 Found 242 hits with Last Name = 'monjas' and Initial = 'l'
Found 242 hits with Last Name = 'monjas' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380548
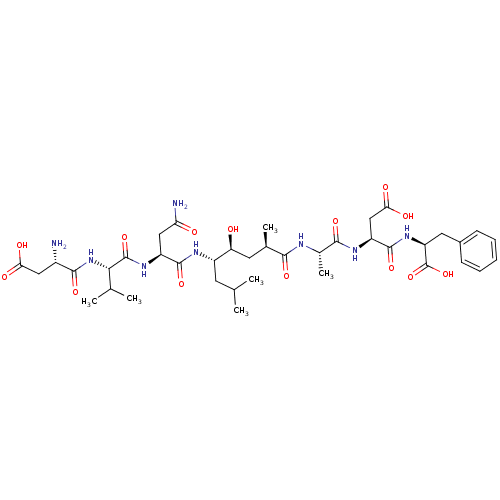
(CHEMBL2019055)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H60N8O14/c1-18(2)12-24(43-36(57)25(16-29(41)49)45-38(59)32(19(3)4)47-35(56)23(40)15-30(50)51)28(48)13-20(5)33(54)42-21(6)34(55)44-26(17-31(52)53)37(58)46-27(39(60)61)14-22-10-8-7-9-11-22/h7-11,18-21,23-28,32,48H,12-17,40H2,1-6H3,(H2,41,49)(H,42,54)(H,43,57)(H,44,55)(H,45,59)(H,46,58)(H,47,56)(H,50,51)(H,52,53)(H,60,61)/t20-,21+,23+,24+,25+,26+,27+,28+,32+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50401238
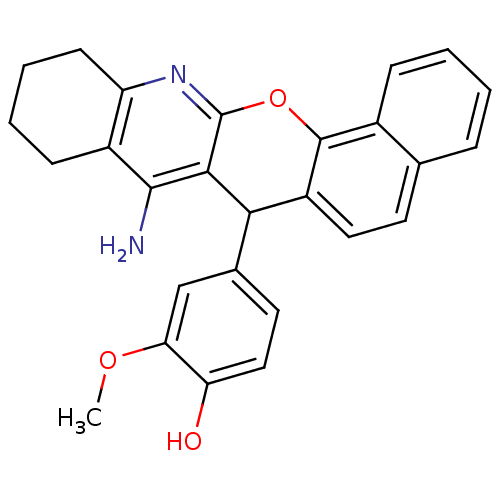
(CHEMBL2206895)Show SMILES COc1cc(ccc1O)C1c2ccc3ccccc3c2Oc2nc3CCCCc3c(N)c12 Show InChI InChI=1S/C27H24N2O3/c1-31-22-14-16(11-13-21(22)30)23-19-12-10-15-6-2-3-7-17(15)26(19)32-27-24(23)25(28)18-8-4-5-9-20(18)29-27/h2-3,6-7,10-14,23,30H,4-5,8-9H2,1H3,(H2,28,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 54: 750-63 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.038
BindingDB Entry DOI: 10.7270/Q2R78GCM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380542
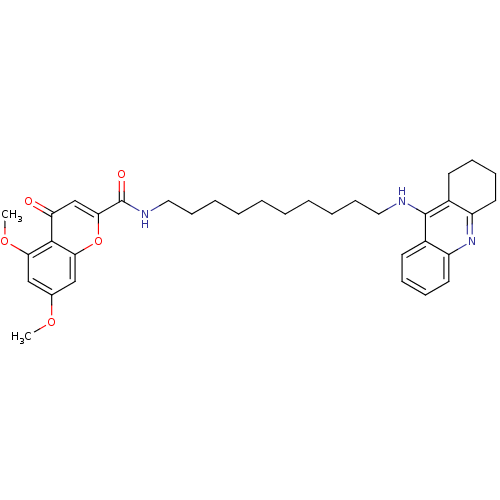
(CHEMBL2019037)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-24-21-30(42-2)33-29(39)23-32(43-31(33)22-24)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-25-15-9-11-17-27(25)38-28-18-12-10-16-26(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380544
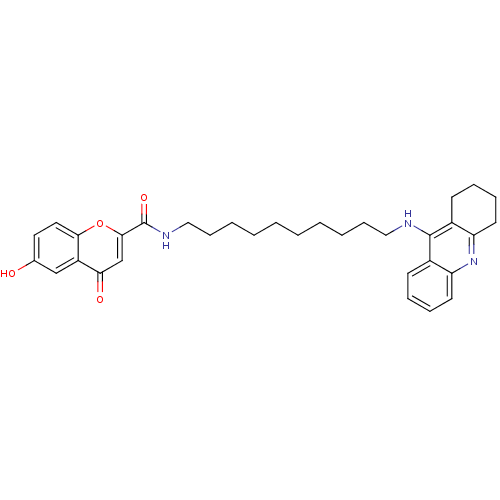
(CHEMBL2019043)Show SMILES Oc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O4/c37-23-17-18-30-26(21-23)29(38)22-31(40-30)33(39)35-20-12-6-4-2-1-3-5-11-19-34-32-24-13-7-9-15-27(24)36-28-16-10-8-14-25(28)32/h7,9,13,15,17-18,21-22,37H,1-6,8,10-12,14,16,19-20H2,(H,34,36)(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380545
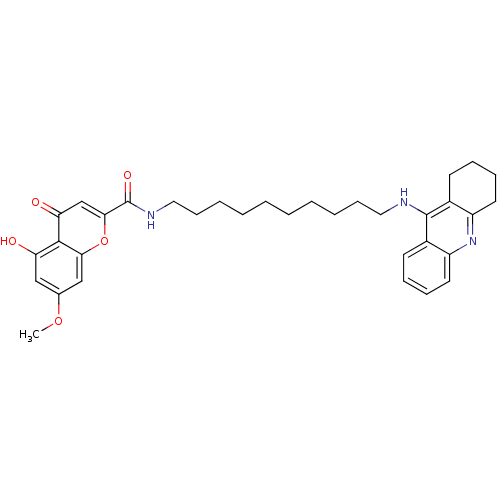
(CHEMBL2019046)Show SMILES COc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O5/c1-41-23-20-28(38)32-29(39)22-31(42-30(32)21-23)34(40)36-19-13-7-5-3-2-4-6-12-18-35-33-24-14-8-10-16-26(24)37-27-17-11-9-15-25(27)33/h8,10,14,16,20-22,38H,2-7,9,11-13,15,17-19H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380543
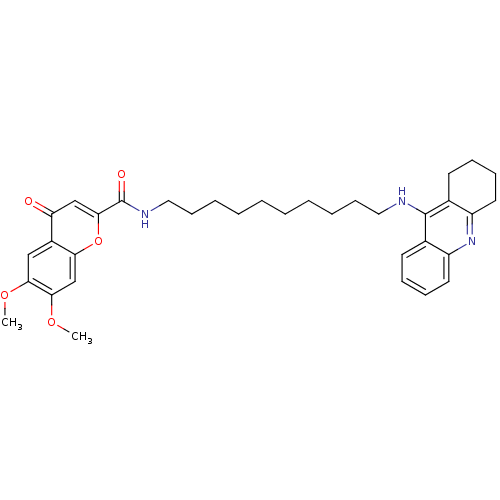
(CHEMBL2019040)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-31-21-26-29(39)22-33(43-30(26)23-32(31)42-2)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-24-15-9-11-17-27(24)38-28-18-12-10-16-25(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380546
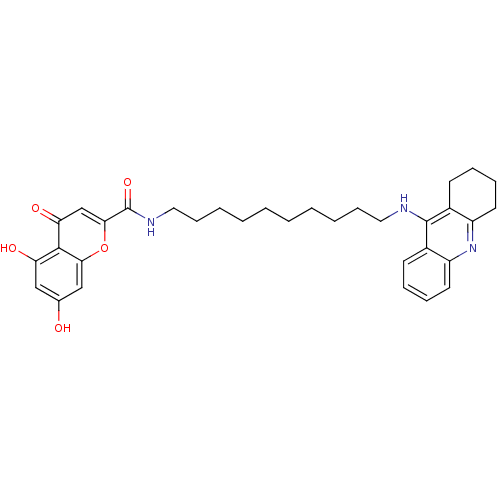
(CHEMBL2019047)Show SMILES Oc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O5/c37-22-19-27(38)31-28(39)21-30(41-29(31)20-22)33(40)35-18-12-6-4-2-1-3-5-11-17-34-32-23-13-7-9-15-25(23)36-26-16-10-8-14-24(26)32/h7,9,13,15,19-21,37-38H,1-6,8,10-12,14,16-18H2,(H,34,36)(H,35,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380547
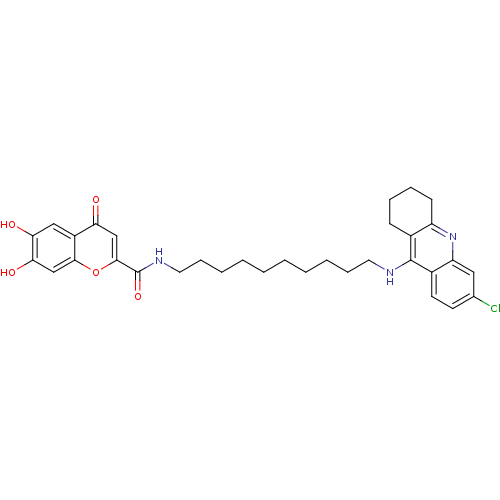
(CHEMBL2019053)Show SMILES Oc1cc2oc(cc(=O)c2cc1O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H38ClN3O5/c34-21-13-14-23-26(17-21)37-25-12-8-7-11-22(25)32(23)35-15-9-5-3-1-2-4-6-10-16-36-33(41)31-19-27(38)24-18-28(39)29(40)20-30(24)42-31/h13-14,17-20,39-40H,1-12,15-16H2,(H,35,37)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380552
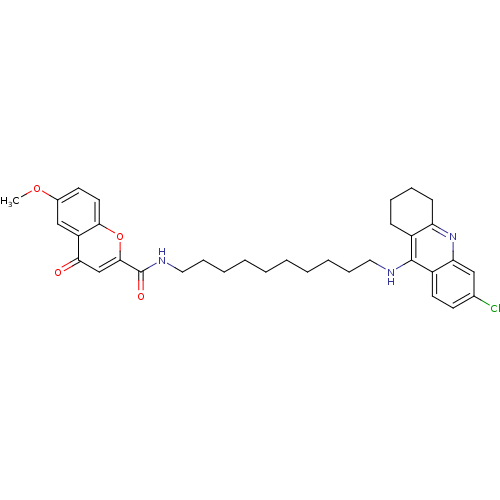
(CHEMBL2019035)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O4/c1-41-24-15-17-31-27(21-24)30(39)22-32(42-31)34(40)37-19-11-7-5-3-2-4-6-10-18-36-33-25-12-8-9-13-28(25)38-29-20-23(35)14-16-26(29)33/h14-17,20-22H,2-13,18-19H2,1H3,(H,36,38)(H,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50380541
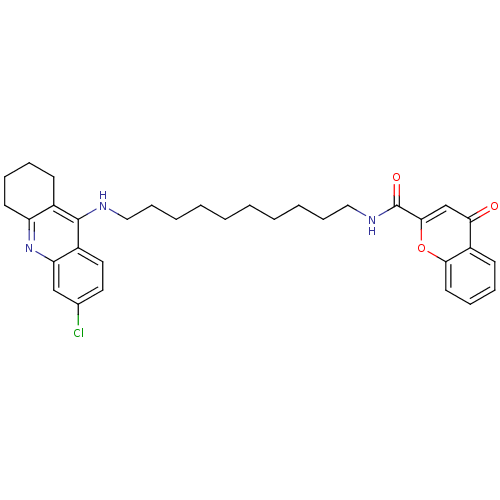
(CHEMBL2019032)Show SMILES Clc1ccc2c(NCCCCCCCCCCNC(=O)c3cc(=O)c4ccccc4o3)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38ClN3O3/c34-23-17-18-25-28(21-23)37-27-15-9-7-13-24(27)32(25)35-19-11-5-3-1-2-4-6-12-20-36-33(39)31-22-29(38)26-14-8-10-16-30(26)40-31/h8,10,14,16-18,21-22H,1-7,9,11-13,15,19-20H2,(H,35,37)(H,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assay |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380559

(CHEMBL2019048)Show SMILES COc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O5/c1-42-23-19-28(39)32-29(40)21-31(43-30(32)20-23)34(41)37-17-11-7-5-3-2-4-6-10-16-36-33-24-12-8-9-13-26(24)38-27-18-22(35)14-15-25(27)33/h14-15,18-21,39H,2-13,16-17H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380553
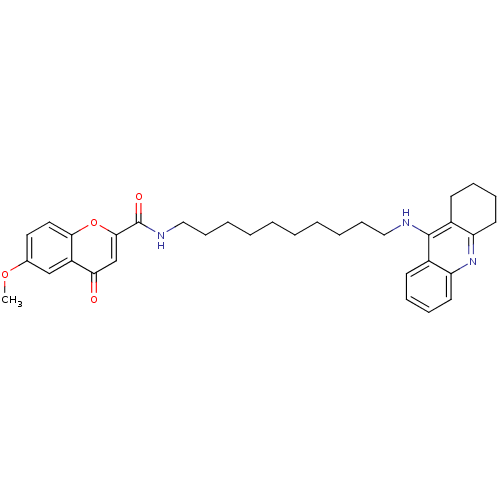
(CHEMBL2019034)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O4/c1-40-24-18-19-31-27(22-24)30(38)23-32(41-31)34(39)36-21-13-7-5-3-2-4-6-12-20-35-33-25-14-8-10-16-28(25)37-29-17-11-9-15-26(29)33/h8,10,14,16,18-19,22-23H,2-7,9,11-13,15,17,20-21H2,1H3,(H,35,37)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380558
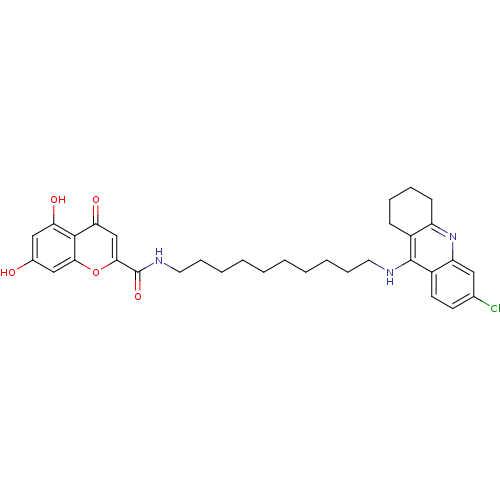
(CHEMBL2019049)Show SMILES Oc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H38ClN3O5/c34-21-13-14-24-26(17-21)37-25-12-8-7-11-23(25)32(24)35-15-9-5-3-1-2-4-6-10-16-36-33(41)30-20-28(40)31-27(39)18-22(38)19-29(31)42-30/h13-14,17-20,38-39H,1-12,15-16H2,(H,35,37)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380541

(CHEMBL2019032)Show SMILES Clc1ccc2c(NCCCCCCCCCCNC(=O)c3cc(=O)c4ccccc4o3)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38ClN3O3/c34-23-17-18-25-28(21-23)37-27-15-9-7-13-24(27)32(25)35-19-11-5-3-1-2-4-6-12-20-36-33(39)31-22-29(38)26-14-8-10-16-30(26)40-31/h8,10,14,16-18,21-22H,1-7,9,11-13,15,19-20H2,(H,35,37)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380547

(CHEMBL2019053)Show SMILES Oc1cc2oc(cc(=O)c2cc1O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H38ClN3O5/c34-21-13-14-23-26(17-21)37-25-12-8-7-11-22(25)32(23)35-15-9-5-3-1-2-4-6-10-16-36-33(41)31-19-27(38)24-18-28(39)29(40)20-30(24)42-31/h13-14,17-20,39-40H,1-12,15-16H2,(H,35,37)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380552

(CHEMBL2019035)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O4/c1-41-24-15-17-31-27(21-24)30(39)22-32(42-31)34(40)37-19-11-7-5-3-2-4-6-10-18-36-33-25-12-8-9-13-28(25)38-29-20-23(35)14-16-26(29)33/h14-17,20-22H,2-13,18-19H2,1H3,(H,36,38)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380551
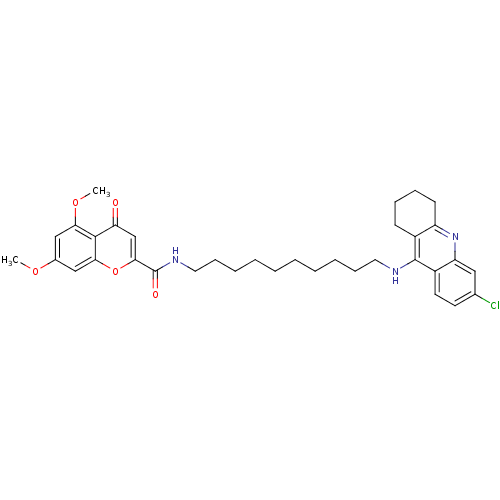
(CHEMBL2019038)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-24-20-30(43-2)33-29(40)22-32(44-31(33)21-24)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-25-13-9-10-14-27(25)39-28-19-23(36)15-16-26(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380552

(CHEMBL2019035)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O4/c1-41-24-15-17-31-27(21-24)30(39)22-32(42-31)34(40)37-19-11-7-5-3-2-4-6-10-18-36-33-25-12-8-9-13-28(25)38-29-20-23(35)14-16-26(29)33/h14-17,20-22H,2-13,18-19H2,1H3,(H,36,38)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380542

(CHEMBL2019037)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-24-21-30(42-2)33-29(39)23-32(43-31(33)22-24)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-25-15-9-11-17-27(25)38-28-18-12-10-16-26(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380537
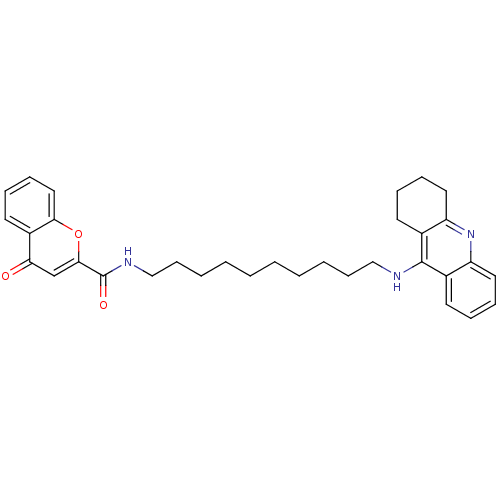
(CHEMBL2019030)Show SMILES O=C(NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C33H39N3O3/c37-29-23-31(39-30-20-12-9-17-26(29)30)33(38)35-22-14-6-4-2-1-3-5-13-21-34-32-24-15-7-10-18-27(24)36-28-19-11-8-16-25(28)32/h7,9-10,12,15,17-18,20,23H,1-6,8,11,13-14,16,19,21-22H2,(H,34,36)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380559

(CHEMBL2019048)Show SMILES COc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O5/c1-42-23-19-28(39)32-29(40)21-31(43-30(32)20-23)34(41)37-17-11-7-5-3-2-4-6-10-16-36-33-24-12-8-9-13-26(24)38-27-18-22(35)14-15-25(27)33/h14-15,18-21,39H,2-13,16-17H2,1H3,(H,36,38)(H,37,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380541

(CHEMBL2019032)Show SMILES Clc1ccc2c(NCCCCCCCCCCNC(=O)c3cc(=O)c4ccccc4o3)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38ClN3O3/c34-23-17-18-25-28(21-23)37-27-15-9-7-13-24(27)32(25)35-19-11-5-3-1-2-4-6-12-20-36-33(39)31-22-29(38)26-14-8-10-16-30(26)40-31/h8,10,14,16-18,21-22H,1-7,9,11-13,15,19-20H2,(H,35,37)(H,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380537

(CHEMBL2019030)Show SMILES O=C(NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C33H39N3O3/c37-29-23-31(39-30-20-12-9-17-26(29)30)33(38)35-22-14-6-4-2-1-3-5-13-21-34-32-24-15-7-10-18-27(24)36-28-19-11-8-16-25(28)32/h7,9-10,12,15,17-18,20,23H,1-6,8,11,13-14,16,19,21-22H2,(H,34,36)(H,35,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380550
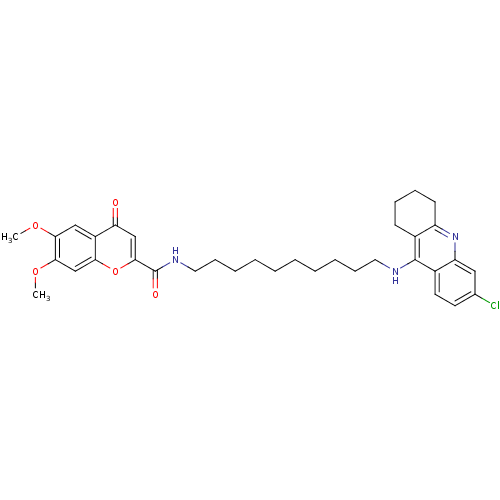
(CHEMBL2019041)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-31-20-26-29(40)21-33(44-30(26)22-32(31)43-2)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-24-13-9-10-14-27(24)39-28-19-23(36)15-16-25(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380536
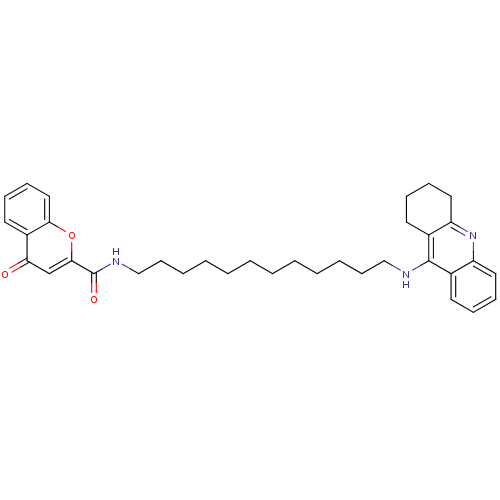
(CHEMBL2019031)Show SMILES O=C(NCCCCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C35H43N3O3/c39-31-25-33(41-32-22-14-11-19-28(31)32)35(40)37-24-16-8-6-4-2-1-3-5-7-15-23-36-34-26-17-9-12-20-29(26)38-30-21-13-10-18-27(30)34/h9,11-12,14,17,19-20,22,25H,1-8,10,13,15-16,18,21,23-24H2,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380541

(CHEMBL2019032)Show SMILES Clc1ccc2c(NCCCCCCCCCCNC(=O)c3cc(=O)c4ccccc4o3)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38ClN3O3/c34-23-17-18-25-28(21-23)37-27-15-9-7-13-24(27)32(25)35-19-11-5-3-1-2-4-6-12-20-36-33(39)31-22-29(38)26-14-8-10-16-30(26)40-31/h8,10,14,16-18,21-22H,1-7,9,11-13,15,19-20H2,(H,35,37)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380553

(CHEMBL2019034)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O4/c1-40-24-18-19-31-27(22-24)30(38)23-32(41-31)34(39)36-21-13-7-5-3-2-4-6-12-20-35-33-25-14-8-10-16-28(25)37-29-17-11-9-15-26(29)33/h8,10,14,16,18-19,22-23H,2-7,9,11-13,15,17,20-21H2,1H3,(H,35,37)(H,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380542

(CHEMBL2019037)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-24-21-30(42-2)33-29(39)23-32(43-31(33)22-24)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-25-15-9-11-17-27(25)38-28-18-12-10-16-26(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380546

(CHEMBL2019047)Show SMILES Oc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O5/c37-22-19-27(38)31-28(39)21-30(41-29(31)20-22)33(40)35-18-12-6-4-2-1-3-5-11-17-34-32-23-13-7-9-15-25(23)36-26-16-10-8-14-24(26)32/h7,9,13,15,19-21,37-38H,1-6,8,10-12,14,16-18H2,(H,34,36)(H,35,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380551

(CHEMBL2019038)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-24-20-30(43-2)33-29(40)22-32(44-31(33)21-24)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-25-13-9-10-14-27(25)39-28-19-23(36)15-16-26(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380552

(CHEMBL2019035)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O4/c1-41-24-15-17-31-27(21-24)30(39)22-32(42-31)34(40)37-19-11-7-5-3-2-4-6-10-18-36-33-25-12-8-9-13-28(25)38-29-20-23(35)14-16-26(29)33/h14-17,20-22H,2-13,18-19H2,1H3,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380539
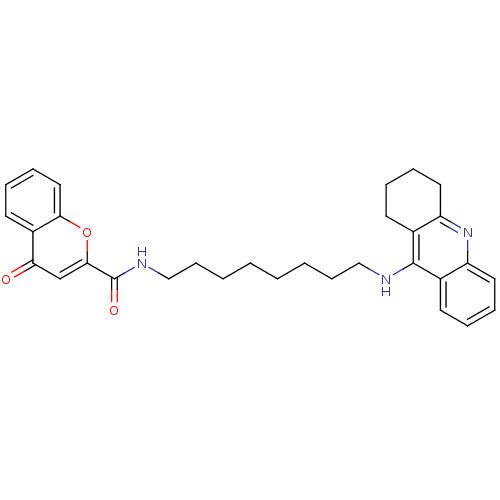
(CHEMBL2019028)Show SMILES O=C(NCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C31H35N3O3/c35-27-21-29(37-28-18-10-7-15-24(27)28)31(36)33-20-12-4-2-1-3-11-19-32-30-22-13-5-8-16-25(22)34-26-17-9-6-14-23(26)30/h5,7-8,10,13,15-16,18,21H,1-4,6,9,11-12,14,17,19-20H2,(H,32,34)(H,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380543

(CHEMBL2019040)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-31-21-26-29(39)22-33(43-30(26)23-32(31)42-2)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-24-15-9-11-17-27(24)38-28-18-12-10-16-25(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380538
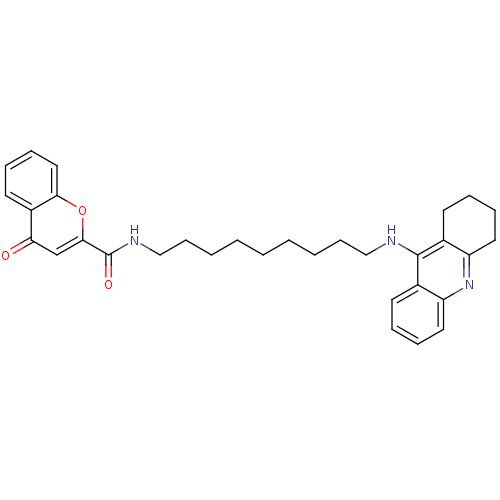
(CHEMBL2019029)Show SMILES O=C(NCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C32H37N3O3/c36-28-22-30(38-29-19-11-8-16-25(28)29)32(37)34-21-13-5-3-1-2-4-12-20-33-31-23-14-6-9-17-26(23)35-27-18-10-7-15-24(27)31/h6,8-9,11,14,16-17,19,22H,1-5,7,10,12-13,15,18,20-21H2,(H,33,35)(H,34,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380562
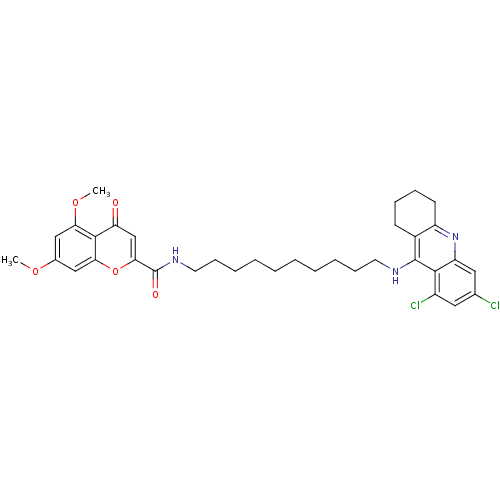
(CHEMBL2019039)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)cc(Cl)c12 Show InChI InChI=1S/C35H41Cl2N3O5/c1-43-23-19-29(44-2)33-28(41)21-31(45-30(33)20-23)35(42)39-16-12-8-6-4-3-5-7-11-15-38-34-24-13-9-10-14-26(24)40-27-18-22(36)17-25(37)32(27)34/h17-21H,3-16H2,1-2H3,(H,38,40)(H,39,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380553

(CHEMBL2019034)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O4/c1-40-24-18-19-31-27(22-24)30(38)23-32(41-31)34(39)36-21-13-7-5-3-2-4-6-12-20-35-33-25-14-8-10-16-28(25)37-29-17-11-9-15-26(29)33/h8,10,14,16,18-19,22-23H,2-7,9,11-13,15,17,20-21H2,1H3,(H,35,37)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380546

(CHEMBL2019047)Show SMILES Oc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O5/c37-22-19-27(38)31-28(39)21-30(41-29(31)20-22)33(40)35-18-12-6-4-2-1-3-5-11-17-34-32-23-13-7-9-15-25(23)36-26-16-10-8-14-24(26)32/h7,9,13,15,19-21,37-38H,1-6,8,10-12,14,16-18H2,(H,34,36)(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380550

(CHEMBL2019041)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-31-20-26-29(40)21-33(44-30(26)22-32(31)43-2)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-24-13-9-10-14-27(24)39-28-19-23(36)15-16-25(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380549

(CHEMBL2019044)Show SMILES Oc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H38ClN3O4/c34-22-13-15-25-28(19-22)37-27-12-8-7-11-24(27)32(25)35-17-9-5-3-1-2-4-6-10-18-36-33(40)31-21-29(39)26-20-23(38)14-16-30(26)41-31/h13-16,19-21,38H,1-12,17-18H2,(H,35,37)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380545

(CHEMBL2019046)Show SMILES COc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O5/c1-41-23-20-28(38)32-29(39)22-31(42-30(32)21-23)34(40)36-19-13-7-5-3-2-4-6-12-18-35-33-24-14-8-10-16-26(24)37-27-17-11-9-15-25(27)33/h8,10,14,16,20-22,38H,2-7,9,11-13,15,17-19H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380545

(CHEMBL2019046)Show SMILES COc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O5/c1-41-23-20-28(38)32-29(39)22-31(42-30(32)21-23)34(40)36-19-13-7-5-3-2-4-6-12-18-35-33-24-14-8-10-16-26(24)37-27-17-11-9-15-25(27)33/h8,10,14,16,20-22,38H,2-7,9,11-13,15,17-19H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380544

(CHEMBL2019043)Show SMILES Oc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O4/c37-23-17-18-30-26(21-23)29(38)22-31(40-30)33(39)35-20-12-6-4-2-1-3-5-11-19-34-32-24-13-7-9-15-27(24)36-28-16-10-8-14-25(28)32/h7,9,13,15,17-18,21-22,37H,1-6,8,10-12,14,16,19-20H2,(H,34,36)(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380551

(CHEMBL2019038)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-24-20-30(43-2)33-29(40)22-32(44-31(33)21-24)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-25-13-9-10-14-27(25)39-28-19-23(36)15-16-26(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380544

(CHEMBL2019043)Show SMILES Oc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H39N3O4/c37-23-17-18-30-26(21-23)29(38)22-31(40-30)33(39)35-20-12-6-4-2-1-3-5-11-19-34-32-24-13-7-9-15-27(24)36-28-16-10-8-14-25(28)32/h7,9,13,15,17-18,21-22,37H,1-6,8,10-12,14,16,19-20H2,(H,34,36)(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380549

(CHEMBL2019044)Show SMILES Oc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H38ClN3O4/c34-22-13-15-25-28(19-22)37-27-12-8-7-11-24(27)32(25)35-17-9-5-3-1-2-4-6-10-18-36-33(40)31-21-29(39)26-20-23(38)14-16-30(26)41-31/h13-16,19-21,38H,1-12,17-18H2,(H,35,37)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380540

(CHEMBL2019027)Show SMILES O=C(NCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C30H33N3O3/c34-26-20-28(36-27-17-9-6-14-23(26)27)30(35)32-19-11-3-1-2-10-18-31-29-21-12-4-7-15-24(21)33-25-16-8-5-13-22(25)29/h4,6-7,9,12,14-15,17,20H,1-3,5,8,10-11,13,16,18-19H2,(H,31,33)(H,32,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380543

(CHEMBL2019040)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-31-21-26-29(39)22-33(43-30(26)23-32(31)42-2)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-24-15-9-11-17-27(24)38-28-18-12-10-16-25(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50380545

(CHEMBL2019046)Show SMILES COc1cc(O)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O5/c1-41-23-20-28(38)32-29(39)22-31(42-30(32)21-23)34(40)36-19-13-7-5-3-2-4-6-12-18-35-33-24-14-8-10-16-26(24)37-27-17-11-9-15-25(27)33/h8,10,14,16,20-22,38H,2-7,9,11-13,15,17-19H2,1H3,(H,35,37)(H,36,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50380542

(CHEMBL2019037)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-24-21-30(42-2)33-29(39)23-32(43-31(33)22-24)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-25-15-9-11-17-27(25)38-28-18-12-10-16-26(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data