 Found 212 hits with Last Name = 'moore' and Initial = 'de'
Found 212 hits with Last Name = 'moore' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086246
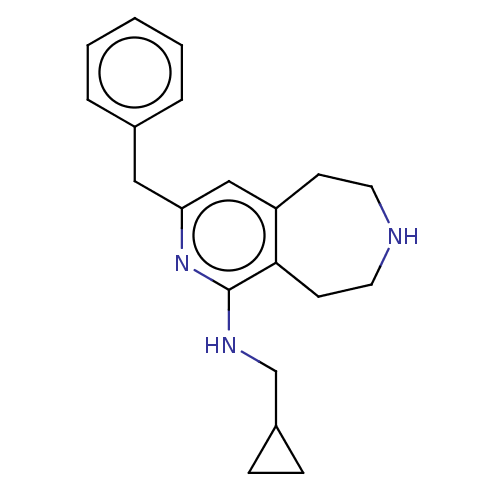
(CHEMBL3425758)Show InChI InChI=1S/C20H25N3/c1-2-4-15(5-3-1)12-18-13-17-8-10-21-11-9-19(17)20(23-18)22-14-16-6-7-16/h1-5,13,16,21H,6-12,14H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086249
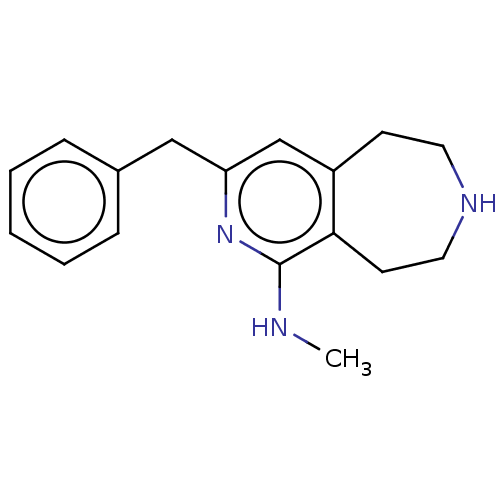
(CHEMBL3425755)Show InChI InChI=1S/C17H21N3/c1-18-17-16-8-10-19-9-7-14(16)12-15(20-17)11-13-5-3-2-4-6-13/h2-6,12,19H,7-11H2,1H3,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086248
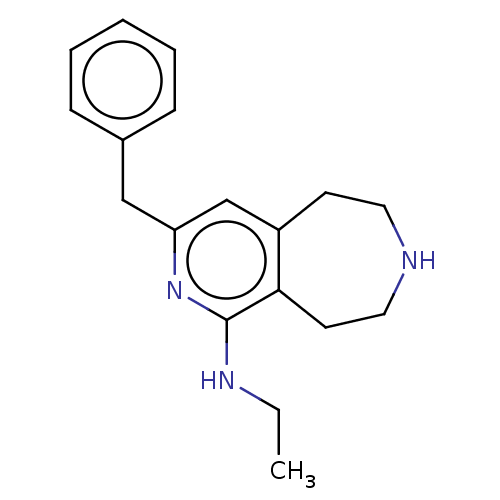
(CHEMBL3425756)Show InChI InChI=1S/C18H23N3/c1-2-20-18-17-9-11-19-10-8-15(17)13-16(21-18)12-14-6-4-3-5-7-14/h3-7,13,19H,2,8-12H2,1H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086250

(CHEMBL3425759)Show InChI InChI=1S/C19H25N3/c1-2-10-21-19-18-9-12-20-11-8-16(18)14-17(22-19)13-15-6-4-3-5-7-15/h3-7,14,20H,2,8-13H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086252
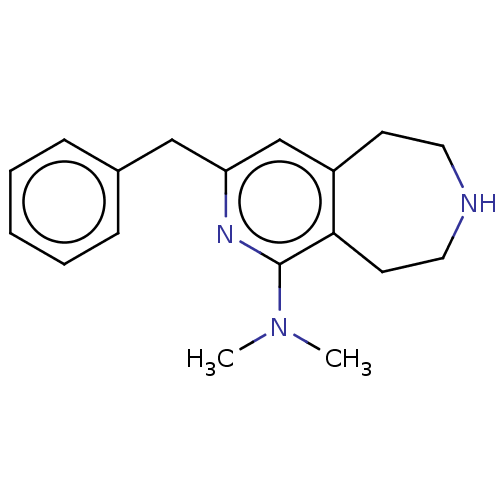
(CHEMBL3425762)Show InChI InChI=1S/C18H23N3/c1-21(2)18-17-9-11-19-10-8-15(17)13-16(20-18)12-14-6-4-3-5-7-14/h3-7,13,19H,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086247
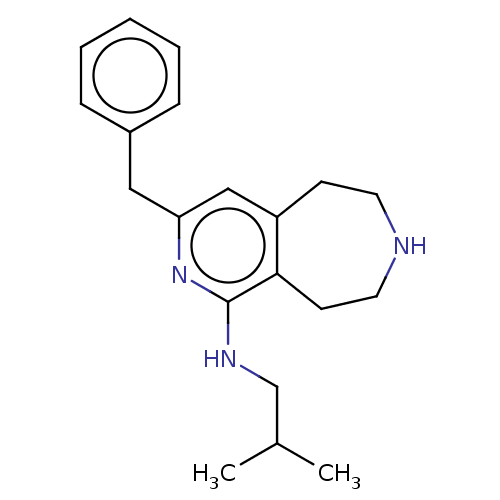
(CHEMBL3425757)Show InChI InChI=1S/C20H27N3/c1-15(2)14-22-20-19-9-11-21-10-8-17(19)13-18(23-20)12-16-6-4-3-5-7-16/h3-7,13,15,21H,8-12,14H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086251
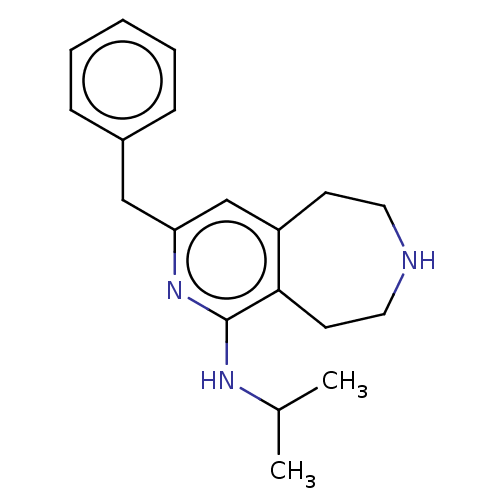
(CHEMBL3425760)Show InChI InChI=1S/C19H25N3/c1-14(2)21-19-18-9-11-20-10-8-16(18)13-17(22-19)12-15-6-4-3-5-7-15/h3-7,13-14,20H,8-12H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086245
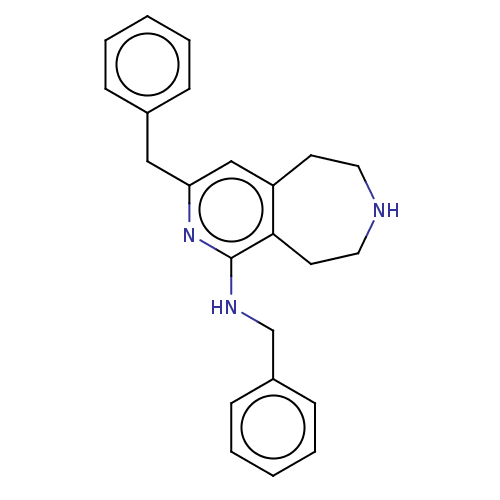
(CHEMBL3425761)Show InChI InChI=1S/C23H25N3/c1-3-7-18(8-4-1)15-21-16-20-11-13-24-14-12-22(20)23(26-21)25-17-19-9-5-2-6-10-19/h1-10,16,24H,11-15,17H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50341309
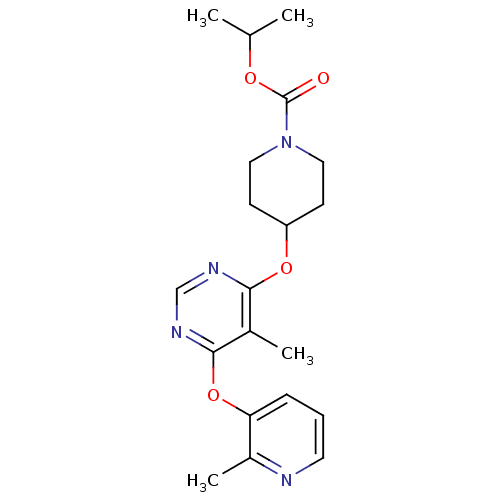
(CHEMBL1766081 | isopropyl 4-(5-methyl-6-(2-methylp...)Show SMILES CC(C)OC(=O)N1CCC(CC1)Oc1ncnc(Oc2cccnc2C)c1C Show InChI InChI=1S/C20H26N4O4/c1-13(2)26-20(25)24-10-7-16(8-11-24)27-18-14(3)19(23-12-22-18)28-17-6-5-9-21-15(17)4/h5-6,9,12-13,16H,7-8,10-11H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50341310

(CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...)Show SMILES CC(C)OC(=O)N1CC2COCC(C1)C2Oc1ncnc(Oc2cccnc2C)c1C |TLB:15:14:9.10.11:13.6.7| Show InChI InChI=1S/C22H28N4O5/c1-13(2)29-22(27)26-8-16-10-28-11-17(9-26)19(16)31-21-14(3)20(24-12-25-21)30-18-6-5-7-23-15(18)4/h5-7,12-13,16-17,19H,8-11H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50341310

(CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...)Show SMILES CC(C)OC(=O)N1CC2COCC(C1)C2Oc1ncnc(Oc2cccnc2C)c1C |TLB:15:14:9.10.11:13.6.7| Show InChI InChI=1S/C22H28N4O5/c1-13(2)29-22(27)26-8-16-10-28-11-17(9-26)19(16)31-21-14(3)20(24-12-25-21)30-18-6-5-7-23-15(18)4/h5-7,12-13,16-17,19H,8-11H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50086253
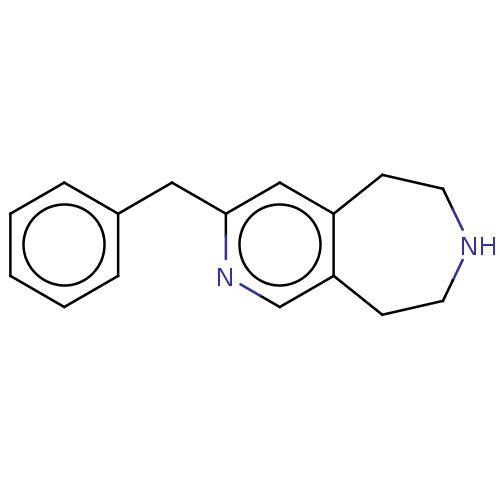
(CHEMBL3425763)Show InChI InChI=1S/C16H18N2/c1-2-4-13(5-3-1)10-16-11-14-6-8-17-9-7-15(14)12-18-16/h1-5,11-12,17H,6-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi... |
ACS Med Chem Lett 6: 329-33 (2015)
Article DOI: 10.1021/ml500507v
BindingDB Entry DOI: 10.7270/Q29K4CZW |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Rattus norvegicus) | BDBM50341310

(CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...)Show SMILES CC(C)OC(=O)N1CC2COCC(C1)C2Oc1ncnc(Oc2cccnc2C)c1C |TLB:15:14:9.10.11:13.6.7| Show InChI InChI=1S/C22H28N4O5/c1-13(2)29-22(27)26-8-16-10-28-11-17(9-26)19(16)31-21-14(3)20(24-12-25-21)30-18-6-5-7-23-15(18)4/h5-7,12-13,16-17,19H,8-11H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Rattus norvegicus) | BDBM50341310

(CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...)Show SMILES CC(C)OC(=O)N1CC2COCC(C1)C2Oc1ncnc(Oc2cccnc2C)c1C |TLB:15:14:9.10.11:13.6.7| Show InChI InChI=1S/C22H28N4O5/c1-13(2)29-22(27)26-8-16-10-28-11-17(9-26)19(16)31-21-14(3)20(24-12-25-21)30-18-6-5-7-23-15(18)4/h5-7,12-13,16-17,19H,8-11H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Rattus norvegicus) | BDBM50341309

(CHEMBL1766081 | isopropyl 4-(5-methyl-6-(2-methylp...)Show SMILES CC(C)OC(=O)N1CCC(CC1)Oc1ncnc(Oc2cccnc2C)c1C Show InChI InChI=1S/C20H26N4O4/c1-13(2)26-20(25)24-10-7-16(8-11-24)27-18-14(3)19(23-12-22-18)28-17-6-5-9-21-15(17)4/h5-6,9,12-13,16H,7-8,10-11H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50341312
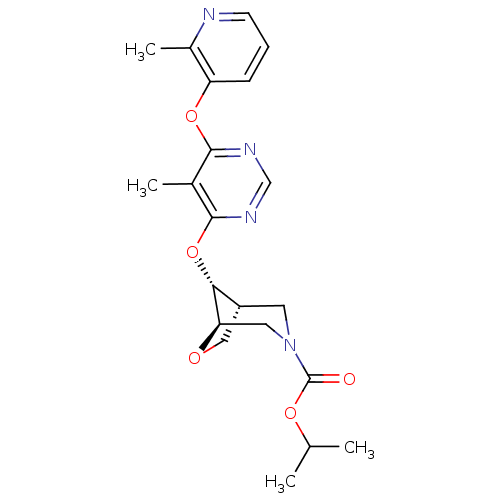
((1R,5R,8R)-Isopropyl 8-(5-Methyl-6-(2-methylpyridi...)Show SMILES CC(C)OC(=O)N1C[C@@H]2CO[C@H](C1)[C@@H]2Oc1ncnc(Oc2cccnc2C)c1C |r| Show InChI InChI=1S/C21H26N4O5/c1-12(2)28-21(26)25-8-15-10-27-17(9-25)18(15)30-20-13(3)19(23-11-24-20)29-16-6-5-7-22-14(16)4/h5-7,11-12,15,17-18H,8-10H2,1-4H3/t15-,17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50341311

((+/-)-isopropyl 8-(5-methyl-6-(2-methylpyridin-3-y...)Show SMILES CC(C)OC(=O)N1CC2COC(C1)C2Oc1ncnc(Oc2cccnc2C)c1C Show InChI InChI=1S/C21H26N4O5/c1-12(2)28-21(26)25-8-15-10-27-17(9-25)18(15)30-20-13(3)19(23-11-24-20)29-16-6-5-7-22-14(16)4/h5-7,11-12,15,17-18H,8-10H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50341313

((1S,5S,8S)-Isopropyl 8-(5-Methyl-6-(2-methylpyridi...)Show SMILES CC(C)OC(=O)N1C[C@H]2CO[C@@H](C1)[C@H]2Oc1ncnc(Oc2cccnc2C)c1C |r| Show InChI InChI=1S/C21H26N4O5/c1-12(2)28-21(26)25-8-15-10-27-17(9-25)18(15)30-20-13(3)19(23-11-24-20)29-16-6-5-7-22-14(16)4/h5-7,11-12,15,17-18H,8-10H2,1-4H3/t15-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... |
J Med Chem 54: 1948-52 (2011)
Article DOI: 10.1021/jm200003p
BindingDB Entry DOI: 10.7270/Q2CZ37G0 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344013
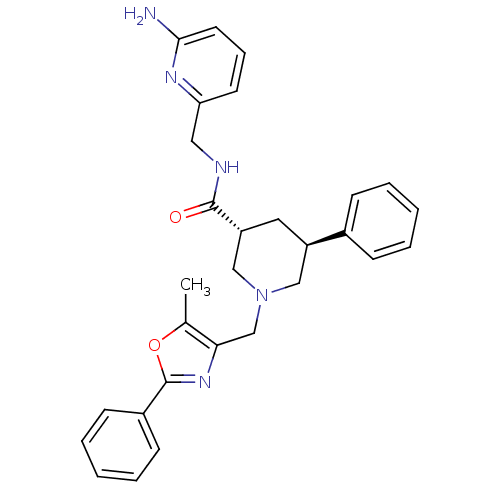
(CHEMBL1780094 | trans-(3R,5S)-N-((6-aminopyridin-2...)Show SMILES Cc1oc(nc1CN1C[C@@H](C[C@H](C1)c1ccccc1)C(=O)NCc1cccc(N)n1)-c1ccccc1 |r| Show InChI InChI=1S/C29H31N5O2/c1-20-26(33-29(36-20)22-11-6-3-7-12-22)19-34-17-23(21-9-4-2-5-10-21)15-24(18-34)28(35)31-16-25-13-8-14-27(30)32-25/h2-14,23-24H,15-19H2,1H3,(H2,30,32)(H,31,35)/t23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344014
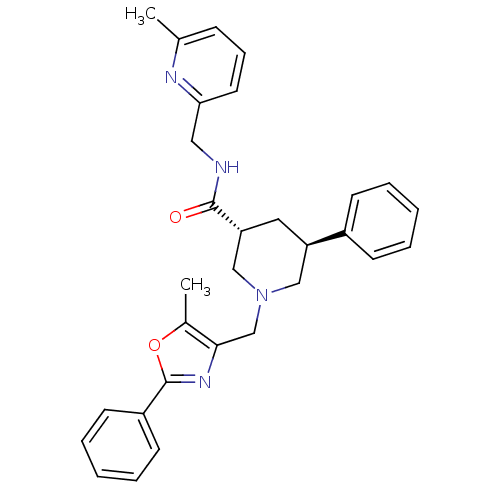
(CHEMBL1779987 | trans-(3R,5S)-1-((5-methyl-2-pheny...)Show SMILES Cc1oc(nc1CN1C[C@@H](C[C@H](C1)c1ccccc1)C(=O)NCc1cccc(C)n1)-c1ccccc1 |r| Show InChI InChI=1S/C30H32N4O2/c1-21-10-9-15-27(32-21)17-31-29(35)26-16-25(23-11-5-3-6-12-23)18-34(19-26)20-28-22(2)36-30(33-28)24-13-7-4-8-14-24/h3-15,25-26H,16-20H2,1-2H3,(H,31,35)/t25-,26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344015
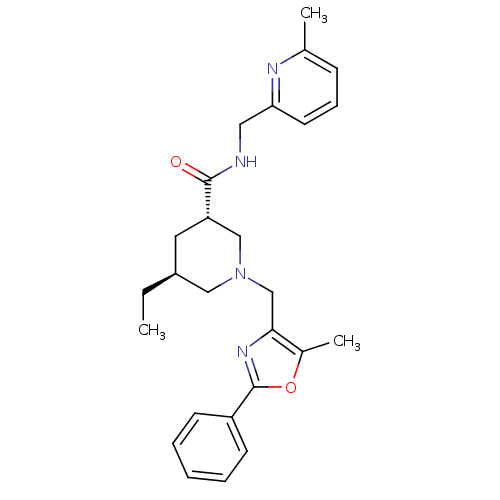
(CHEMBL1780096 | trans-(3S,5S)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@H]1C[C@@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O2/c1-4-20-13-22(25(31)27-14-23-12-8-9-18(2)28-23)16-30(15-20)17-24-19(3)32-26(29-24)21-10-6-5-7-11-21/h5-12,20,22H,4,13-17H2,1-3H3,(H,27,31)/t20-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439646

(CHEMBL2419600 | US8993586, 110)Show SMILES CCCNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C27H34N6O2/c1-5-12-28-22-9-8-18-6-7-19(15-21(18)29-22)25(35)32-13-10-27(11-14-32)16-20-17-33(26(2,3)4)31-23(20)24(34)30-27/h6-9,15,17H,5,10-14,16H2,1-4H3,(H,28,29)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344018
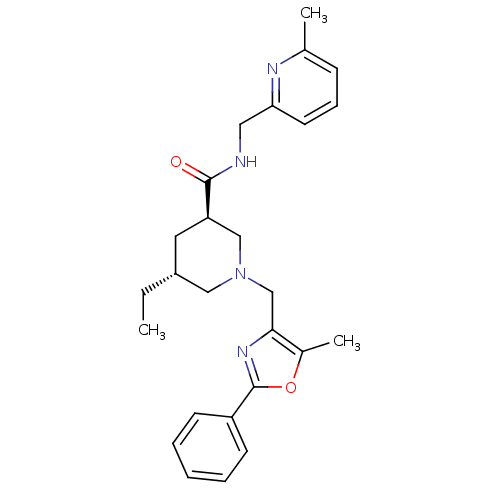
(CHEMBL1779984 | trans-(3R,5R)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O2/c1-4-20-13-22(25(31)27-14-23-12-8-9-18(2)28-23)16-30(15-20)17-24-19(3)32-26(29-24)21-10-6-5-7-11-21/h5-12,20,22H,4,13-17H2,1-3H3,(H,27,31)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344016

(2-((5-methyl-2-phenyloxazol-4-yl)methyl)-N-((6-met...)Show SMILES Cc1oc(nc1CN1CC(C(=O)NCc2cccc(C)n2)c2ccccc2C1)-c1ccccc1 Show InChI InChI=1S/C28H28N4O2/c1-19-9-8-13-23(30-19)15-29-27(33)25-17-32(16-22-12-6-7-14-24(22)25)18-26-20(2)34-28(31-26)21-10-4-3-5-11-21/h3-14,25H,15-18H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50439646

(CHEMBL2419600 | US8993586, 110)Show SMILES CCCNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C27H34N6O2/c1-5-12-28-22-9-8-18-6-7-19(15-21(18)29-22)25(35)32-13-10-27(11-14-32)16-20-17-33(26(2,3)4)31-23(20)24(34)30-27/h6-9,15,17H,5,10-14,16H2,1-4H3,(H,28,29)(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50344017

(1-((5-methyl-2-phenyloxazol-4-yl)methyl)-N-((6-met...)Show SMILES Cc1oc(nc1CN1CCCC(C1)C(=O)NCc1cccc(C)n1)-c1ccccc1 Show InChI InChI=1S/C24H28N4O2/c1-17-8-6-12-21(26-17)14-25-23(29)20-11-7-13-28(15-20)16-22-18(2)30-24(27-22)19-9-4-3-5-10-19/h3-6,8-10,12,20H,7,11,13-16H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344019
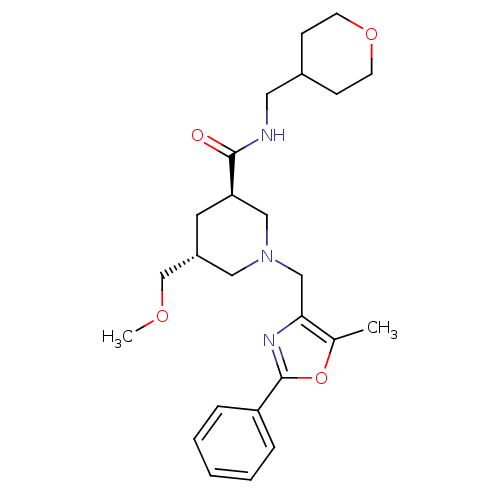
(CHEMBL1780085 | trans-(3R,5R)-5-(methoxymethyl)-1-...)Show SMILES COC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCC1CCOCC1 |r| Show InChI InChI=1S/C25H35N3O4/c1-18-23(27-25(32-18)21-6-4-3-5-7-21)16-28-14-20(17-30-2)12-22(15-28)24(29)26-13-19-8-10-31-11-9-19/h3-7,19-20,22H,8-17H2,1-2H3,(H,26,29)/t20-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439642
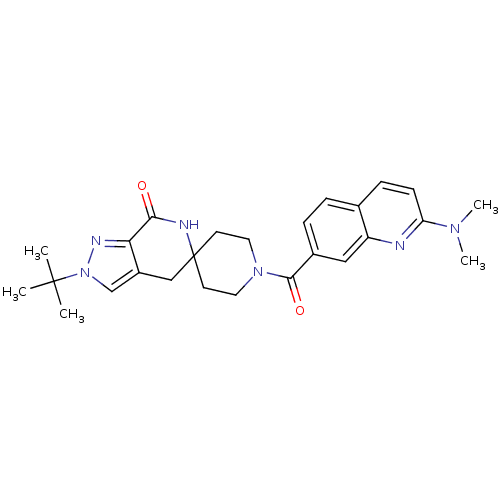
(CHEMBL2419589 | US8993586, 105)Show SMILES CN(C)c1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C26H32N6O2/c1-25(2,3)32-16-19-15-26(28-23(33)22(19)29-32)10-12-31(13-11-26)24(34)18-7-6-17-8-9-21(30(4)5)27-20(17)14-18/h6-9,14,16H,10-13,15H2,1-5H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344020
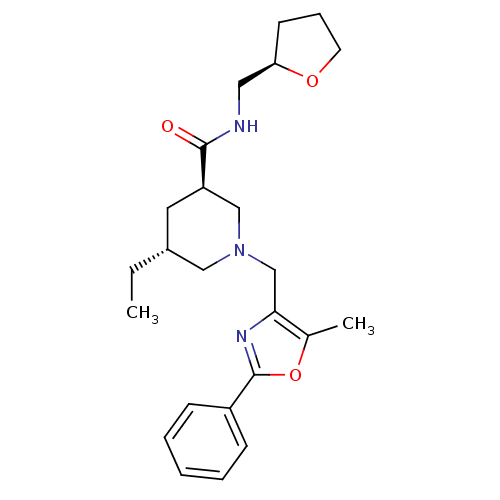
(CHEMBL1780116 | trans-(3R,5R)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NC[C@H]1CCCO1 |r| Show InChI InChI=1S/C24H33N3O3/c1-3-18-12-20(23(28)25-13-21-10-7-11-29-21)15-27(14-18)16-22-17(2)30-24(26-22)19-8-5-4-6-9-19/h4-6,8-9,18,20-21H,3,7,10-16H2,1-2H3,(H,25,28)/t18-,20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439644
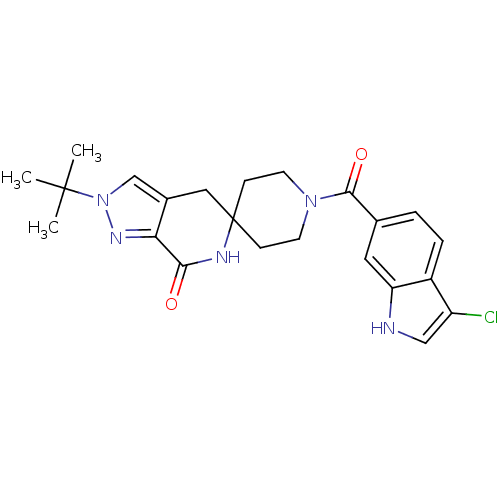
(CHEMBL2419593 | US8993586, 86)Show SMILES CC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3ccc4c(Cl)c[nH]c4c3)NC(=O)c2n1 Show InChI InChI=1S/C23H26ClN5O2/c1-22(2,3)29-13-15-11-23(26-20(30)19(15)27-29)6-8-28(9-7-23)21(31)14-4-5-16-17(24)12-25-18(16)10-14/h4-5,10,12-13,25H,6-9,11H2,1-3H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344021
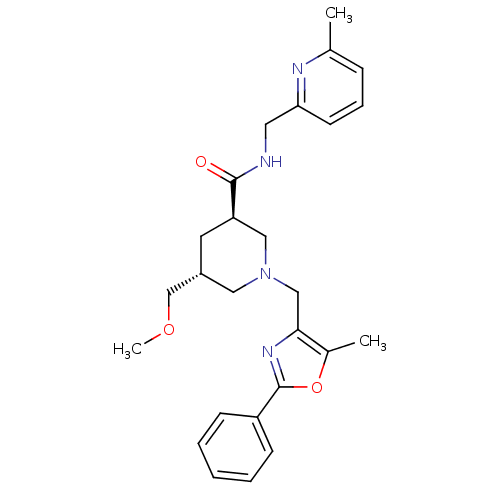
(CHEMBL1779988 | trans-(3R,5R)-5-(methoxymethyl)-1-...)Show SMILES COC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O3/c1-18-8-7-11-23(28-18)13-27-25(31)22-12-20(17-32-3)14-30(15-22)16-24-19(2)33-26(29-24)21-9-5-4-6-10-21/h4-11,20,22H,12-17H2,1-3H3,(H,27,31)/t20-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344022
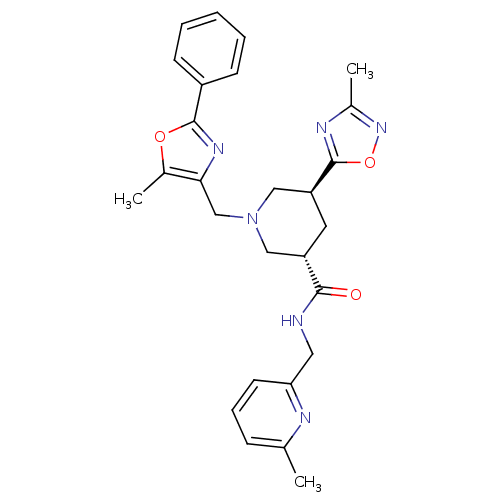
(CHEMBL1780100 | trans-(3S,5S)-5-(3-methyl-1,2,4-ox...)Show SMILES Cc1noc(n1)[C@H]1C[C@@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C27H30N6O3/c1-17-8-7-11-23(29-17)13-28-25(34)21-12-22(27-30-19(3)32-36-27)15-33(14-21)16-24-18(2)35-26(31-24)20-9-5-4-6-10-20/h4-11,21-22H,12-16H2,1-3H3,(H,28,34)/t21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344023

(CHEMBL1780089 | trans-(3R,5R)-5-(3-methyl-1,2,4-ox...)Show SMILES Cc1noc(n1)[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C27H30N6O3/c1-17-8-7-11-23(29-17)13-28-25(34)21-12-22(27-30-19(3)32-36-27)15-33(14-21)16-24-18(2)35-26(31-24)20-9-5-4-6-10-20/h4-11,21-22H,12-16H2,1-3H3,(H,28,34)/t21-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439634
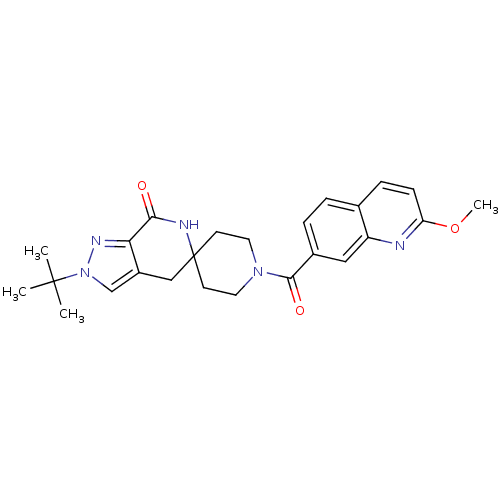
(CHEMBL2419596 | US8993586, 71)Show SMILES COc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H29N5O3/c1-24(2,3)30-15-18-14-25(27-22(31)21(18)28-30)9-11-29(12-10-25)23(32)17-6-5-16-7-8-20(33-4)26-19(16)13-17/h5-8,13,15H,9-12,14H2,1-4H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50259015

(5-(3-chlorobenzyl)-3-isopropyl-1H-pyrazolo[4,3-d]p...)Show InChI InChI=1S/C15H15ClN4O/c1-8(2)12-13-14(20-19-12)15(21)18-11(17-13)7-9-4-3-5-10(16)6-9/h3-6,8H,7H2,1-2H3,(H,19,20)(H,17,18,21) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PDE1c |
Bioorg Med Chem Lett 19: 2537-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.024
BindingDB Entry DOI: 10.7270/Q2RB74GG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439643
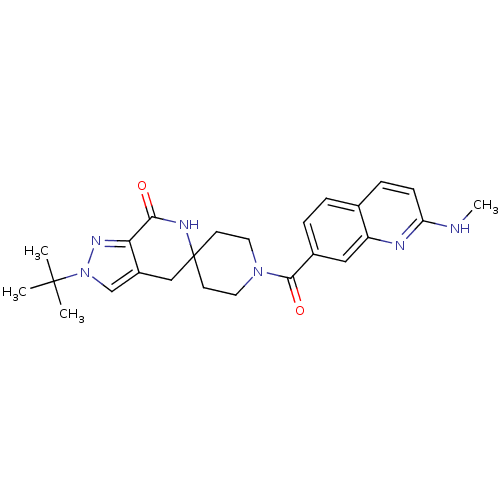
(CHEMBL2419598 | US8993586, 76)Show SMILES CNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H30N6O2/c1-24(2,3)31-15-18-14-25(28-22(32)21(18)29-31)9-11-30(12-10-25)23(33)17-6-5-16-7-8-20(26-4)27-19(16)13-17/h5-8,13,15H,9-12,14H2,1-4H3,(H,26,27)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439641
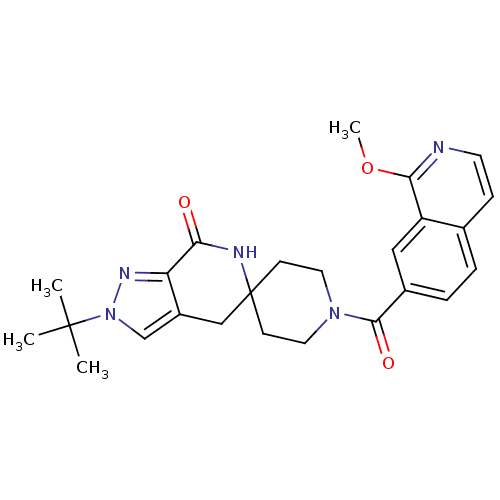
(CHEMBL2419597 | US8993586, 55)Show SMILES COc1nccc2ccc(cc12)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H29N5O3/c1-24(2,3)30-15-18-14-25(27-21(31)20(18)28-30)8-11-29(12-9-25)23(32)17-6-5-16-7-10-26-22(33-4)19(16)13-17/h5-7,10,13,15H,8-9,11-12,14H2,1-4H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344024

(CHEMBL1780097 | trans-(3S,5S)-5-(methoxymethyl)-1-...)Show SMILES COC[C@H]1C[C@@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O3/c1-18-8-7-11-23(28-18)13-27-25(31)22-12-20(17-32-3)14-30(15-22)16-24-19(2)33-26(29-24)21-9-5-4-6-10-21/h4-11,20,22H,12-17H2,1-3H3,(H,27,31)/t20-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344025
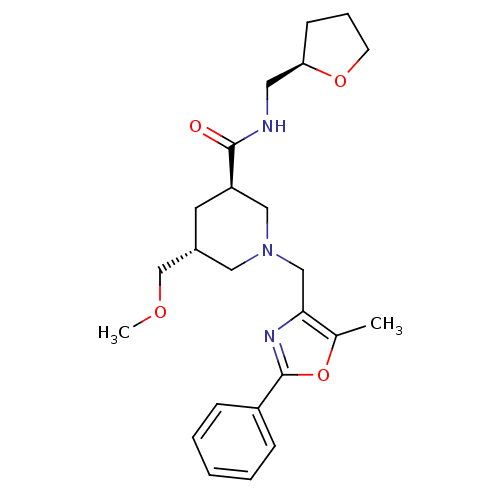
(CHEMBL1780105 | trans-(3R,5R)-5-(methoxymethyl)-1-...)Show SMILES COC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NC[C@H]1CCCO1 |r| Show InChI InChI=1S/C24H33N3O4/c1-17-22(26-24(31-17)19-7-4-3-5-8-19)15-27-13-18(16-29-2)11-20(14-27)23(28)25-12-21-9-6-10-30-21/h3-5,7-8,18,20-21H,6,9-16H2,1-2H3,(H,25,28)/t18-,20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344026
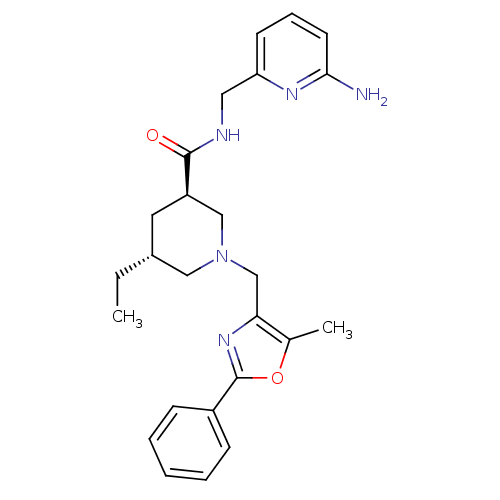
(CHEMBL1780111 | trans-(3R,5R)-N-((6-aminopyridin-2...)Show SMILES CC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(N)n1 |r| Show InChI InChI=1S/C25H31N5O2/c1-3-18-12-20(24(31)27-13-21-10-7-11-23(26)28-21)15-30(14-18)16-22-17(2)32-25(29-22)19-8-5-4-6-9-19/h4-11,18,20H,3,12-16H2,1-2H3,(H2,26,28)(H,27,31)/t18-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439645
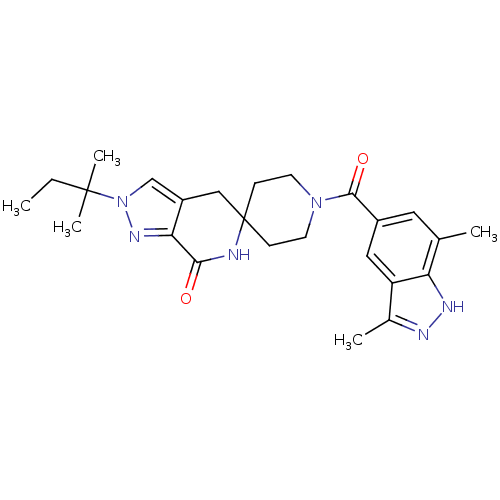
(CHEMBL2419607)Show SMILES CCC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3cc(C)c4[nH]nc(C)c4c3)NC(=O)c2n1 Show InChI InChI=1S/C25H32N6O2/c1-6-24(4,5)31-14-18-13-25(26-22(32)21(18)29-31)7-9-30(10-8-25)23(33)17-11-15(2)20-19(12-17)16(3)27-28-20/h11-12,14H,6-10,13H2,1-5H3,(H,26,32)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50439642

(CHEMBL2419589 | US8993586, 105)Show SMILES CN(C)c1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C26H32N6O2/c1-25(2,3)32-16-19-15-26(28-23(33)22(19)29-32)10-12-31(13-11-26)24(34)18-7-6-17-8-9-21(30(4)5)27-20(17)14-18/h6-9,14,16H,10-13,15H2,1-5H3,(H,28,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344016

(2-((5-methyl-2-phenyloxazol-4-yl)methyl)-N-((6-met...)Show SMILES Cc1oc(nc1CN1CC(C(=O)NCc2cccc(C)n2)c2ccccc2C1)-c1ccccc1 Show InChI InChI=1S/C28H28N4O2/c1-19-9-8-13-23(30-19)15-29-27(33)25-17-32(16-22-12-6-7-14-24(22)25)18-26-20(2)34-28(31-26)21-10-4-3-5-11-21/h3-14,25H,15-18H2,1-2H3,(H,29,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439635
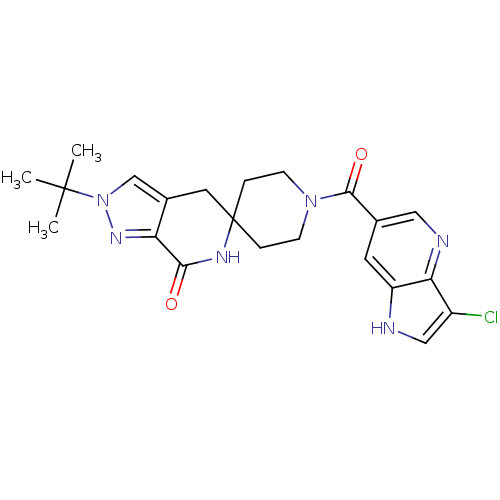
(CHEMBL2419594 | US8993586, 88)Show SMILES CC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3cnc4c(Cl)c[nH]c4c3)NC(=O)c2n1 Show InChI InChI=1S/C22H25ClN6O2/c1-21(2,3)29-12-14-9-22(26-19(30)17(14)27-29)4-6-28(7-5-22)20(31)13-8-16-18(25-10-13)15(23)11-24-16/h8,10-12,24H,4-7,9H2,1-3H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344027
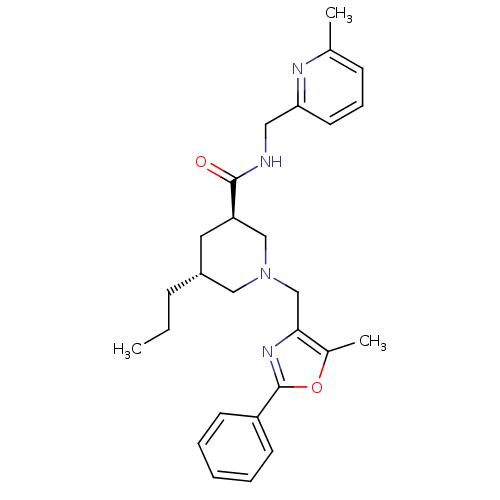
(CHEMBL1779985 | trans-(3R,5R)-1-((5-methyl-2-pheny...)Show SMILES CCC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C27H34N4O2/c1-4-9-21-14-23(26(32)28-15-24-13-8-10-19(2)29-24)17-31(16-21)18-25-20(3)33-27(30-25)22-11-6-5-7-12-22/h5-8,10-13,21,23H,4,9,14-18H2,1-3H3,(H,28,32)/t21-,23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50439643

(CHEMBL2419598 | US8993586, 76)Show SMILES CNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H30N6O2/c1-24(2,3)31-15-18-14-25(28-22(32)21(18)29-31)9-11-30(12-10-25)23(33)17-6-5-16-7-8-20(26-4)27-19(16)13-17/h5-8,13,15H,9-12,14H2,1-4H3,(H,26,27)(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439638
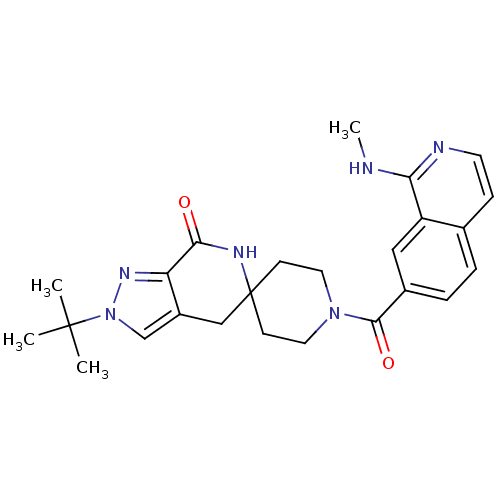
(CHEMBL2419599 | US8993586, 82)Show SMILES CNc1nccc2ccc(cc12)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H30N6O2/c1-24(2,3)31-15-18-14-25(28-22(32)20(18)29-31)8-11-30(12-9-25)23(33)17-6-5-16-7-10-27-21(26-4)19(16)13-17/h5-7,10,13,15H,8-9,11-12,14H2,1-4H3,(H,26,27)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439633
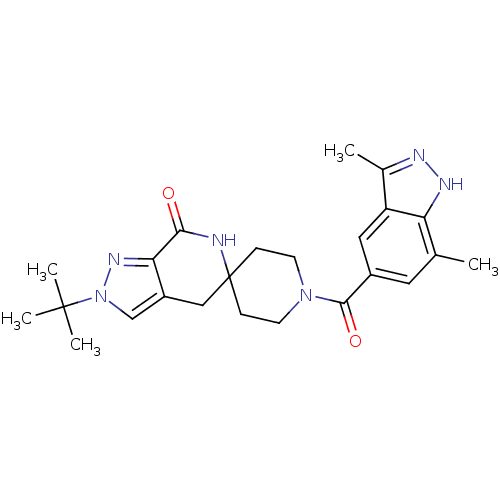
(CHEMBL2419604)Show SMILES Cc1n[nH]c2c(C)cc(cc12)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C24H30N6O2/c1-14-10-16(11-18-15(2)26-27-19(14)18)22(32)29-8-6-24(7-9-29)12-17-13-30(23(3,4)5)28-20(17)21(31)25-24/h10-11,13H,6-9,12H2,1-5H3,(H,25,31)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50344018

(CHEMBL1779984 | trans-(3R,5R)-5-ethyl-1-((5-methyl...)Show SMILES CC[C@@H]1C[C@H](CN(Cc2nc(oc2C)-c2ccccc2)C1)C(=O)NCc1cccc(C)n1 |r| Show InChI InChI=1S/C26H32N4O2/c1-4-20-13-22(25(31)27-14-23-12-8-9-18(2)28-23)16-30(15-20)17-24-19(3)32-26(29-24)21-10-6-5-7-11-21/h5-12,20,22H,4,13-17H2,1-3H3,(H,27,31)/t20-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B |
Bioorg Med Chem Lett 21: 3095-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.022
BindingDB Entry DOI: 10.7270/Q20002F8 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439639
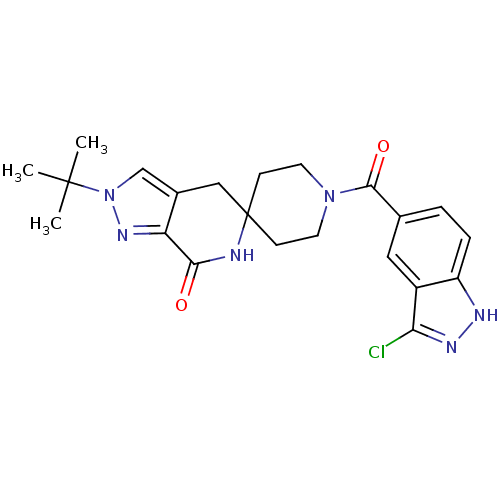
(CHEMBL2419591 | US8993586, 64)Show SMILES CC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3ccc4[nH]nc(Cl)c4c3)NC(=O)c2n1 Show InChI InChI=1S/C22H25ClN6O2/c1-21(2,3)29-12-14-11-22(24-19(30)17(14)27-29)6-8-28(9-7-22)20(31)13-4-5-16-15(10-13)18(23)26-25-16/h4-5,10,12H,6-9,11H2,1-3H3,(H,24,30)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data