 Found 201 hits with Last Name = 'moilanen' and Initial = 'a'
Found 201 hits with Last Name = 'moilanen' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017931

(CHEMBL3289302)Show SMILES NCCC1CCN(CC1)C(=O)[C@@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)C1CCC(N)=NC1 |r,c:39| Show InChI InChI=1S/C28H39N7O3S/c29-12-9-19-10-13-35(14-11-19)28(36)25(16-20-3-1-5-22(15-20)27(31)32)34-39(37,38)24-6-2-4-21(17-24)23-7-8-26(30)33-18-23/h1-6,15,17,19,23,25,34H,7-14,16,18,29H2,(H2,30,33)(H3,31,32)/t23?,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017916
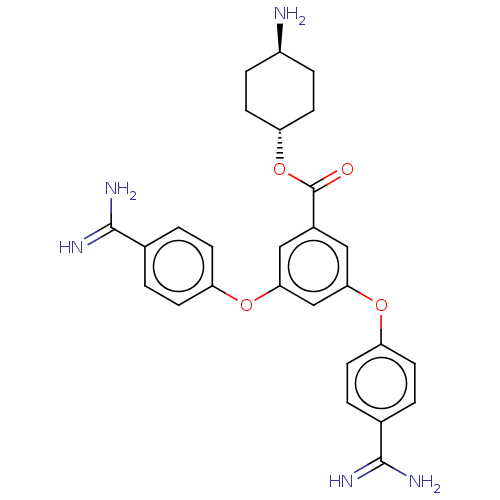
(CHEMBL3289038)Show SMILES N[C@H]1CC[C@@H](CC1)OC(=O)c1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(22.46,-8.49,;21.13,-7.72,;21.13,-6.18,;19.8,-5.41,;18.46,-6.18,;18.46,-7.72,;19.8,-8.49,;17.13,-5.41,;15.8,-6.18,;15.8,-7.72,;14.46,-5.41,;14.46,-3.87,;13.13,-3.1,;13.13,-1.56,;14.46,-.79,;14.46,.75,;15.8,1.52,;17.13,.75,;17.13,-.79,;15.8,-1.56,;18.46,1.52,;19.8,.75,;18.46,3.06,;11.79,-3.87,;11.79,-5.41,;10.46,-6.18,;9.13,-5.41,;7.79,-6.18,;6.46,-5.41,;6.46,-3.87,;7.79,-3.1,;9.13,-3.87,;5.13,-3.1,;3.79,-3.87,;5.13,-1.56,;13.13,-6.18,)| Show InChI InChI=1S/C27H29N5O4/c28-19-5-11-22(12-6-19)36-27(33)18-13-23(34-20-7-1-16(2-8-20)25(29)30)15-24(14-18)35-21-9-3-17(4-10-21)26(31)32/h1-4,7-10,13-15,19,22H,5-6,11-12,28H2,(H3,29,30)(H3,31,32)/t19-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017902
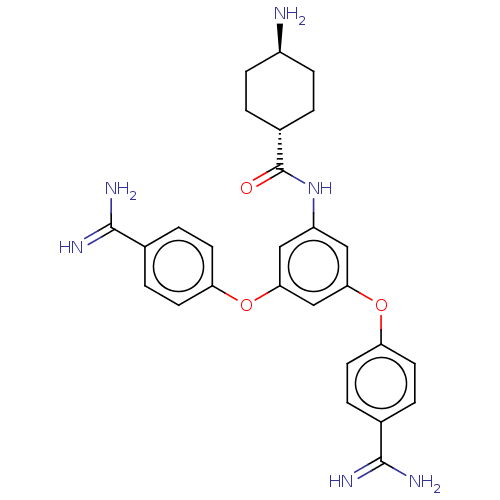
(CHEMBL3289034)Show SMILES N[C@H]1CC[C@@H](CC1)C(=O)Nc1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:1.0,wD:4.7,(15.95,-2.09,;15.95,-3.63,;17.28,-4.4,;17.28,-5.94,;15.95,-6.71,;14.62,-5.94,;14.62,-4.4,;15.95,-8.25,;17.28,-9.02,;14.62,-9.02,;13.28,-8.25,;13.28,-6.71,;11.95,-5.94,;11.95,-4.4,;10.61,-3.63,;10.61,-2.09,;9.28,-1.32,;7.95,-2.09,;7.95,-3.63,;9.28,-4.4,;6.61,-1.32,;5.28,-2.09,;6.61,.22,;10.61,-6.71,;10.61,-8.25,;9.28,-9.02,;7.95,-8.25,;6.61,-9.02,;5.28,-8.25,;5.28,-6.71,;6.61,-5.94,;7.95,-6.71,;3.95,-5.94,;2.61,-6.71,;3.95,-4.4,;11.95,-9.02,)| Show InChI InChI=1S/C27H30N6O3/c28-19-7-1-18(2-8-19)27(34)33-20-13-23(35-21-9-3-16(4-10-21)25(29)30)15-24(14-20)36-22-11-5-17(6-12-22)26(31)32/h3-6,9-15,18-19H,1-2,7-8,28H2,(H3,29,30)(H3,31,32)(H,33,34)/t18-,19- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017934
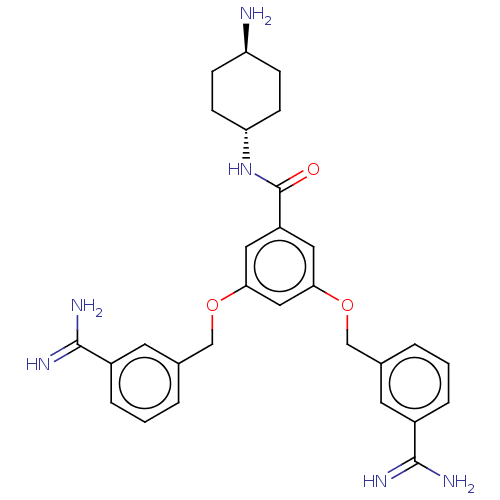
(CHEMBL3289301)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(OCc2cccc(c2)C(N)=N)cc(OCc2cccc(c2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(-.73,-10.04,;.81,-10.04,;1.58,-11.38,;3.12,-11.38,;3.89,-10.04,;3.12,-8.71,;1.58,-8.71,;5.43,-10.04,;6.2,-8.71,;5.43,-7.38,;7.74,-8.71,;8.51,-10.04,;10.05,-10.04,;10.82,-11.38,;12.36,-11.38,;13.13,-12.71,;12.36,-14.04,;13.13,-15.38,;14.67,-15.38,;15.44,-14.04,;14.67,-12.71,;16.98,-14.04,;17.75,-15.38,;17.75,-12.71,;10.82,-8.71,;10.05,-7.38,;10.82,-6.04,;12.36,-6.04,;13.13,-4.71,;14.67,-4.71,;15.44,-3.37,;14.67,-2.04,;13.13,-2.04,;12.36,-3.37,;12.36,-.71,;13.13,.63,;10.82,-.71,;8.51,-7.38,)| Show InChI InChI=1S/C29H34N6O3/c30-23-7-9-24(10-8-23)35-29(36)22-13-25(37-16-18-3-1-5-20(11-18)27(31)32)15-26(14-22)38-17-19-4-2-6-21(12-19)28(33)34/h1-6,11-15,23-24H,7-10,16-17,30H2,(H3,31,32)(H3,33,34)(H,35,36)/t23-,24- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017919
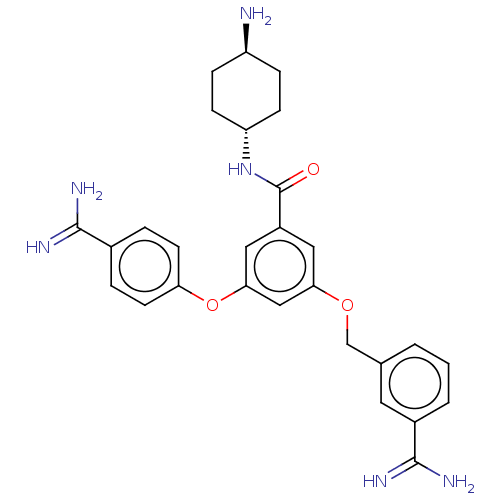
(CHEMBL3289043)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(OCc2cccc(c2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(6.16,1.78,;6.93,.45,;6.16,-.88,;6.93,-2.22,;8.47,-2.22,;9.24,-.88,;8.47,.45,;9.24,-3.55,;10.78,-3.55,;11.55,-2.22,;11.55,-4.88,;13.09,-4.88,;13.86,-6.22,;15.4,-6.22,;16.17,-4.88,;17.71,-4.88,;18.48,-6.22,;20.02,-6.22,;20.79,-4.88,;20.02,-3.55,;18.48,-3.55,;20.79,-2.22,;22.33,-2.22,;20.02,-.88,;13.09,-7.55,;11.55,-7.55,;10.78,-8.89,;9.24,-8.89,;8.47,-10.22,;6.93,-10.22,;6.16,-8.89,;6.93,-7.55,;8.47,-7.55,;4.62,-8.89,;3.85,-10.22,;3.85,-7.55,;10.78,-6.22,)| Show InChI InChI=1S/C28H32N6O3/c29-21-6-8-22(9-7-21)34-28(35)20-13-24(36-16-17-2-1-3-19(12-17)27(32)33)15-25(14-20)37-23-10-4-18(5-11-23)26(30)31/h1-5,10-15,21-22H,6-9,16,29H2,(H3,30,31)(H3,32,33)(H,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017897
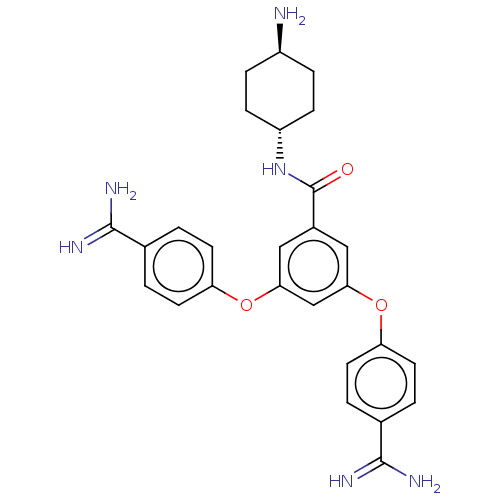
(CHEMBL3289023)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:1.0,wD:4.7,(22.23,-9.88,;20.9,-9.11,;19.57,-9.88,;18.23,-9.11,;18.23,-7.57,;19.57,-6.8,;20.9,-7.57,;16.9,-6.8,;15.56,-7.57,;15.56,-9.11,;14.23,-6.8,;14.23,-5.26,;12.9,-4.49,;12.9,-2.95,;14.23,-2.18,;14.23,-.64,;15.56,.13,;16.9,-.64,;16.9,-2.18,;15.56,-2.95,;18.23,.13,;19.57,-.64,;18.23,1.67,;11.56,-5.26,;11.56,-6.8,;10.23,-7.57,;8.9,-6.8,;7.56,-7.57,;6.23,-6.8,;6.23,-5.26,;7.56,-4.49,;8.9,-5.26,;4.9,-4.49,;3.56,-5.26,;4.9,-2.95,;12.9,-7.57,)| Show InChI InChI=1S/C27H30N6O3/c28-19-5-7-20(8-6-19)33-27(34)18-13-23(35-21-9-1-16(2-10-21)25(29)30)15-24(14-18)36-22-11-3-17(4-12-22)26(31)32/h1-4,9-15,19-20H,5-8,28H2,(H3,29,30)(H3,31,32)(H,33,34)/t19-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017920
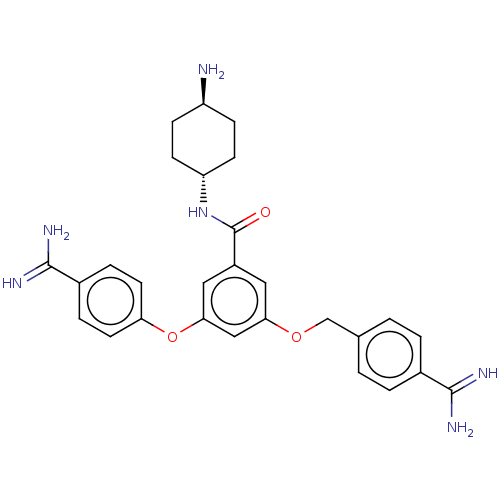
(CHEMBL3289042)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(OCc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(3.93,1.19,;4.7,-.15,;3.93,-1.48,;4.7,-2.81,;6.24,-2.81,;7.01,-1.48,;6.24,-.15,;7.01,-4.15,;8.55,-4.15,;9.32,-2.81,;9.32,-5.48,;10.86,-5.48,;11.63,-6.81,;13.17,-6.81,;13.94,-5.48,;15.48,-5.48,;16.25,-4.15,;17.79,-4.15,;18.56,-5.48,;17.79,-6.81,;16.25,-6.81,;20.1,-5.48,;20.87,-6.81,;20.87,-4.15,;10.86,-8.15,;9.32,-8.15,;8.55,-9.48,;7.01,-9.48,;6.24,-10.82,;4.7,-10.82,;3.93,-9.48,;4.7,-8.15,;6.24,-8.15,;2.39,-9.48,;1.62,-8.15,;1.62,-10.82,;8.55,-6.81,)| Show InChI InChI=1S/C28H32N6O3/c29-21-7-9-22(10-8-21)34-28(35)20-13-24(36-16-17-1-3-18(4-2-17)26(30)31)15-25(14-20)37-23-11-5-19(6-12-23)27(32)33/h1-6,11-15,21-22H,7-10,16,29H2,(H3,30,31)(H3,32,33)(H,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017911
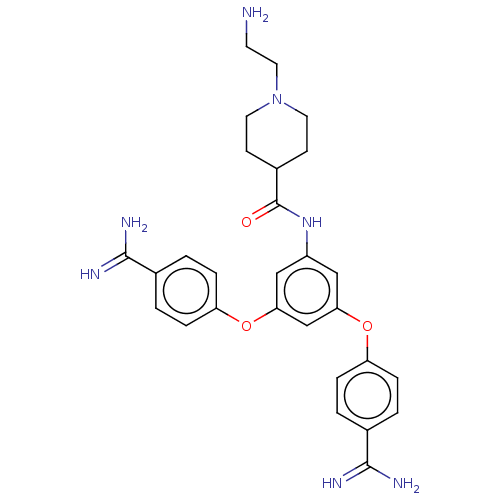
(CHEMBL3289033)Show SMILES NCCN1CCC(CC1)C(=O)Nc1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C28H33N7O3/c29-11-14-35-12-9-20(10-13-35)28(36)34-21-15-24(37-22-5-1-18(2-6-22)26(30)31)17-25(16-21)38-23-7-3-19(4-8-23)27(32)33/h1-8,15-17,20H,9-14,29H2,(H3,30,31)(H3,32,33)(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017930
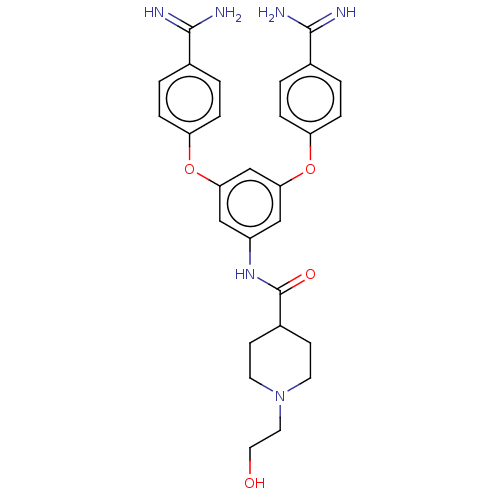
(CHEMBL3289035)Show SMILES NC(=N)c1ccc(Oc2cc(NC(=O)C3CCN(CCO)CC3)cc(Oc3ccc(cc3)C(N)=N)c2)cc1 Show InChI InChI=1S/C28H32N6O4/c29-26(30)18-1-5-22(6-2-18)37-24-15-21(33-28(36)20-9-11-34(12-10-20)13-14-35)16-25(17-24)38-23-7-3-19(4-8-23)27(31)32/h1-8,15-17,20,35H,9-14H2,(H3,29,30)(H3,31,32)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017943
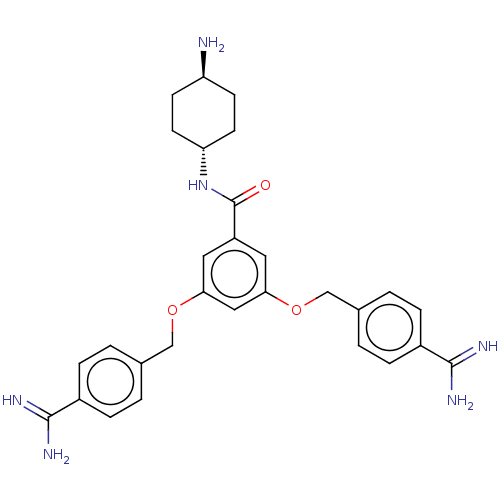
(CHEMBL3289300)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(OCc2ccc(cc2)C(N)=N)cc(OCc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(-.89,-7.94,;.65,-7.94,;1.42,-9.27,;2.96,-9.27,;3.73,-7.94,;2.96,-6.61,;1.42,-6.61,;5.27,-7.94,;6.04,-6.61,;5.27,-5.27,;7.58,-6.61,;8.35,-7.94,;9.89,-7.94,;10.66,-9.27,;12.2,-9.27,;12.97,-10.61,;14.51,-10.61,;15.28,-11.94,;14.51,-13.27,;12.97,-13.27,;12.2,-11.94,;15.28,-14.61,;14.51,-15.94,;16.82,-14.61,;10.66,-6.61,;9.89,-5.27,;10.66,-3.94,;12.2,-3.94,;12.97,-2.6,;12.2,-1.27,;12.97,.06,;14.51,.06,;15.28,-1.27,;14.51,-2.6,;15.28,1.4,;16.82,1.4,;14.51,2.73,;8.35,-5.27,)| Show InChI InChI=1S/C29H34N6O3/c30-23-9-11-24(12-10-23)35-29(36)22-13-25(37-16-18-1-5-20(6-2-18)27(31)32)15-26(14-22)38-17-19-3-7-21(8-4-19)28(33)34/h1-8,13-15,23-24H,9-12,16-17,30H2,(H3,31,32)(H3,33,34)(H,35,36)/t23-,24- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444526
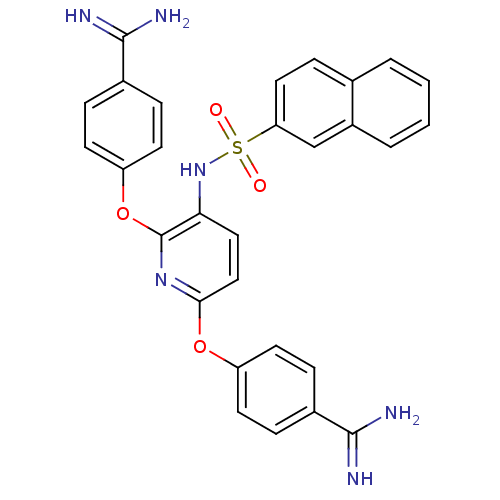
(CHEMBL3099812)Show SMILES NC(=N)c1ccc(Oc2ccc(NS(=O)(=O)c3ccc4ccccc4c3)c(Oc3ccc(cc3)C(N)=N)n2)cc1 Show InChI InChI=1S/C29H24N6O4S/c30-27(31)19-5-10-22(11-6-19)38-26-16-15-25(29(34-26)39-23-12-7-20(8-13-23)28(32)33)35-40(36,37)24-14-9-18-3-1-2-4-21(18)17-24/h1-17,35H,(H3,30,31)(H3,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017923
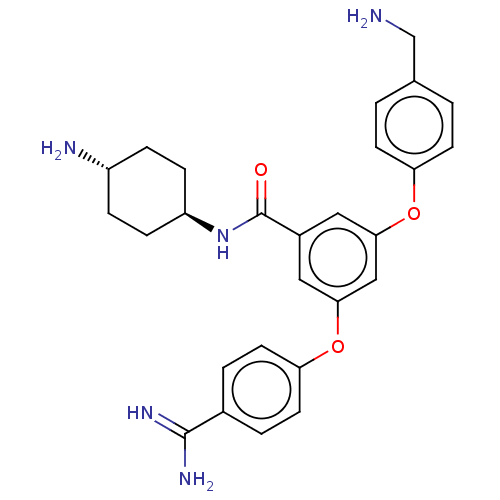
(CHEMBL3289298)Show SMILES NCc1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)N[C@H]2CC[C@H](N)CC2)cc1 |r,wU:26.27,wD:29.31,(-.67,-7.68,;.66,-6.91,;1.99,-7.68,;1.99,-9.22,;3.33,-9.99,;4.66,-9.22,;6,-9.99,;7.33,-9.22,;7.33,-7.68,;8.66,-6.91,;8.66,-5.37,;10,-4.6,;10,-3.06,;11.33,-2.29,;12.66,-3.06,;12.66,-4.6,;11.33,-5.37,;14,-2.29,;15.33,-3.06,;14,-.75,;10,-7.68,;10,-9.22,;8.66,-9.99,;11.33,-9.99,;11.33,-11.53,;12.66,-9.22,;14,-9.99,;15.33,-9.22,;16.66,-9.99,;16.66,-11.53,;18,-12.3,;15.33,-12.3,;14,-11.53,;4.66,-7.68,;3.33,-6.91,)| Show InChI InChI=1S/C27H31N5O3/c28-16-17-1-9-22(10-2-17)34-24-13-19(27(33)32-21-7-5-20(29)6-8-21)14-25(15-24)35-23-11-3-18(4-12-23)26(30)31/h1-4,9-15,20-21H,5-8,16,28-29H2,(H3,30,31)(H,32,33)/t20-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444519
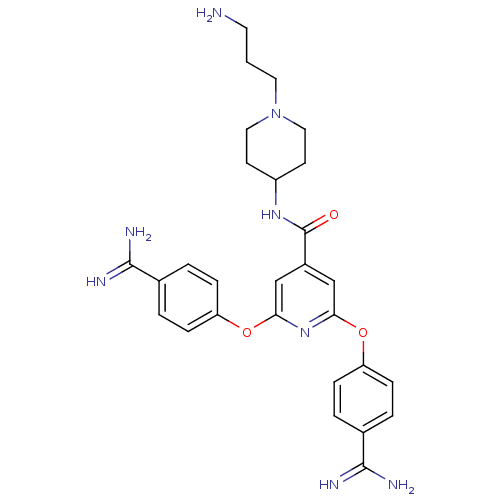
(CHEMBL3099586)Show SMILES NCCCN1CCC(CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)nc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C28H34N8O3/c29-12-1-13-36-14-10-21(11-15-36)34-28(37)20-16-24(38-22-6-2-18(3-7-22)26(30)31)35-25(17-20)39-23-8-4-19(5-9-23)27(32)33/h2-9,16-17,21H,1,10-15,29H2,(H3,30,31)(H3,32,33)(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444518
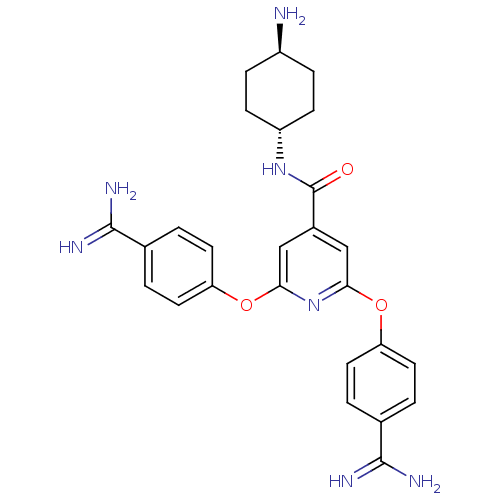
(CHEMBL3099587)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)nc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(26.14,-7.69,;26.15,-9.23,;24.81,-9.99,;24.81,-11.54,;26.14,-12.31,;27.48,-11.54,;27.47,-9.99,;26.15,-13.85,;27.48,-14.62,;28.81,-13.85,;27.48,-16.16,;28.82,-16.93,;28.82,-18.47,;30.16,-19.26,;31.5,-18.49,;31.51,-16.95,;32.84,-16.18,;34.19,-16.96,;34.17,-18.5,;32.83,-19.26,;35.55,-16.2,;35.59,-14.65,;36.88,-17,;27.48,-19.24,;26.15,-18.47,;24.81,-19.25,;24.82,-20.8,;26.15,-21.56,;26.15,-23.11,;24.82,-23.88,;23.49,-23.11,;23.49,-21.57,;24.81,-25.41,;23.47,-26.17,;26.13,-26.2,;26.16,-16.92,)| Show InChI InChI=1S/C26H29N7O3/c27-18-5-7-19(8-6-18)32-26(34)17-13-22(35-20-9-1-15(2-10-20)24(28)29)33-23(14-17)36-21-11-3-16(4-12-21)25(30)31/h1-4,9-14,18-19H,5-8,27H2,(H3,28,29)(H3,30,31)(H,32,34)/t18-,19- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Bos taurus) | BDBM50017916

(CHEMBL3289038)Show SMILES N[C@H]1CC[C@@H](CC1)OC(=O)c1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(22.46,-8.49,;21.13,-7.72,;21.13,-6.18,;19.8,-5.41,;18.46,-6.18,;18.46,-7.72,;19.8,-8.49,;17.13,-5.41,;15.8,-6.18,;15.8,-7.72,;14.46,-5.41,;14.46,-3.87,;13.13,-3.1,;13.13,-1.56,;14.46,-.79,;14.46,.75,;15.8,1.52,;17.13,.75,;17.13,-.79,;15.8,-1.56,;18.46,1.52,;19.8,.75,;18.46,3.06,;11.79,-3.87,;11.79,-5.41,;10.46,-6.18,;9.13,-5.41,;7.79,-6.18,;6.46,-5.41,;6.46,-3.87,;7.79,-3.1,;9.13,-3.87,;5.13,-3.1,;3.79,-3.87,;5.13,-1.56,;13.13,-6.18,)| Show InChI InChI=1S/C27H29N5O4/c28-19-5-11-22(12-6-19)36-27(33)18-13-23(34-20-7-1-16(2-8-20)25(29)30)15-24(14-18)35-21-9-3-17(4-10-21)26(31)32/h1-4,7-10,13-15,19,22H,5-6,11-12,28H2,(H3,29,30)(H3,31,32)/t19-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017917
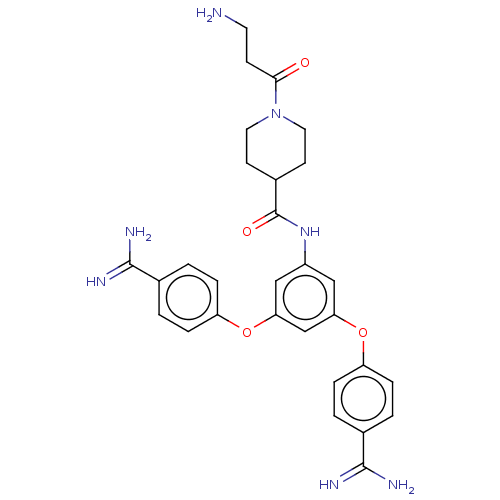
(CHEMBL3289032)Show SMILES NCCC(=O)N1CCC(CC1)C(=O)Nc1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C29H33N7O4/c30-12-9-26(37)36-13-10-20(11-14-36)29(38)35-21-15-24(39-22-5-1-18(2-6-22)27(31)32)17-25(16-21)40-23-7-3-19(4-8-23)28(33)34/h1-8,15-17,20H,9-14,30H2,(H3,31,32)(H3,33,34)(H,35,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017911

(CHEMBL3289033)Show SMILES NCCN1CCC(CC1)C(=O)Nc1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C28H33N7O3/c29-11-14-35-12-9-20(10-13-35)28(36)34-21-15-24(37-22-5-1-18(2-6-22)26(30)31)17-25(16-21)38-23-7-3-19(4-8-23)27(32)33/h1-8,15-17,20H,9-14,29H2,(H3,30,31)(H3,32,33)(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017900
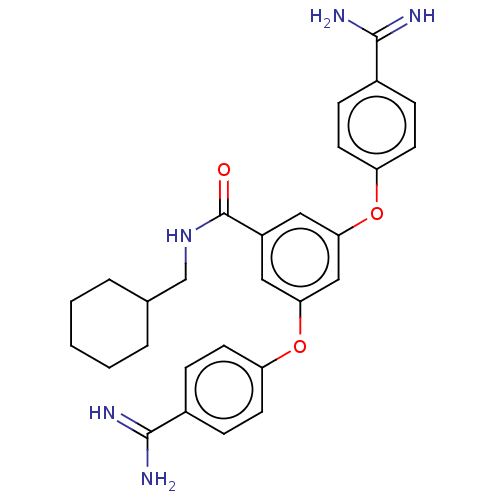
(CHEMBL3289018)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C28H31N5O3/c29-26(30)19-6-10-22(11-7-19)35-24-14-21(28(34)33-17-18-4-2-1-3-5-18)15-25(16-24)36-23-12-8-20(9-13-23)27(31)32/h6-16,18H,1-5,17H2,(H3,29,30)(H3,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017929
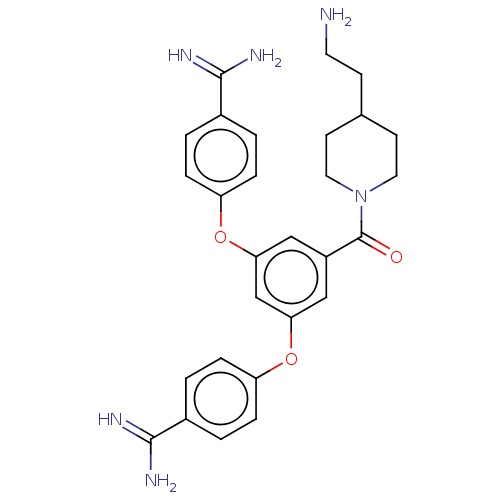
(CHEMBL3289024)Show SMILES NCCC1CCN(CC1)C(=O)c1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C28H32N6O3/c29-12-9-18-10-13-34(14-11-18)28(35)21-15-24(36-22-5-1-19(2-6-22)26(30)31)17-25(16-21)37-23-7-3-20(4-8-23)27(32)33/h1-8,15-18H,9-14,29H2,(H3,30,31)(H3,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017917

(CHEMBL3289032)Show SMILES NCCC(=O)N1CCC(CC1)C(=O)Nc1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C29H33N7O4/c30-12-9-26(37)36-13-10-20(11-14-36)29(38)35-21-15-24(39-22-5-1-18(2-6-22)27(31)32)17-25(16-21)40-23-7-3-19(4-8-23)28(33)34/h1-8,15-17,20H,9-14,30H2,(H3,31,32)(H3,33,34)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444520

(CHEMBL3099585)Show SMILES NCCC1CCN(CC1)C(=O)c1cc(Oc2ccc(cc2)C(N)=N)nc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C27H31N7O3/c28-12-9-17-10-13-34(14-11-17)27(35)20-15-23(36-21-5-1-18(2-6-21)25(29)30)33-24(16-20)37-22-7-3-19(4-8-22)26(31)32/h1-8,15-17H,9-14,28H2,(H3,29,30)(H3,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50444519

(CHEMBL3099586)Show SMILES NCCCN1CCC(CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)nc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C28H34N8O3/c29-12-1-13-36-14-10-21(11-15-36)34-28(37)20-16-24(38-22-6-2-18(3-7-22)26(30)31)35-25(17-20)39-23-8-4-19(5-9-23)27(32)33/h2-9,16-17,21H,1,10-15,29H2,(H3,30,31)(H3,32,33)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a (unknown origin) using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrate |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Serine protease hepsin
(Homo sapiens (Human)) | BDBM50130874

(CHEMBL3632761)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc([n+]1[O-])C1(O)CCCCC1 Show InChI InChI=1S/C20H22N4O2/c21-19(22)13-7-8-15-14(11-13)12-16(23-15)17-5-4-6-18(24(17)26)20(25)9-2-1-3-10-20/h4-8,11-12,23,25H,1-3,9-10H2,(H3,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant hepsin by fluorescence based assay using 65 uM BOC-Gln-Arg-Arg -AMC as substrate |
Bioorg Med Chem Lett 25: 5309-14 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.042
BindingDB Entry DOI: 10.7270/Q24B335X |
More data for this
Ligand-Target Pair | |
Serine protease hepsin
(Homo sapiens (Human)) | BDBM50130870
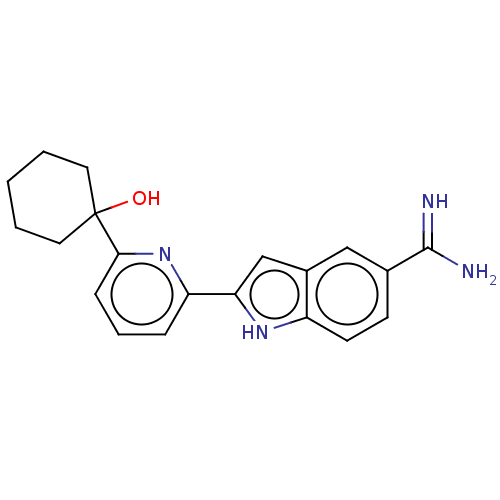
(CHEMBL3632759)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(n1)C1(O)CCCCC1 Show InChI InChI=1S/C20H22N4O/c21-19(22)13-7-8-15-14(11-13)12-17(23-15)16-5-4-6-18(24-16)20(25)9-2-1-3-10-20/h4-8,11-12,23,25H,1-3,9-10H2,(H3,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant hepsin by fluorescence based assay using 65 uM BOC-Gln-Arg-Arg -AMC as substrate |
Bioorg Med Chem Lett 25: 5309-14 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.042
BindingDB Entry DOI: 10.7270/Q24B335X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017924
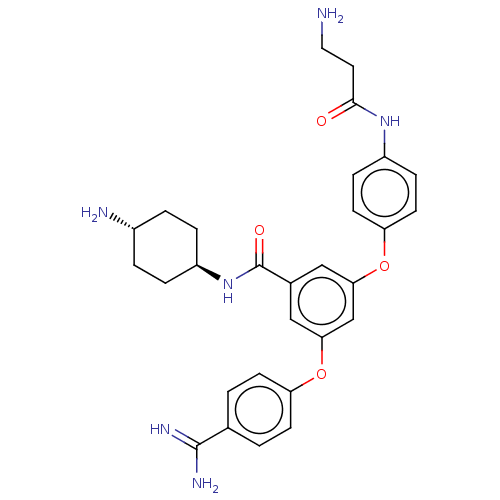
(CHEMBL3289044)Show SMILES NCCC(=O)Nc1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)N[C@H]2CC[C@H](N)CC2)cc1 |r,wU:30.31,wD:33.35,(-.16,-9.38,;-.16,-7.84,;1.17,-7.07,;1.17,-5.53,;-.16,-4.76,;2.51,-4.76,;3.84,-5.53,;3.84,-7.07,;5.17,-7.84,;6.51,-7.07,;7.84,-7.84,;9.17,-7.07,;9.17,-5.53,;10.51,-4.76,;10.51,-3.22,;11.84,-2.45,;11.84,-.91,;13.18,-.14,;14.51,-.91,;14.51,-2.45,;13.18,-3.22,;15.84,-.14,;17.18,-.91,;15.84,1.4,;11.84,-5.53,;11.84,-7.07,;10.51,-7.84,;13.18,-7.84,;13.18,-9.38,;14.51,-7.07,;15.84,-7.84,;17.18,-7.07,;18.51,-7.84,;18.51,-9.38,;19.84,-10.15,;17.18,-10.15,;15.84,-9.38,;6.51,-5.53,;5.17,-4.76,)| Show InChI InChI=1S/C29H34N6O4/c30-14-13-27(36)34-21-7-11-24(12-8-21)39-26-16-19(29(37)35-22-5-3-20(31)4-6-22)15-25(17-26)38-23-9-1-18(2-10-23)28(32)33/h1-2,7-12,15-17,20,22H,3-6,13-14,30-31H2,(H3,32,33)(H,34,36)(H,35,37)/t20-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017936
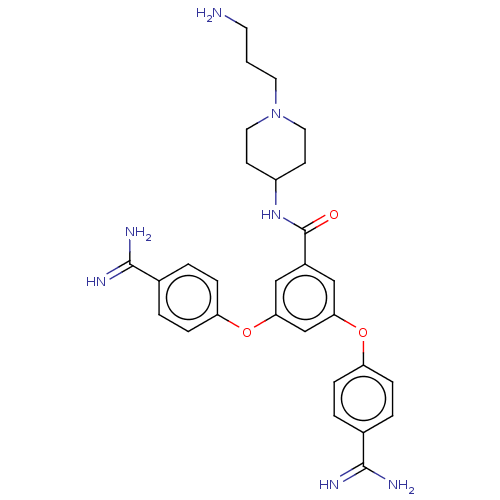
(CHEMBL3289022)Show SMILES NCCCN1CCC(CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C29H35N7O3/c30-12-1-13-36-14-10-22(11-15-36)35-29(37)21-16-25(38-23-6-2-19(3-7-23)27(31)32)18-26(17-21)39-24-8-4-20(5-9-24)28(33)34/h2-9,16-18,22H,1,10-15,30H2,(H3,31,32)(H3,33,34)(H,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017934

(CHEMBL3289301)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(OCc2cccc(c2)C(N)=N)cc(OCc2cccc(c2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(-.73,-10.04,;.81,-10.04,;1.58,-11.38,;3.12,-11.38,;3.89,-10.04,;3.12,-8.71,;1.58,-8.71,;5.43,-10.04,;6.2,-8.71,;5.43,-7.38,;7.74,-8.71,;8.51,-10.04,;10.05,-10.04,;10.82,-11.38,;12.36,-11.38,;13.13,-12.71,;12.36,-14.04,;13.13,-15.38,;14.67,-15.38,;15.44,-14.04,;14.67,-12.71,;16.98,-14.04,;17.75,-15.38,;17.75,-12.71,;10.82,-8.71,;10.05,-7.38,;10.82,-6.04,;12.36,-6.04,;13.13,-4.71,;14.67,-4.71,;15.44,-3.37,;14.67,-2.04,;13.13,-2.04,;12.36,-3.37,;12.36,-.71,;13.13,.63,;10.82,-.71,;8.51,-7.38,)| Show InChI InChI=1S/C29H34N6O3/c30-23-7-9-24(10-8-23)35-29(36)22-13-25(37-16-18-3-1-5-20(11-18)27(31)32)15-26(14-22)38-17-19-4-2-6-21(12-19)28(33)34/h1-6,11-15,23-24H,7-10,16-17,30H2,(H3,31,32)(H3,33,34)(H,35,36)/t23-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017906
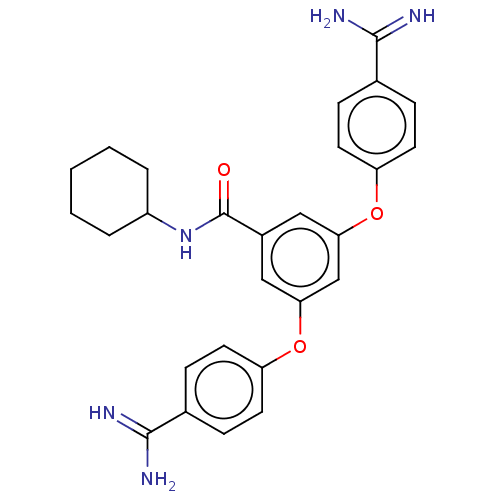
(CHEMBL3289017)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)NC2CCCCC2)cc1 Show InChI InChI=1S/C27H29N5O3/c28-25(29)17-6-10-21(11-7-17)34-23-14-19(27(33)32-20-4-2-1-3-5-20)15-24(16-23)35-22-12-8-18(9-13-22)26(30)31/h6-16,20H,1-5H2,(H3,28,29)(H3,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444521
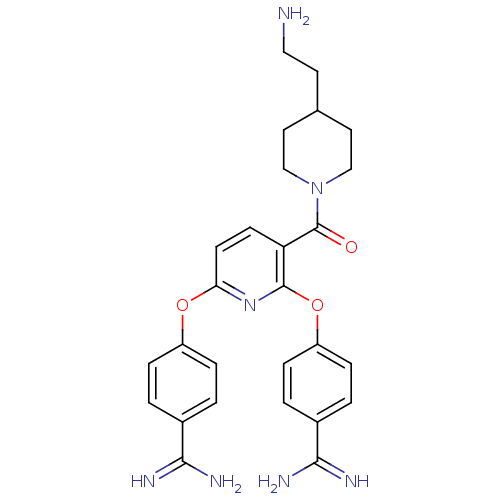
(CHEMBL3099584)Show SMILES NCCC1CCN(CC1)C(=O)c1ccc(Oc2ccc(cc2)C(N)=N)nc1Oc1ccc(cc1)C(N)=N Show InChI InChI=1S/C27H31N7O3/c28-14-11-17-12-15-34(16-13-17)27(35)22-9-10-23(36-20-5-1-18(2-6-20)24(29)30)33-26(22)37-21-7-3-19(4-8-21)25(31)32/h1-10,17H,11-16,28H2,(H3,29,30)(H3,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50444520

(CHEMBL3099585)Show SMILES NCCC1CCN(CC1)C(=O)c1cc(Oc2ccc(cc2)C(N)=N)nc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C27H31N7O3/c28-12-9-17-10-13-34(14-11-17)27(35)20-15-23(36-21-5-1-18(2-6-21)25(29)30)33-24(16-20)37-22-7-3-19(4-8-22)26(31)32/h1-8,15-17H,9-14,28H2,(H3,29,30)(H3,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a (unknown origin) using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrate |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444525
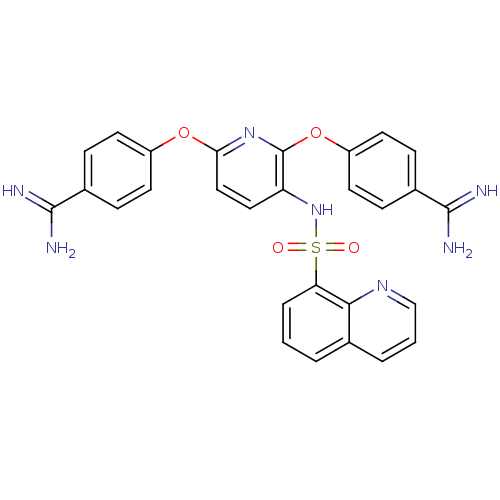
(CHEMBL3099813)Show SMILES NC(=N)c1ccc(Oc2ccc(NS(=O)(=O)c3cccc4cccnc34)c(Oc3ccc(cc3)C(N)=N)n2)cc1 Show InChI InChI=1S/C28H23N7O4S/c29-26(30)18-6-10-20(11-7-18)38-24-15-14-22(28(34-24)39-21-12-8-19(9-13-21)27(31)32)35-40(36,37)23-5-1-3-17-4-2-16-33-25(17)23/h1-16,35H,(H3,29,30)(H3,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50098553
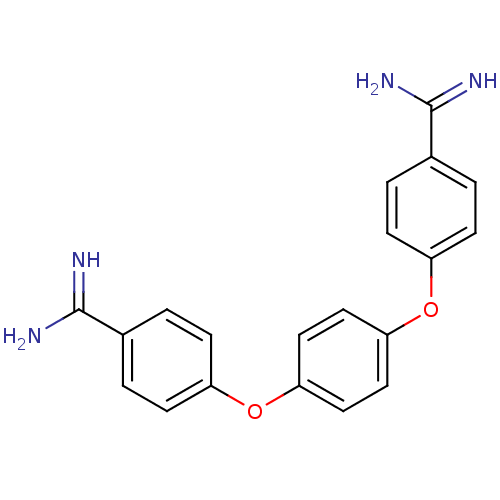
(4-(4-{4-[amino(imino)methyl]phenoxy}phenoxy)benzen...)Show SMILES NC(=N)c1ccc(Oc2ccc(Oc3ccc(cc3)C(N)=N)cc2)cc1 Show InChI InChI=1S/C20H18N4O2/c21-19(22)13-1-5-15(6-2-13)25-17-9-11-18(12-10-17)26-16-7-3-14(4-8-16)20(23)24/h1-12H,(H3,21,22)(H3,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444528
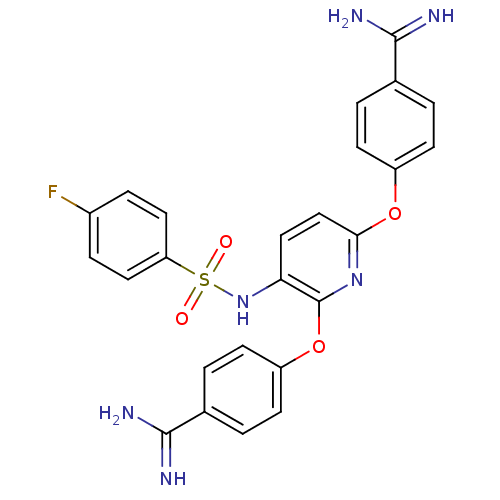
(CHEMBL3099589)Show SMILES NC(=N)c1ccc(Oc2ccc(NS(=O)(=O)c3ccc(F)cc3)c(Oc3ccc(cc3)C(N)=N)n2)cc1 Show InChI InChI=1S/C25H21FN6O4S/c26-17-5-11-20(12-6-17)37(33,34)32-21-13-14-22(35-18-7-1-15(2-8-18)23(27)28)31-25(21)36-19-9-3-16(4-10-19)24(29)30/h1-14,32H,(H3,27,28)(H3,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017935
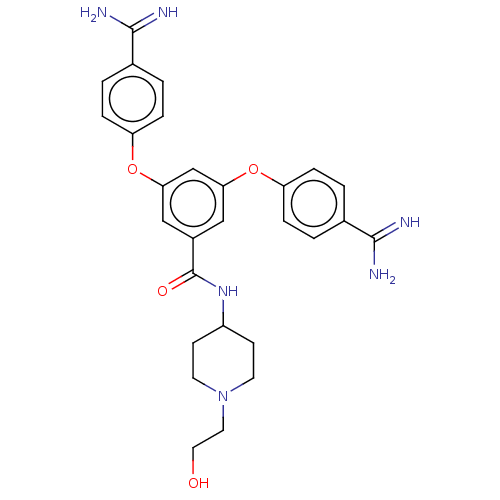
(CHEMBL3289021)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)NC2CCN(CCO)CC2)cc1 Show InChI InChI=1S/C28H32N6O4/c29-26(30)18-1-5-22(6-2-18)37-24-15-20(28(36)33-21-9-11-34(12-10-21)13-14-35)16-25(17-24)38-23-7-3-19(4-8-23)27(31)32/h1-8,15-17,21,35H,9-14H2,(H3,29,30)(H3,31,32)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017895
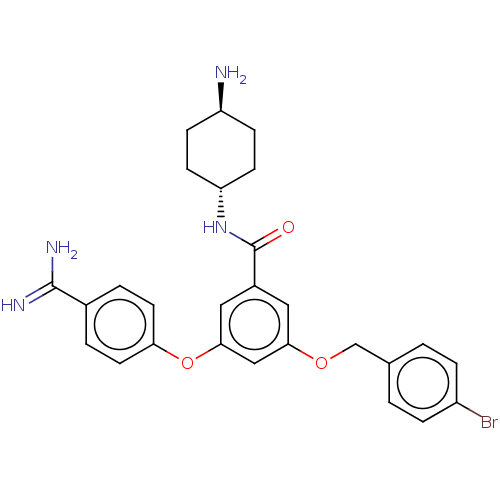
(CHEMBL3289297)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(OCc2ccc(Br)cc2)cc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(3.91,.71,;4.68,-.63,;3.91,-1.96,;4.68,-3.3,;6.22,-3.3,;6.99,-1.96,;6.22,-.63,;6.99,-4.63,;8.53,-4.63,;9.3,-3.3,;9.3,-5.96,;10.84,-5.96,;11.61,-7.3,;13.15,-7.3,;13.92,-5.96,;15.46,-5.96,;16.23,-4.63,;17.77,-4.63,;18.54,-5.96,;20.08,-5.96,;17.77,-7.3,;16.23,-7.3,;10.84,-8.63,;9.3,-8.63,;8.53,-9.96,;6.99,-9.96,;6.22,-11.3,;4.68,-11.3,;3.91,-9.96,;4.68,-8.63,;6.22,-8.63,;2.37,-9.96,;1.6,-11.3,;1.6,-8.63,;8.53,-7.3,)| Show InChI InChI=1S/C27H29BrN4O3/c28-20-5-1-17(2-6-20)16-34-24-13-19(27(33)32-22-9-7-21(29)8-10-22)14-25(15-24)35-23-11-3-18(4-12-23)26(30)31/h1-6,11-15,21-22H,7-10,16,29H2,(H3,30,31)(H,32,33)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017928
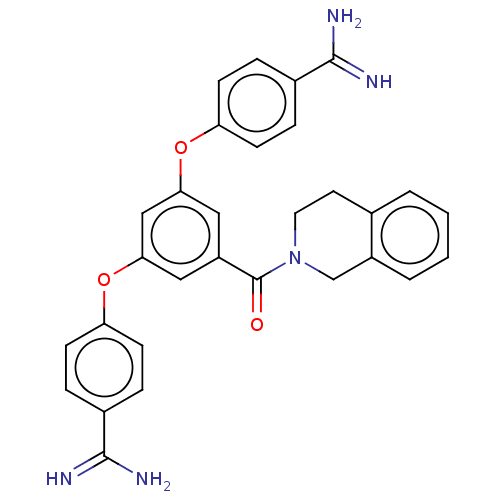
(CHEMBL3289029)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)N2CCc3ccccc3C2)cc1 Show InChI InChI=1S/C30H27N5O3/c31-28(32)20-5-9-24(10-6-20)37-26-15-23(30(36)35-14-13-19-3-1-2-4-22(19)18-35)16-27(17-26)38-25-11-7-21(8-12-25)29(33)34/h1-12,15-17H,13-14,18H2,(H3,31,32)(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017926
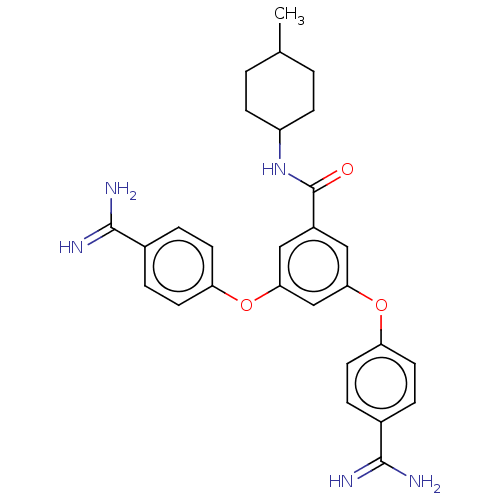
(CHEMBL3289019)Show SMILES CC1CCC(CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 |(22.23,-8.98,;20.89,-8.21,;20.89,-6.67,;19.56,-5.9,;18.23,-6.67,;18.23,-8.21,;19.56,-8.98,;16.89,-5.9,;15.56,-6.67,;15.56,-8.21,;14.22,-5.9,;14.22,-4.36,;12.89,-3.59,;12.89,-2.05,;14.22,-1.28,;14.22,.26,;15.56,1.03,;16.89,.26,;16.89,-1.28,;15.56,-2.05,;18.23,1.03,;19.56,.26,;18.23,2.57,;11.56,-4.36,;11.56,-5.9,;10.22,-6.67,;8.89,-5.9,;7.56,-6.67,;6.22,-5.9,;6.22,-4.36,;7.56,-3.59,;8.89,-4.36,;4.89,-3.59,;3.55,-4.36,;4.89,-2.05,;12.89,-6.67,)| Show InChI InChI=1S/C28H31N5O3/c1-17-2-8-21(9-3-17)33-28(34)20-14-24(35-22-10-4-18(5-11-22)26(29)30)16-25(15-20)36-23-12-6-19(7-13-23)27(31)32/h4-7,10-17,21H,2-3,8-9H2,1H3,(H3,29,30)(H3,31,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50444522
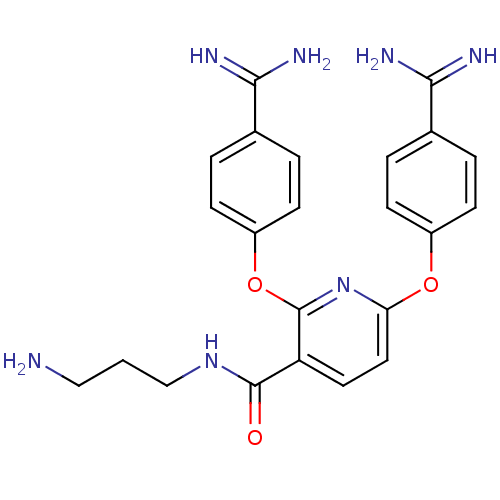
(CHEMBL3099583)Show SMILES NCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=N)nc1Oc1ccc(cc1)C(N)=N Show InChI InChI=1S/C23H25N7O3/c24-12-1-13-29-22(31)18-10-11-19(32-16-6-2-14(3-7-16)20(25)26)30-23(18)33-17-8-4-15(5-9-17)21(27)28/h2-11H,1,12-13,24H2,(H3,25,26)(H3,27,28)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a (unknown origin) using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrate |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50444528

(CHEMBL3099589)Show SMILES NC(=N)c1ccc(Oc2ccc(NS(=O)(=O)c3ccc(F)cc3)c(Oc3ccc(cc3)C(N)=N)n2)cc1 Show InChI InChI=1S/C25H21FN6O4S/c26-17-5-11-20(12-6-17)37(33,34)32-21-13-14-22(35-18-7-1-15(2-8-18)23(27)28)31-25(21)36-19-9-3-16(4-10-19)24(29)30/h1-14,32H,(H3,27,28)(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a (unknown origin) using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrate |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50444526

(CHEMBL3099812)Show SMILES NC(=N)c1ccc(Oc2ccc(NS(=O)(=O)c3ccc4ccccc4c3)c(Oc3ccc(cc3)C(N)=N)n2)cc1 Show InChI InChI=1S/C29H24N6O4S/c30-27(31)19-5-10-22(11-6-19)38-26-16-15-25(29(34-26)39-23-12-7-20(8-13-23)28(32)33)35-40(36,37)24-14-9-18-3-1-2-4-21(18)17-24/h1-17,35H,(H3,30,31)(H3,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a (unknown origin) using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrate |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017941
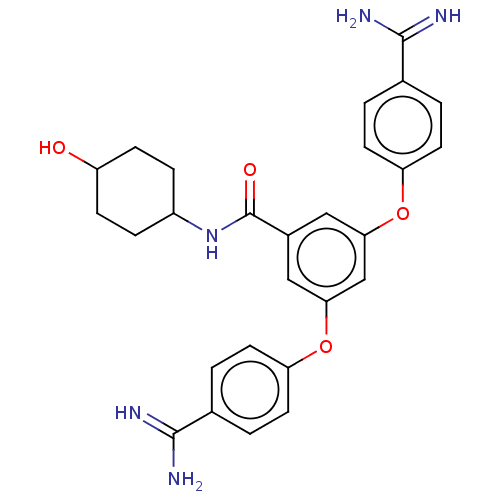
(CHEMBL3289020)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)NC2CCC(O)CC2)cc1 |(20.63,-.34,;19.29,.43,;19.29,1.97,;17.96,-.34,;16.63,.43,;15.29,-.34,;15.29,-1.88,;13.96,-2.65,;13.96,-4.19,;12.63,-4.96,;12.63,-6.5,;11.29,-7.27,;9.96,-6.5,;8.62,-7.27,;7.29,-6.5,;7.29,-4.96,;8.62,-4.19,;9.96,-4.96,;5.96,-4.19,;4.62,-4.96,;5.96,-2.65,;13.96,-7.27,;15.29,-6.5,;15.29,-4.96,;16.63,-7.27,;16.63,-8.81,;17.96,-6.5,;19.29,-7.27,;20.63,-6.5,;21.96,-7.27,;21.96,-8.81,;23.3,-9.58,;20.63,-9.58,;19.29,-8.81,;16.63,-2.65,;17.96,-1.88,)| Show InChI InChI=1S/C27H29N5O4/c28-25(29)16-1-9-21(10-2-16)35-23-13-18(27(34)32-19-5-7-20(33)8-6-19)14-24(15-23)36-22-11-3-17(4-12-22)26(30)31/h1-4,9-15,19-20,33H,5-8H2,(H3,28,29)(H3,30,31)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017939

(CHEMBL3289028)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)N2CCC3CCCCC3C2)cc1 Show InChI InChI=1S/C30H33N5O3/c31-28(32)20-5-9-24(10-6-20)37-26-15-23(30(36)35-14-13-19-3-1-2-4-22(19)18-35)16-27(17-26)38-25-11-7-21(8-12-25)29(33)34/h5-12,15-17,19,22H,1-4,13-14,18H2,(H3,31,32)(H3,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017939

(CHEMBL3289028)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)N2CCC3CCCCC3C2)cc1 Show InChI InChI=1S/C30H33N5O3/c31-28(32)20-5-9-24(10-6-20)37-26-15-23(30(36)35-14-13-19-3-1-2-4-22(19)18-35)16-27(17-26)38-25-11-7-21(8-12-25)29(33)34/h5-12,15-17,19,22H,1-4,13-14,18H2,(H3,31,32)(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50444518

(CHEMBL3099587)Show SMILES N[C@H]1CC[C@@H](CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)nc(Oc2ccc(cc2)C(N)=N)c1 |r,wU:4.7,wD:1.0,(26.14,-7.69,;26.15,-9.23,;24.81,-9.99,;24.81,-11.54,;26.14,-12.31,;27.48,-11.54,;27.47,-9.99,;26.15,-13.85,;27.48,-14.62,;28.81,-13.85,;27.48,-16.16,;28.82,-16.93,;28.82,-18.47,;30.16,-19.26,;31.5,-18.49,;31.51,-16.95,;32.84,-16.18,;34.19,-16.96,;34.17,-18.5,;32.83,-19.26,;35.55,-16.2,;35.59,-14.65,;36.88,-17,;27.48,-19.24,;26.15,-18.47,;24.81,-19.25,;24.82,-20.8,;26.15,-21.56,;26.15,-23.11,;24.82,-23.88,;23.49,-23.11,;23.49,-21.57,;24.81,-25.41,;23.47,-26.17,;26.13,-26.2,;26.16,-16.92,)| Show InChI InChI=1S/C26H29N7O3/c27-18-5-7-19(8-6-18)32-26(34)17-13-22(35-20-9-1-15(2-10-20)24(28)29)33-23(14-17)36-21-11-3-16(4-12-21)25(30)31/h1-4,9-14,18-19H,5-8,27H2,(H3,28,29)(H3,30,31)(H,32,34)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a (unknown origin) using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrate |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066618

(4-{6-[4-amino(imino)methylphenoxy]-2-pyridyloxy}ph...)Show SMILES NC(=N)c1ccc(Oc2cccc(Oc3ccc(cc3)C(N)=N)n2)cc1 Show InChI InChI=1S/C19H17N5O2/c20-18(21)12-4-8-14(9-5-12)25-16-2-1-3-17(24-16)26-15-10-6-13(7-11-15)19(22)23/h1-11H,(H3,20,21)(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a (unknown origin) using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH as substrate |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Serine protease hepsin
(Homo sapiens (Human)) | BDBM50130871
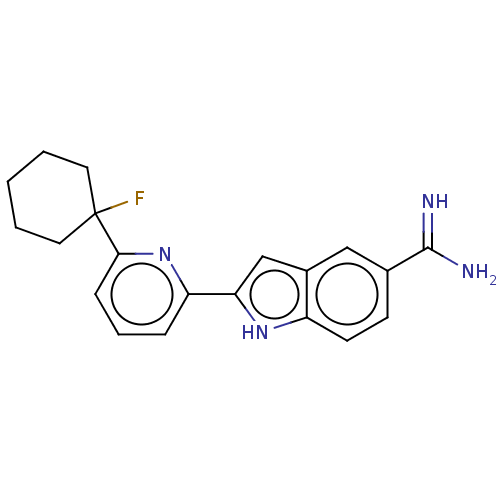
(CHEMBL3632760)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(n1)C1(F)CCCCC1 Show InChI InChI=1S/C20H21FN4/c21-20(9-2-1-3-10-20)18-6-4-5-16(25-18)17-12-14-11-13(19(22)23)7-8-15(14)24-17/h4-8,11-12,24H,1-3,9-10H2,(H3,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant hepsin by fluorescence based assay using 65 uM BOC-Gln-Arg-Arg -AMC as substrate |
Bioorg Med Chem Lett 25: 5309-14 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.042
BindingDB Entry DOI: 10.7270/Q24B335X |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50444522

(CHEMBL3099583)Show SMILES NCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=N)nc1Oc1ccc(cc1)C(N)=N Show InChI InChI=1S/C23H25N7O3/c24-12-1-13-29-22(31)18-10-11-19(32-16-6-2-14(3-7-16)20(25)26)30-23(18)33-17-8-4-15(5-9-17)21(27)28/h2-11H,1,12-13,24H2,(H3,25,26)(H3,27,28)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of matriptase-SP1 (615 to 855) (unknown origin) expressed in Escherichia coli BL21(DE3) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hy... |
ACS Med Chem Lett 4: 1152-7 (2013)
Article DOI: 10.1021/ml400213v
BindingDB Entry DOI: 10.7270/Q29S1SHZ |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50130870

(CHEMBL3632759)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(n1)C1(O)CCCCC1 Show InChI InChI=1S/C20H22N4O/c21-19(22)13-7-8-15-14(11-13)12-17(23-15)16-5-4-6-18(24-16)20(25)9-2-1-3-10-20/h4-8,11-12,23,25H,1-3,9-10H2,(H3,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of human trypsin by fluorescence based assay using 25 uM Boc-Gln-Ala-Arg-7-amido-4-methyl coumarinhydrobromide as substrate |
Bioorg Med Chem Lett 25: 5309-14 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.042
BindingDB Entry DOI: 10.7270/Q24B335X |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017900

(CHEMBL3289018)Show SMILES NC(=N)c1ccc(Oc2cc(Oc3ccc(cc3)C(N)=N)cc(c2)C(=O)NCC2CCCCC2)cc1 Show InChI InChI=1S/C28H31N5O3/c29-26(30)19-6-10-22(11-7-19)35-24-14-21(28(34)33-17-18-4-2-1-3-5-18)15-25(16-24)36-23-12-8-20(9-13-23)27(31)32/h6-16,18H,1-5,17H2,(H3,29,30)(H3,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 411 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50017936

(CHEMBL3289022)Show SMILES NCCCN1CCC(CC1)NC(=O)c1cc(Oc2ccc(cc2)C(N)=N)cc(Oc2ccc(cc2)C(N)=N)c1 Show InChI InChI=1S/C29H35N7O3/c30-12-1-13-36-14-10-22(11-15-36)35-29(37)21-16-25(38-23-6-2-19(3-7-23)27(31)32)18-26(17-21)39-24-8-4-20(5-9-24)28(33)34/h2-9,16-18,22H,1,10-15,30H2,(H3,31,32)(H3,33,34)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of bovine plasma factor 10a using CH3OCO-D-CHA-Gly-Arg-pNA.AcoH substrate |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data