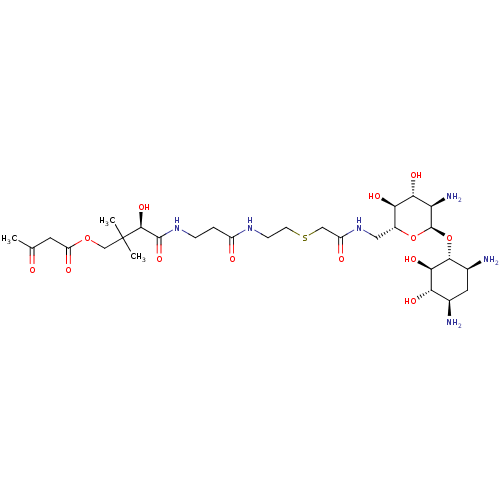
((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1R,2...)Show SMILES CC(=O)CC(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C29H52N6O13S/c1-13(36)8-19(39)46-12-29(2,3)26(44)27(45)34-5-4-17(37)33-6-7-49-11-18(38)35-10-16-22(41)23(42)20(32)28(47-16)48-25-15(31)9-14(30)21(40)24(25)43/h14-16,20-26,28,40-44H,4-12,30-32H2,1-3H3,(H,33,37)(H,34,45)(H,35,38)/t14-,15+,16-,20-,21+,22-,23-,24-,25-,26+,28-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50309855

((3S,6S,9S,12S,15S,18S)-1-((S)-1-((S)-4-amino-2-((2...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H90N16O13/c1-7-31(5)45(59)52(82)70-41(29-44(58)76)54(84)73-25-11-14-43(73)51(81)72-46(32(6)8-2)53(83)69-40(27-33-15-19-35(74)20-16-33)50(80)67-37(12-9-23-64-56(60)61)47(77)68-39(26-30(3)4)49(79)66-38(13-10-24-65-57(62)63)48(78)71-42(55(85)86)28-34-17-21-36(75)22-18-34/h15-22,30-32,37-43,45-46,74-75H,7-14,23-29,59H2,1-6H3,(H2,58,76)(H,66,79)(H,67,80)(H,68,77)(H,69,83)(H,70,82)(H,71,78)(H,72,81)(H,85,86)(H4,60,61,64)(H4,62,63,65)/t31-,32-,37-,38-,39-,40-,41-,42-,43-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human neuropeptide Y2 receptor expressed in human SK-N-BE2 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193480
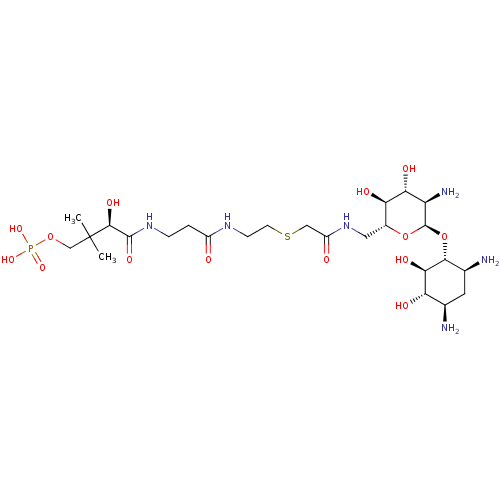
((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1R,2...)Show SMILES CC(C)(COP(O)(O)=O)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C25H49N6O14PS/c1-25(2,10-43-46(40,41)42)22(38)23(39)30-4-3-14(32)29-5-6-47-9-15(33)31-8-13-18(35)19(36)16(28)24(44-13)45-21-12(27)7-11(26)17(34)20(21)37/h11-13,16-22,24,34-38H,3-10,26-28H2,1-2H3,(H,29,32)(H,30,39)(H,31,33)(H2,40,41,42)/t11-,12+,13-,16-,17+,18-,19-,20-,21-,22+,24-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330961

((2R,3S,4S)-1-((2S,3S,4R,5R,6S)-2,3,4,5,6,7-hexahyd...)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)C[Se+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C12H25O9Se/c13-1-5(15)10(19)12(21)11(20)7(17)4-22-3-6(16)9(18)8(22)2-14/h5-21H,1-4H2/q+1/t5-,6+,7+,8+,9-,10+,11+,12+,22?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330954
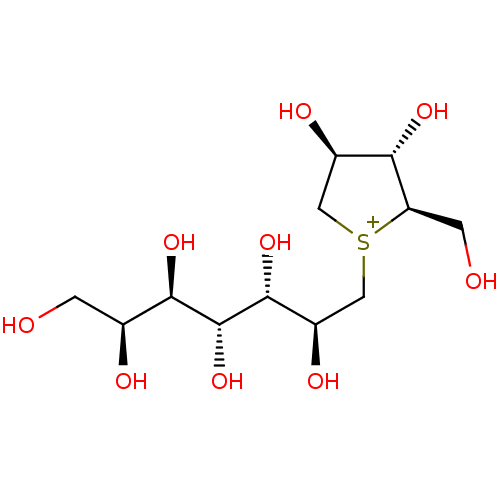
(CHEMBL1276973 | de-O-sulfonated kotalanol)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C12H25O9S/c13-1-5(15)10(19)12(21)11(20)7(17)4-22-3-6(16)9(18)8(22)2-14/h5-21H,1-4H2/q+1/t5-,6+,7+,8+,9-,10+,11+,12+,22?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193476
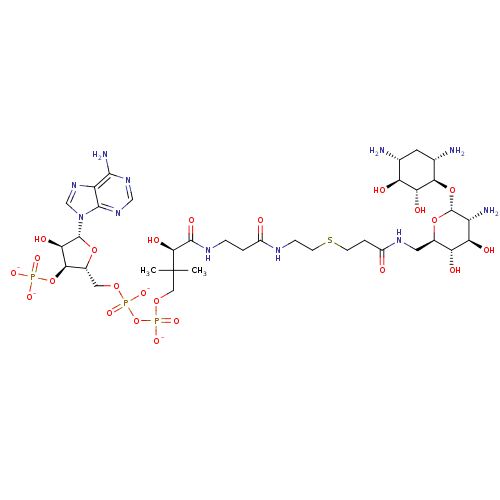
(CID44415023 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C36H64N11O23P3S/c1-36(2,12-65-73(62,63)70-72(60,61)64-11-18-29(69-71(57,58)59)27(54)34(66-18)47-14-46-22-31(40)44-13-45-32(22)47)30(55)33(56)42-5-3-19(48)41-6-8-74-7-4-20(49)43-10-17-24(51)25(52)21(39)35(67-17)68-28-16(38)9-15(37)23(50)26(28)53/h13-18,21,23-30,34-35,50-55H,3-12,37-39H2,1-2H3,(H,41,48)(H,42,56)(H,43,49)(H,60,61)(H,62,63)(H2,40,44,45)(H2,57,58,59)/p-4/t15-,16+,17-,18-,21-,23+,24-,25-,26-,27-,28-,29-,30+,34-,35-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330960
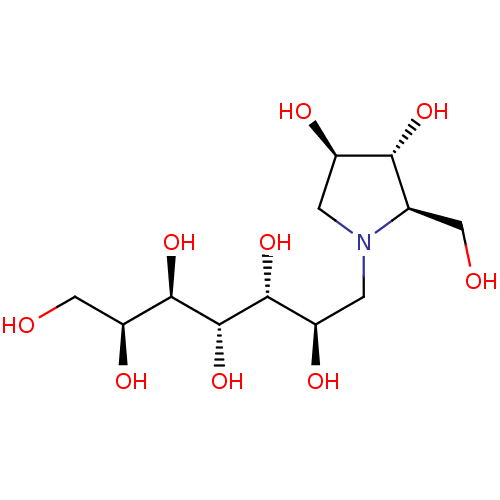
((2R,3R,4R)-1-((2R,3R,4S,5R,6S)-2,3,4,5,6,7-hexahyd...)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CN1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C12H25NO9/c14-3-5-9(19)6(16)1-13(5)2-7(17)10(20)12(22)11(21)8(18)4-15/h5-12,14-22H,1-4H2/t5-,6-,7-,8+,9-,10-,11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309857

(2,2',2''-(10-((6S,9S,12S,15S,18S)-1-amino-18-((S)-...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6](-[#8])=O)-[#6]-[#6]-[#7](-[#6]-[#6](-[#8])=O)-[#6]-[#6]-[#7](-[#6]-[#6](-[#8])=O)-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C73H117N21O20/c1-5-44(4)62(75)69(111)88-54(38-57(74)97)70(112)94-26-10-14-56(94)68(110)85-49(11-6-7-23-80-58(98)39-90-27-29-91(40-59(99)100)31-33-93(42-61(103)104)34-32-92(30-28-90)41-60(101)102)63(105)87-53(36-45-15-19-47(95)20-16-45)67(109)84-50(12-8-24-81-72(76)77)64(106)86-52(35-43(2)3)66(108)83-51(13-9-25-82-73(78)79)65(107)89-55(71(113)114)37-46-17-21-48(96)22-18-46/h15-22,43-44,49-56,62,95-96H,5-14,23-42,75H2,1-4H3,(H2,74,97)(H,80,98)(H,83,108)(H,84,109)(H,85,110)(H,86,106)(H,87,105)(H,88,111)(H,89,107)(H,99,100)(H,101,102)(H,103,104)(H,113,114)(H4,76,77,81)(H4,78,79,82)/t44-,49-,50-,51-,52-,53-,54-,55-,56-,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343684
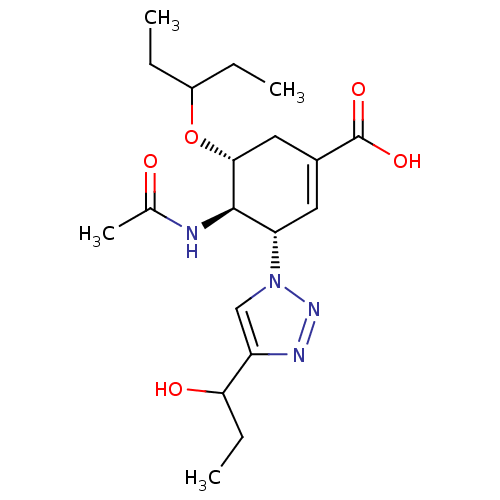
((3S,4R,5R)-4-Acetamido-5-(1-ethyl-propoxy)-3-[4-(1...)Show SMILES CCC(CC)O[C@@H]1CC(=C[C@@H]([C@H]1NC(C)=O)n1cc(nn1)C(O)CC)C(O)=O |r,c:8| Show InChI InChI=1S/C19H30N4O5/c1-5-13(6-2)28-17-9-12(19(26)27)8-15(18(17)20-11(4)24)23-10-14(21-22-23)16(25)7-3/h8,10,13,15-18,25H,5-7,9H2,1-4H3,(H,20,24)(H,26,27)/t15-,16?,17+,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193486

(CID44415022 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C35H62N11O23P3S/c1-35(2,11-64-72(61,62)69-71(59,60)63-9-17-28(68-70(56,57)58)26(53)33(65-17)46-13-45-21-30(39)43-12-44-31(21)46)29(54)32(55)41-4-3-18(47)40-5-6-73-10-19(48)42-8-16-23(50)24(51)20(38)34(66-16)67-27-15(37)7-14(36)22(49)25(27)52/h12-17,20,22-29,33-34,49-54H,3-11,36-38H2,1-2H3,(H,40,47)(H,41,55)(H,42,48)(H,59,60)(H,61,62)(H2,39,43,44)(H2,56,57,58)/p-4/t14-,15+,16-,17-,20-,22+,23-,24-,25-,26-,27-,28-,29+,33-,34-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330959
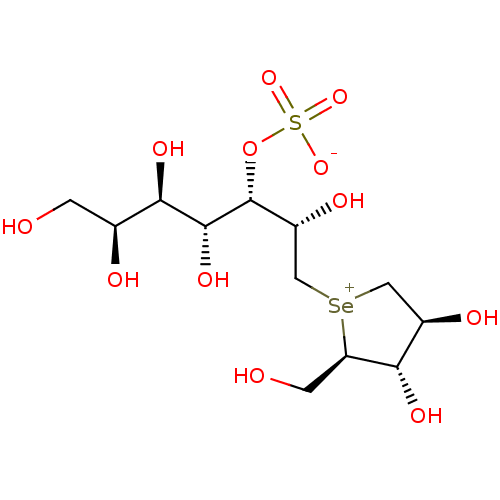
(CHEMBL1277153)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](OS([O-])(=O)=O)[C@H](O)C[Se+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C12H24O12SSe/c13-1-5(15)10(19)11(20)12(24-25(21,22)23)7(17)4-26-3-6(16)9(18)8(26)2-14/h5-20H,1-4H2/t5-,6+,7+,8+,9-,10+,11+,12+,26?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193482
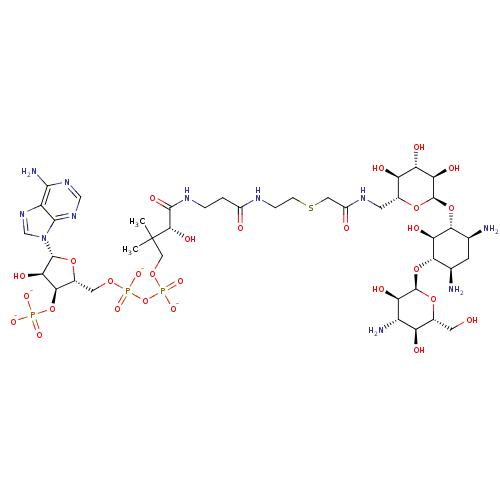
(CID44414946 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C41H72N11O28P3S/c1-41(2,12-73-83(70,71)80-82(68,69)72-10-19-33(79-81(65,66)67)30(62)38(74-19)52-14-51-23-35(45)49-13-50-36(23)52)34(63)37(64)47-4-3-20(54)46-5-6-84-11-21(55)48-8-17-25(57)27(59)28(60)40(75-17)78-32-16(43)7-15(42)31(29(32)61)77-39-26(58)22(44)24(56)18(9-53)76-39/h13-19,22,24-34,38-40,53,56-63H,3-12,42-44H2,1-2H3,(H,46,54)(H,47,64)(H,48,55)(H,68,69)(H,70,71)(H2,45,49,50)(H2,65,66,67)/p-4/t15-,16+,17-,18-,19-,22+,24-,25-,26-,27+,28-,29-,30-,31+,32-,33-,34+,38-,39-,40-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193479
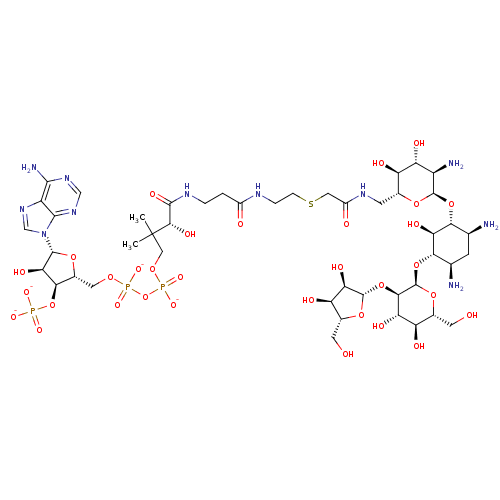
(CID44414949 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C46H80N11O32P3S/c1-46(2,13-80-92(77,78)89-91(75,76)79-11-21-36(88-90(72,73)74)33(69)42(81-21)57-15-56-25-39(50)54-14-55-40(25)57)38(70)41(71)52-4-3-22(60)51-5-6-93-12-23(61)53-8-18-26(62)29(65)24(49)43(82-18)85-34-16(47)7-17(48)35(32(34)68)86-45-37(30(66)27(63)19(9-58)84-45)87-44-31(67)28(64)20(10-59)83-44/h14-21,24,26-38,42-45,58-59,62-70H,3-13,47-49H2,1-2H3,(H,51,60)(H,52,71)(H,53,61)(H,75,76)(H,77,78)(H2,50,54,55)(H2,72,73,74)/p-4/t16-,17+,18+,19+,20+,21+,24+,26+,27+,28+,29+,30-,31+,32-,33+,34+,35-,36+,37+,38-,42+,43+,44-,45+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309859
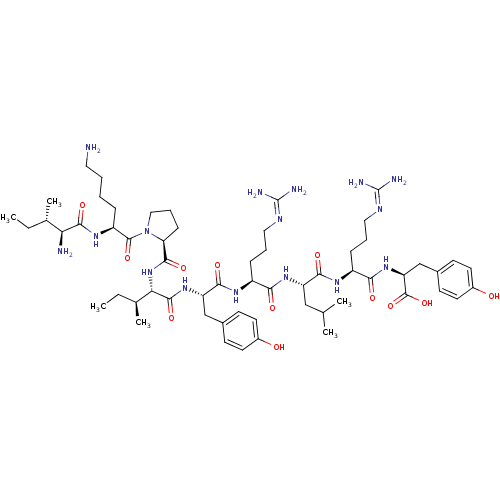
((3S,6S,9S,12S,15S,18S)-1-((S)-1-((S)-6-amino-2-((2...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C59H96N16O12/c1-7-34(5)47(61)54(83)70-42(14-9-10-26-60)56(85)75-29-13-17-46(75)53(82)74-48(35(6)8-2)55(84)72-44(31-36-18-22-38(76)23-19-36)52(81)69-40(15-11-27-66-58(62)63)49(78)71-43(30-33(3)4)51(80)68-41(16-12-28-67-59(64)65)50(79)73-45(57(86)87)32-37-20-24-39(77)25-21-37/h18-25,33-35,40-48,76-77H,7-17,26-32,60-61H2,1-6H3,(H,68,80)(H,69,81)(H,70,83)(H,71,78)(H,72,84)(H,73,79)(H,74,82)(H,86,87)(H4,62,63,66)(H4,64,65,67)/t34-,35-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343685
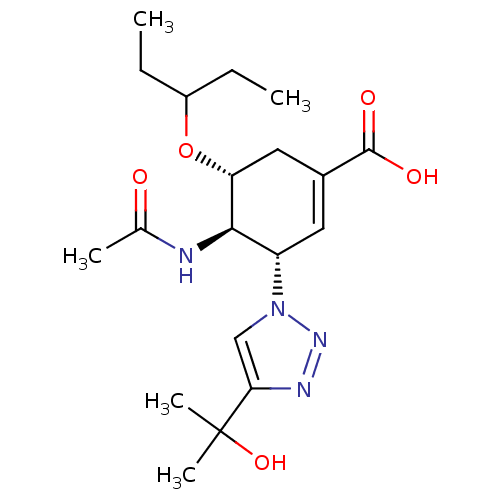
((3S,4R,5R)-4-Acetamido-5-(1-ethylpropoxy)-3-[4-(1-...)Show SMILES CCC(CC)O[C@@H]1CC(=C[C@@H]([C@H]1NC(C)=O)n1cc(nn1)C(C)(C)O)C(O)=O |r,c:8| Show InChI InChI=1S/C19H30N4O5/c1-6-13(7-2)28-15-9-12(18(25)26)8-14(17(15)20-11(3)24)23-10-16(21-22-23)19(4,5)27/h8,10,13-15,17,27H,6-7,9H2,1-5H3,(H,20,24)(H,25,26)/t14-,15+,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193485
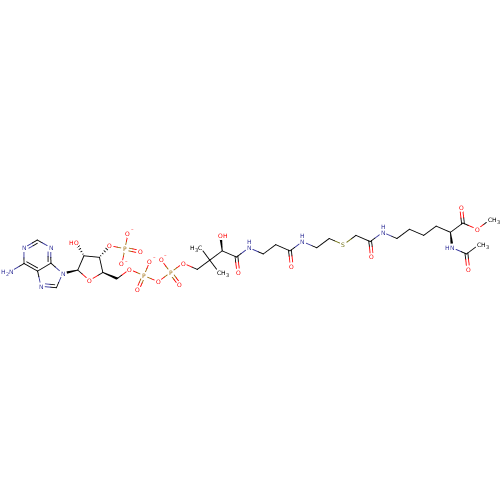
((3R)-3-{[2-({2-[({[(5S)-5-acetamido-6-methoxy-6-ox...)Show SMILES COC(=O)[C@H](CCCCNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)NC(C)=O Show InChI InChI=1S/C32H54N9O20P3S/c1-18(42)40-19(31(48)56-4)7-5-6-9-34-22(44)14-65-12-11-35-21(43)8-10-36-29(47)26(46)32(2,3)15-58-64(54,55)61-63(52,53)57-13-20-25(60-62(49,50)51)24(45)30(59-20)41-17-39-23-27(33)37-16-38-28(23)41/h16-17,19-20,24-26,30,45-46H,5-15H2,1-4H3,(H,34,44)(H,35,43)(H,36,47)(H,40,42)(H,52,53)(H,54,55)(H2,33,37,38)(H2,49,50,51)/p-4/t19-,20+,24+,25+,26-,30+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193483
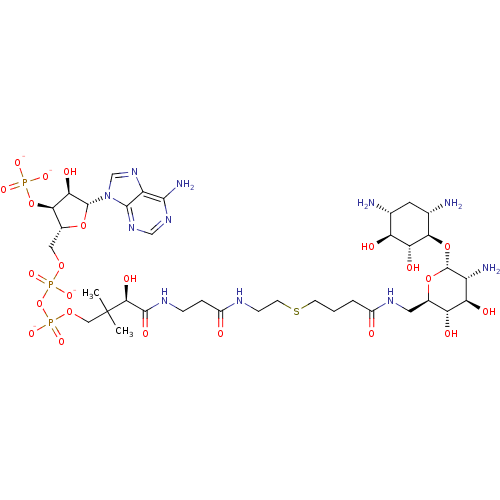
(CID44415024 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCCCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C37H66N11O23P3S/c1-37(2,13-66-74(63,64)71-73(61,62)65-12-19-30(70-72(58,59)60)28(55)35(67-19)48-15-47-23-32(41)45-14-46-33(23)48)31(56)34(57)43-6-5-21(50)42-7-9-75-8-3-4-20(49)44-11-18-25(52)26(53)22(40)36(68-18)69-29-17(39)10-16(38)24(51)27(29)54/h14-19,22,24-31,35-36,51-56H,3-13,38-40H2,1-2H3,(H,42,50)(H,43,57)(H,44,49)(H,61,62)(H,63,64)(H2,41,45,46)(H2,58,59,60)/p-4/t16-,17+,18-,19-,22-,24+,25-,26-,27-,28-,29-,30-,31+,35-,36-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50316179
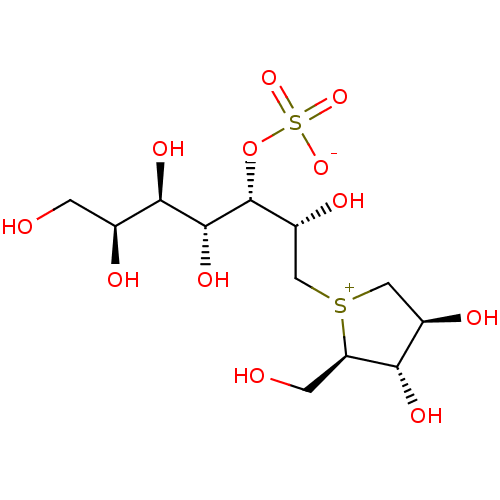
((1S,2R,3R,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](OS([O-])(=O)=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C12H24O12S2/c13-1-5(15)10(19)11(20)12(24-26(21,22)23)7(17)4-25-3-6(16)9(18)8(25)2-14/h5-20H,1-4H2/t5-,6+,7+,8+,9-,10+,11+,12+,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309854
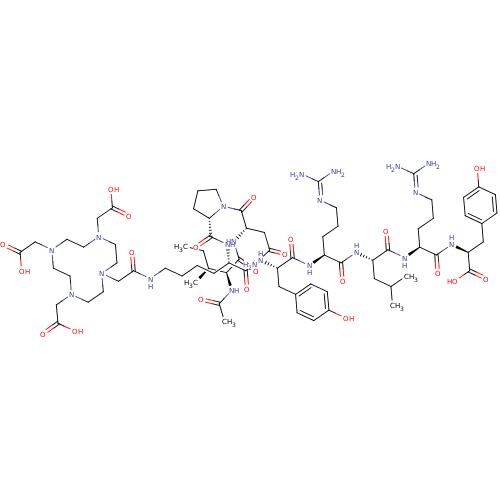
((3S,6S,9S,12S,15S,18S)-1-((S)-1-((S)-2-((2S,3S)-2-...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6](-[#8])=O)-[#6]-[#6]-[#7](-[#6]-[#6](-[#8])=O)-[#6]-[#6]-[#7](-[#6]-[#6](-[#8])=O)-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C75H119N21O21/c1-6-45(4)64(84-46(5)97)71(114)90-56(39-59(76)100)72(115)96-27-11-15-58(96)70(113)87-51(12-7-8-24-81-60(101)40-92-28-30-93(41-61(102)103)32-34-95(43-63(106)107)35-33-94(31-29-92)42-62(104)105)65(108)89-55(37-47-16-20-49(98)21-17-47)69(112)86-52(13-9-25-82-74(77)78)66(109)88-54(36-44(2)3)68(111)85-53(14-10-26-83-75(79)80)67(110)91-57(73(116)117)38-48-18-22-50(99)23-19-48/h16-23,44-45,51-58,64,98-99H,6-15,24-43H2,1-5H3,(H2,76,100)(H,81,101)(H,84,97)(H,85,111)(H,86,112)(H,87,113)(H,88,109)(H,89,108)(H,90,114)(H,91,110)(H,102,103)(H,104,105)(H,106,107)(H,116,117)(H4,77,78,82)(H4,79,80,83)/t45-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343683
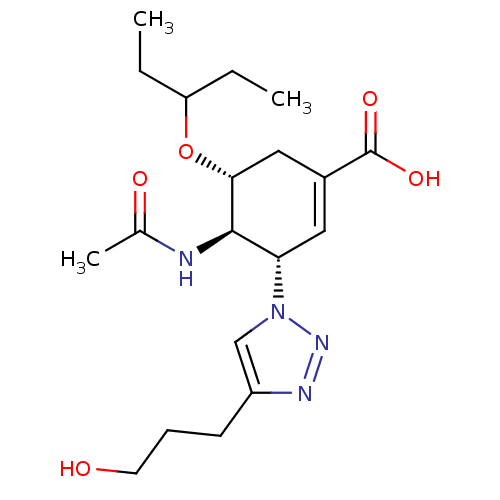
((3S,4R,5R)-4-Acetamido-5-(1-ethylpropoxy)-3-[4-(3-...)Show SMILES CCC(CC)O[C@@H]1CC(=C[C@@H]([C@H]1NC(C)=O)n1cc(CCCO)nn1)C(O)=O |r,c:8| Show InChI InChI=1S/C19H30N4O5/c1-4-15(5-2)28-17-10-13(19(26)27)9-16(18(17)20-12(3)25)23-11-14(21-22-23)7-6-8-24/h9,11,15-18,24H,4-8,10H2,1-3H3,(H,20,25)(H,26,27)/t16-,17+,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330955
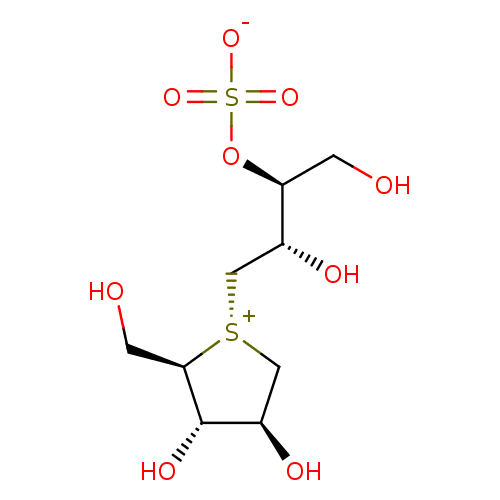
((1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymeth...)Show SMILES OC[C@H](OS([O-])(=O)=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C9H18O9S2/c10-1-7(18-20(15,16)17)5(12)3-19-4-6(13)9(14)8(19)2-11/h5-14H,1-4H2/t5-,6-,7+,8-,9+,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50242271
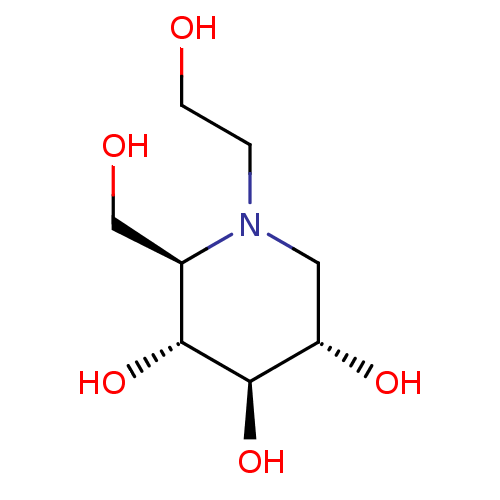
((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...)Show SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C8H17NO5/c10-2-1-9-3-6(12)8(14)7(13)5(9)4-11/h5-8,10-14H,1-4H2/t5-,6+,7-,8-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50343686
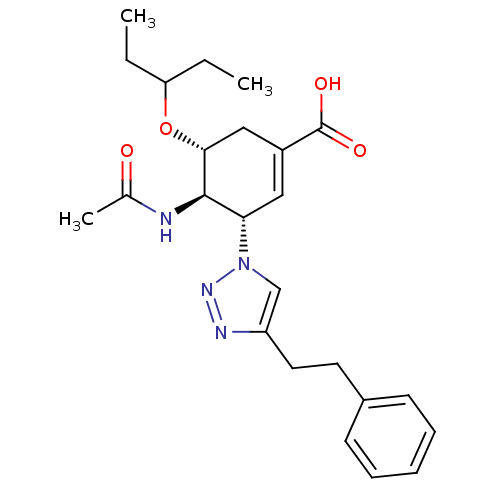
((3S,4R,5R)-4-acetamido-5-(pentan-3-yloxy)-3-(4-phe...)Show SMILES CCC(CC)O[C@@H]1CC(=C[C@@H]([C@H]1NC(C)=O)n1cc(CCc2ccccc2)nn1)C(O)=O |r,c:8| Show InChI InChI=1S/C24H32N4O4/c1-4-20(5-2)32-22-14-18(24(30)31)13-21(23(22)25-16(3)29)28-15-19(26-27-28)12-11-17-9-7-6-8-10-17/h6-10,13,15,20-23H,4-5,11-12,14H2,1-3H3,(H,25,29)(H,30,31)/t21-,22+,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330957
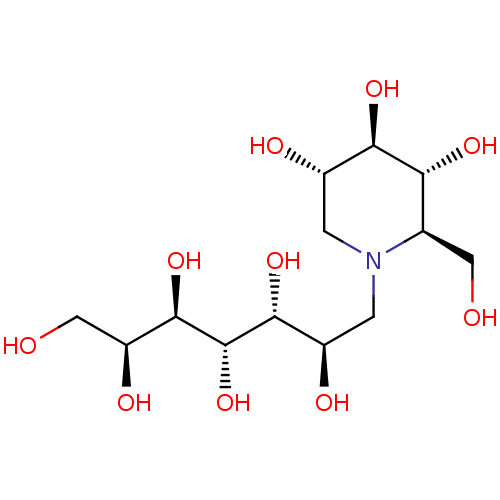
(7'-[(1,5-Dideoxy-1,5-imino-D-glucitol)-5-N-ammoniu...)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C13H27NO10/c15-3-5-9(20)10(21)6(17)1-14(5)2-7(18)11(22)13(24)12(23)8(19)4-16/h5-13,15-24H,1-4H2/t5-,6+,7-,8+,9-,10-,11-,12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330956

(7'-[(1,5-Dideoxy-1,5-imino-D-glucitol)-5-N-ammoniu...)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](OS([O-])(=O)=O)[C@H](O)C[NH+]1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C13H27NO13S/c15-3-5-9(20)10(21)6(17)1-14(5)2-7(18)13(27-28(24,25)26)12(23)11(22)8(19)4-16/h5-13,15-23H,1-4H2,(H,24,25,26)/t5-,6+,7-,8+,9-,10-,11-,12-,13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50309858
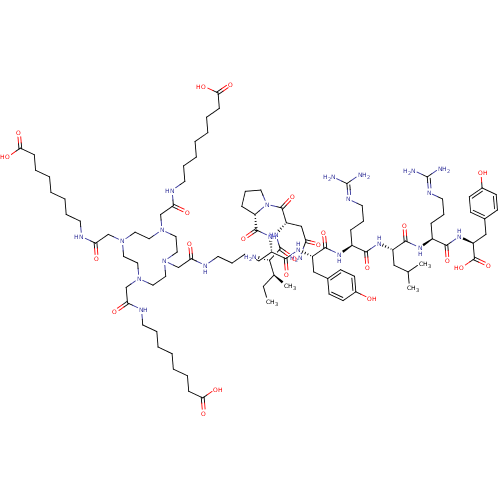
(8-{2-[4-({[(5S)-5-{[(2S)-1-[(2S)-2-[(2S,3S)-2-amin...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](-[#8])=O)-[#6]-[#6]-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](-[#8])=O)-[#6]-[#6]-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](-[#8])=O)-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C97H162N24O23/c1-5-65(4)86(99)93(141)115-75(59-78(98)124)94(142)121-47-25-29-77(121)92(140)112-70(87(135)114-74(57-66-33-37-68(122)38-34-66)91(139)111-71(27-23-45-108-96(100)101)88(136)113-73(56-64(2)3)90(138)110-72(28-24-46-109-97(102)103)89(137)116-76(95(143)144)58-67-35-39-69(123)40-36-67)26-18-22-44-107-82(128)63-120-54-52-118(61-80(126)105-42-20-13-7-10-16-31-84(131)132)50-48-117(60-79(125)104-41-19-12-6-9-15-30-83(129)130)49-51-119(53-55-120)62-81(127)106-43-21-14-8-11-17-32-85(133)134/h33-40,64-65,70-77,86,122-123H,5-32,41-63,99H2,1-4H3,(H2,98,124)(H,104,125)(H,105,126)(H,106,127)(H,107,128)(H,110,138)(H,111,139)(H,112,140)(H,113,136)(H,114,135)(H,115,141)(H,116,137)(H,129,130)(H,131,132)(H,133,134)(H,143,144)(H4,100,101,108)(H4,102,103,109)/t65-,70-,71-,72-,73-,74-,75-,76-,77-,86-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I]NPY from human neuropeptide Y1 receptor expressed in human MCF7 cells after 40 mins |
Bioorg Med Chem Lett 20: 950-3 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.068
BindingDB Entry DOI: 10.7270/Q2V9886H |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343689
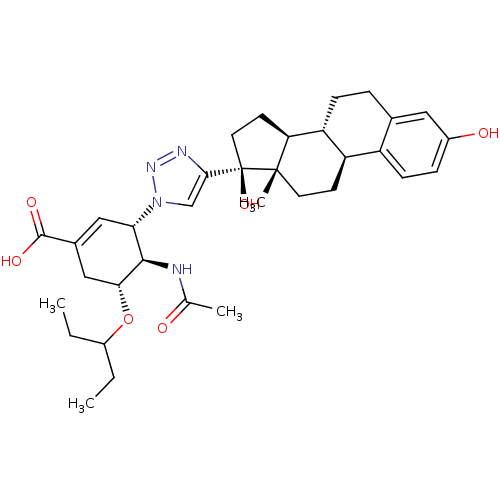
((3S,4R,5R)-4-acetamido-3-(4-((8R,9S,13S,14S,17S)-3...)Show SMILES CCC(CC)O[C@@H]1CC(=C[C@@H]([C@H]1NC(C)=O)n1cc(nn1)[C@]1(O)CC[C@H]2[C@@H]3CCc4cc(O)ccc4[C@H]3CC[C@]12C)C(O)=O |r,c:8| Show InChI InChI=1S/C34H46N4O6/c1-5-23(6-2)44-29-17-21(32(41)42)16-28(31(29)35-19(3)39)38-18-30(36-37-38)34(43)14-12-27-26-9-7-20-15-22(40)8-10-24(20)25(26)11-13-33(27,34)4/h8,10,15-16,18,23,25-29,31,40,43H,5-7,9,11-14,17H2,1-4H3,(H,35,39)(H,41,42)/t25-,26-,27+,28+,29-,31-,33+,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM4706
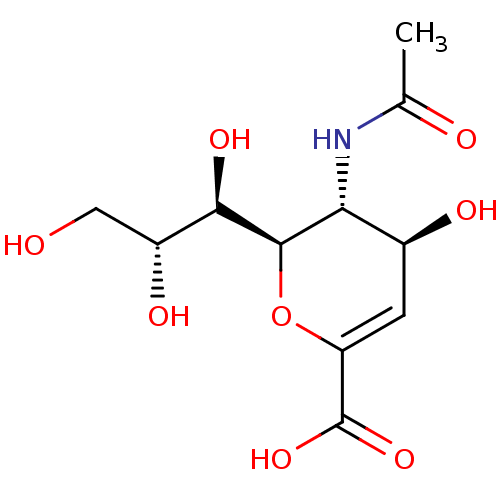
((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |r,c:7| Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193481
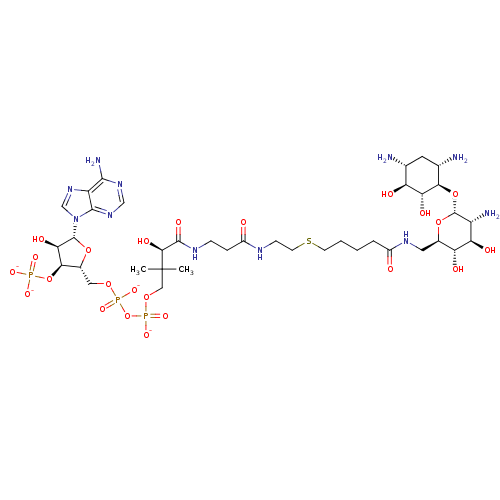
(CID44414945 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCCCCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C38H68N11O23P3S/c1-38(2,14-67-75(64,65)72-74(62,63)66-13-20-31(71-73(59,60)61)29(56)36(68-20)49-16-48-24-33(42)46-15-47-34(24)49)32(57)35(58)44-7-6-22(51)43-8-10-76-9-4-3-5-21(50)45-12-19-26(53)27(54)23(41)37(69-19)70-30-18(40)11-17(39)25(52)28(30)55/h15-20,23,25-32,36-37,52-57H,3-14,39-41H2,1-2H3,(H,43,51)(H,44,58)(H,45,50)(H,62,63)(H,64,65)(H2,42,46,47)(H2,59,60,61)/p-4/t17-,18+,19-,20-,23-,25+,26-,27-,28-,29-,30-,31-,32+,36-,37-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343690
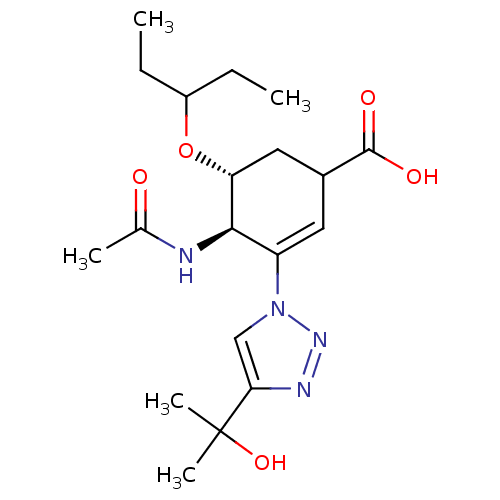
((4R,5R)-4-acetamido-3-(4-(2-hydroxypropan-2-yl)-1H...)Show SMILES CCC(CC)O[C@@H]1CC(C=C([C@H]1NC(C)=O)n1cc(nn1)C(C)(C)O)C(O)=O |r,c:9| Show InChI InChI=1S/C19H30N4O5/c1-6-13(7-2)28-15-9-12(18(25)26)8-14(17(15)20-11(3)24)23-10-16(21-22-23)19(4,5)27/h8,10,12-13,15,17,27H,6-7,9H2,1-5H3,(H,20,24)(H,25,26)/t12?,15-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343680
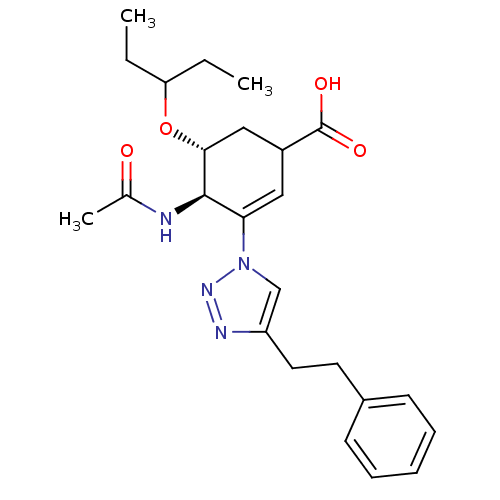
((4R,5R)-4-acetamido-5-(pentan-3-yloxy)-3-(4-phenet...)Show SMILES CCC(CC)O[C@@H]1CC(C=C([C@H]1NC(C)=O)n1cc(CCc2ccccc2)nn1)C(O)=O |r,c:9| Show InChI InChI=1S/C24H32N4O4/c1-4-20(5-2)32-22-14-18(24(30)31)13-21(23(22)25-16(3)29)28-15-19(26-27-28)12-11-17-9-7-6-8-10-17/h6-10,13,15,18,20,22-23H,4-5,11-12,14H2,1-3H3,(H,25,29)(H,30,31)/t18?,22-,23-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330958
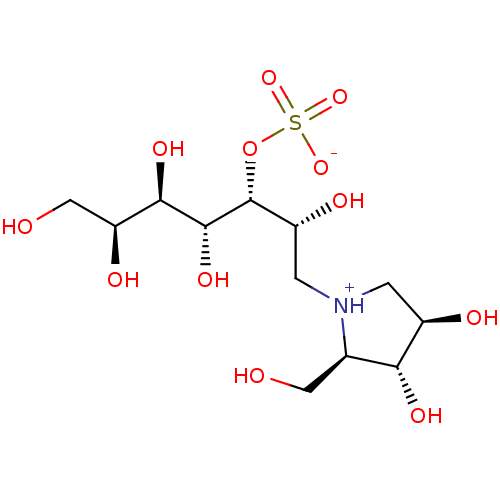
((2R,3R,4R,5R,6S)-1-((2R,3R,4R)-3,4-dihydroxy-2-(hy...)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](OS([O-])(=O)=O)[C@H](O)C[NH+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C12H25NO12S/c14-3-5-9(19)6(16)1-13(5)2-7(17)12(25-26(22,23)24)11(21)10(20)8(18)4-15/h5-12,14-21H,1-4H2,(H,22,23,24)/t5-,6-,7-,8+,9-,10-,11-,12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay |
Bioorg Med Chem 18: 7794-8 (2010)
Article DOI: 10.1016/j.bmc.2010.09.059
BindingDB Entry DOI: 10.7270/Q2TT4RZH |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM50585537
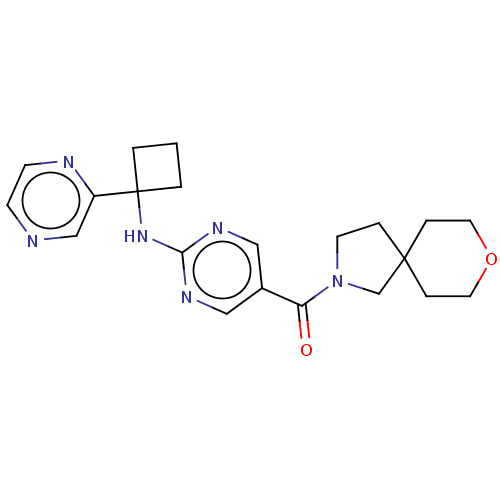
(CHEMBL5084938)Show SMILES O=C(N1CCC2(C1)CCOCC2)c1cnc(NC2(CCC2)c2cnccn2)nc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01849
BindingDB Entry DOI: 10.7270/Q2XD15K6 |
More data for this
Ligand-Target Pair | |
Pantetheinase
(Homo sapiens (Human)) | BDBM480924
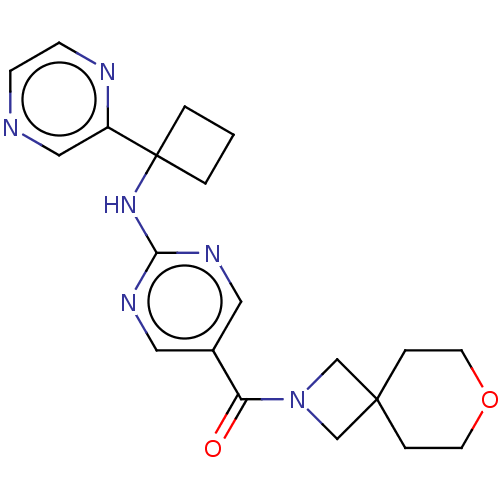
(US10906888, Example 138)Show SMILES O=C(N1CC2(C1)CCOCC2)c1cnc(NC2(CCC2)c2cnccn2)nc1 Show InChI InChI=1S/C20H24N6O2/c27-17(26-13-19(14-26)4-8-28-9-5-19)15-10-23-18(24-11-15)25-20(2-1-3-20)16-12-21-6-7-22-16/h6-7,10-12H,1-5,8-9,13-14H2,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01849
BindingDB Entry DOI: 10.7270/Q2XD15K6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data