 Found 760 hits with Last Name = 'moore' and Initial = 'ml'
Found 760 hits with Last Name = 'moore' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50412728
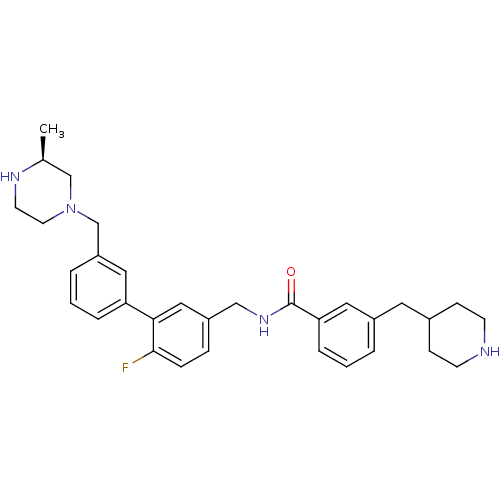
(CHEMBL521523)Show SMILES C[C@H]1CN(Cc2cccc(c2)-c2cc(CNC(=O)c3cccc(CC4CCNCC4)c3)ccc2F)CCN1 |r| Show InChI InChI=1S/C32H39FN4O/c1-23-21-37(15-14-35-23)22-27-5-3-6-28(18-27)30-19-26(8-9-31(30)33)20-36-32(38)29-7-2-4-25(17-29)16-24-10-12-34-13-11-24/h2-9,17-19,23-24,34-35H,10-16,20-22H2,1H3,(H,36,38)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 51: 5915-8 (2008)
Article DOI: 10.1021/jm800935u
BindingDB Entry DOI: 10.7270/Q21G0NHB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50412340
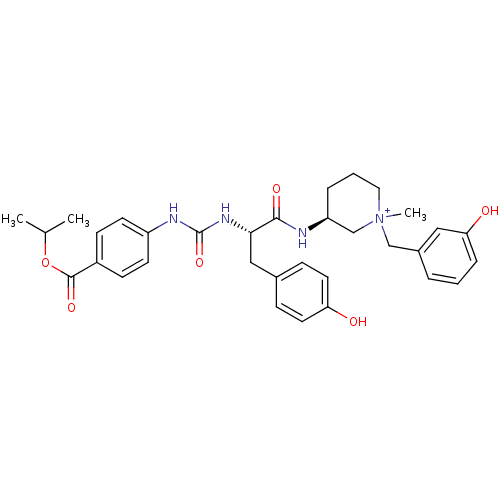
(CHEMBL540359)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H]2CCC[N+](C)(Cc3cccc(O)c3)C2)cc1 |r| Show InChI InChI=1S/C33H40N4O6/c1-22(2)43-32(41)25-11-13-26(14-12-25)35-33(42)36-30(19-23-9-15-28(38)16-10-23)31(40)34-27-7-5-17-37(3,21-27)20-24-6-4-8-29(39)18-24/h4,6,8-16,18,22,27,30H,5,7,17,19-21H2,1-3H3,(H4-,34,35,36,38,39,40,41,42)/p+1/t27-,30-,37?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M1 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 51: 4866-9 (2008)
Article DOI: 10.1021/jm800634k
BindingDB Entry DOI: 10.7270/Q2MG7QQS |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50291823
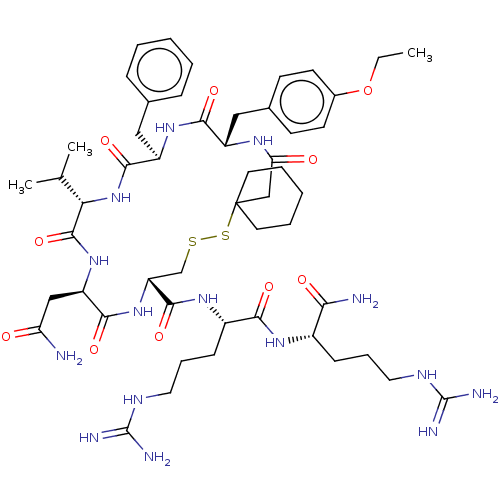
(19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H79N15O10S2/c1-4-77-33-19-17-32(18-20-33)26-36-45(72)64-37(25-31-13-7-5-8-14-31)47(74)67-42(30(2)3)49(76)65-38(27-40(53)68)46(73)66-39(29-78-79-52(28-41(69)61-36)21-9-6-10-22-52)48(75)63-35(16-12-24-60-51(57)58)44(71)62-34(43(54)70)15-11-23-59-50(55)56/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,53,68)(H2,54,70)(H,61,69)(H,62,71)(H,63,75)(H,64,72)(H,65,76)(H,66,73)(H,67,74)(H4,55,56,59)(H4,57,58,60)/t34-,35-,36-,37+,38+,39+,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. |
J Med Chem 32: 880-4 (1989)
BindingDB Entry DOI: 10.7270/Q2ZK5FNZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50291822

(19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CC[C@H](C)CC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 |wU:23.55,63.66,8.7,35.52,wD:59.63,27.28,39.40,16.16,(-5.31,-11.46,;-4.28,-10.32,;-2.76,-10.65,;-1.74,-9.49,;-2.21,-8.02,;-1.19,-6.88,;.33,-7.21,;1.36,-6.06,;2.87,-6.37,;2.8,-7.92,;3.18,-9.41,;1.77,-10.02,;4.03,-10.7,;5.22,-11.67,;6.1,-12.82,;5.32,-14.16,;3.78,-14.16,;3.01,-15.49,;3.01,-12.82,;3.78,-11.49,;7.04,-12.52,;8.2,-12.31,;9.69,-11.9,;10.98,-11.07,;11.95,-9.86,;12.49,-8.42,;14.01,-8.74,;12.58,-6.88,;14.12,-6.73,;15.31,-6.39,;15.75,-4.92,;16.34,-7.53,;12.18,-5.4,;11.34,-4.11,;12.49,-3.08,;10.14,-3.13,;8.71,-2.59,;7.18,-2.5,;6.97,-1.41,;5.68,-2.9,;5.06,-1.49,;5.96,-.24,;7.49,-.4,;8.39,.84,;7.77,2.25,;6.24,2.41,;5.33,1.16,;4.39,-3.74,;3.42,-4.94,;2.08,-4.17,;10.93,-1.8,;12.46,-1.8,;10.14,-.47,;12.02,-12.21,;11.53,-13.67,;13.44,-12.84,;12.3,-13.87,;12.93,-15.27,;14.47,-15.1,;14.77,-13.59,;16.19,-12.96,;16.33,-11.42,;17.43,-13.86,;18.83,-13.22,;18.99,-11.68,;20.39,-11.05,;20.53,-9.51,;21.94,-8.88,;22.11,-7.35,;20.85,-6.45,;23.51,-6.71,;20.09,-14.12,;19.94,-15.65,;21.6,-13.47,;23.16,-13.81,;23.35,-15.31,;22.22,-16.34,;24.82,-15.62,;.8,-8.67,;-.23,-9.82,)| Show InChI InChI=1S/C53H81N15O10S2/c1-5-78-34-17-15-33(16-18-34)26-37-46(73)65-38(25-32-11-7-6-8-12-32)48(75)68-43(30(2)3)50(77)66-39(27-41(54)69)47(74)67-40(29-79-80-53(28-42(70)62-37)21-19-31(4)20-22-53)49(76)64-36(14-10-24-61-52(58)59)45(72)63-35(44(55)71)13-9-23-60-51(56)57/h6-8,11-12,15-18,30-31,35-40,43H,5,9-10,13-14,19-29H2,1-4H3,(H2,54,69)(H2,55,71)(H,62,70)(H,63,72)(H,64,76)(H,65,73)(H,66,77)(H,67,74)(H,68,75)(H4,56,57,60)(H4,58,59,61)/t31?,35-,36-,37-,38-,39-,40+,43-,53?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. |
J Med Chem 32: 880-4 (1989)
BindingDB Entry DOI: 10.7270/Q2ZK5FNZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50452590
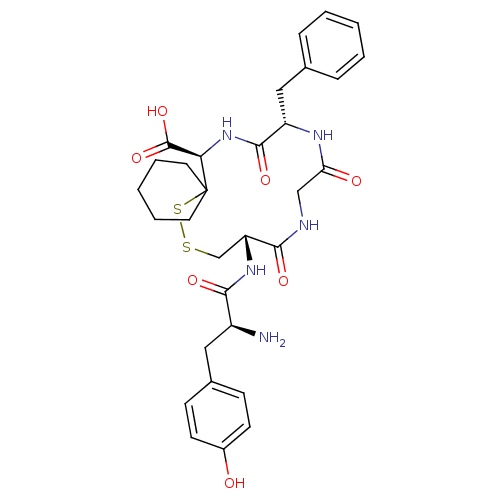
(CHEMBL3084309)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC2(CCCCC2)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O Show InChI InChI=1S/C31H39N5O7S2/c32-22(15-20-9-11-21(37)12-10-20)27(39)35-24-18-44-45-31(13-5-2-6-14-31)26(30(42)43)36-29(41)23(16-19-7-3-1-4-8-19)34-25(38)17-33-28(24)40/h1,3-4,7-12,22-24,26,37H,2,5-6,13-18,32H2,(H,33,40)(H,34,38)(H,35,39)(H,36,41)(H,42,43)/t22-,23-,24+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50291821
(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCC(C)CC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)cc1 |wU:23.55,63.66,8.7,35.52,wD:59.63,27.28,39.40,(-7.06,-8.94,;-6.04,-7.8,;-4.53,-8.13,;-3.51,-6.99,;-3.98,-5.52,;-2.95,-4.36,;-1.43,-4.68,;-.41,-3.54,;1.1,-3.87,;1.02,-5.4,;1.4,-6.89,;.01,-7.52,;2.26,-8.17,;3.45,-9.15,;2.01,-8.96,;1.24,-10.29,;2.01,-11.64,;1.24,-12.97,;3.54,-11.64,;4.31,-10.29,;5.26,-9.99,;6.42,-9.78,;7.92,-9.38,;9.2,-8.55,;10.17,-7.34,;10.71,-5.92,;12.22,-6.24,;10.8,-4.38,;12.34,-4.22,;13.52,-3.89,;13.97,-2.43,;14.55,-5.01,;10.41,-2.89,;9.57,-1.59,;10.71,-.57,;8.36,-.64,;6.93,-.08,;5.4,-.01,;5.2,1.09,;3.91,-.4,;3.28,1.02,;4.19,2.25,;5.71,2.09,;6.61,3.34,;5.99,4.74,;4.47,4.9,;3.54,3.67,;2.61,-1.24,;1.65,-2.43,;.31,-1.66,;9.14,.71,;10.68,.71,;8.36,2.04,;10.24,-9.69,;9.76,-11.15,;11.65,-10.31,;10.52,-11.34,;11.15,-12.76,;12.68,-12.57,;12.99,-11.08,;14.4,-10.43,;14.55,-8.89,;15.64,-11.34,;17.04,-10.71,;17.2,-9.17,;18.6,-8.55,;18.75,-7.01,;20.15,-6.38,;20.31,-4.85,;19.06,-3.94,;21.7,-4.2,;18.29,-11.59,;19.69,-10.96,;18.13,-13.13,;-.97,-6.15,;-1.99,-7.29,)| Show InChI InChI=1S/C52H76N12O10S2/c1-5-74-34-17-15-33(16-18-34)26-36-45(68)60-37(25-32-11-7-6-8-12-32)47(70)63-43(30(2)3)49(72)61-38(27-41(53)65)46(69)62-39(29-75-76-52(28-42(66)58-36)21-19-31(4)20-22-52)50(73)64-24-10-14-40(64)48(71)59-35(44(54)67)13-9-23-57-51(55)56/h6-8,11-12,15-18,30-31,35-40,43H,5,9-10,13-14,19-29H2,1-4H3,(H2,53,65)(H2,54,67)(H,58,66)(H,59,71)(H,60,68)(H,61,72)(H,62,69)(H,63,70)(H4,55,56,57)/t31?,35-,36-,37+,38+,39+,40-,43-,52?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. |
J Med Chem 32: 880-4 (1989)
BindingDB Entry DOI: 10.7270/Q2ZK5FNZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50291821

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCC(C)CC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)cc1 |wU:23.55,63.66,8.7,35.52,wD:59.63,27.28,39.40,(-7.06,-8.94,;-6.04,-7.8,;-4.53,-8.13,;-3.51,-6.99,;-3.98,-5.52,;-2.95,-4.36,;-1.43,-4.68,;-.41,-3.54,;1.1,-3.87,;1.02,-5.4,;1.4,-6.89,;.01,-7.52,;2.26,-8.17,;3.45,-9.15,;2.01,-8.96,;1.24,-10.29,;2.01,-11.64,;1.24,-12.97,;3.54,-11.64,;4.31,-10.29,;5.26,-9.99,;6.42,-9.78,;7.92,-9.38,;9.2,-8.55,;10.17,-7.34,;10.71,-5.92,;12.22,-6.24,;10.8,-4.38,;12.34,-4.22,;13.52,-3.89,;13.97,-2.43,;14.55,-5.01,;10.41,-2.89,;9.57,-1.59,;10.71,-.57,;8.36,-.64,;6.93,-.08,;5.4,-.01,;5.2,1.09,;3.91,-.4,;3.28,1.02,;4.19,2.25,;5.71,2.09,;6.61,3.34,;5.99,4.74,;4.47,4.9,;3.54,3.67,;2.61,-1.24,;1.65,-2.43,;.31,-1.66,;9.14,.71,;10.68,.71,;8.36,2.04,;10.24,-9.69,;9.76,-11.15,;11.65,-10.31,;10.52,-11.34,;11.15,-12.76,;12.68,-12.57,;12.99,-11.08,;14.4,-10.43,;14.55,-8.89,;15.64,-11.34,;17.04,-10.71,;17.2,-9.17,;18.6,-8.55,;18.75,-7.01,;20.15,-6.38,;20.31,-4.85,;19.06,-3.94,;21.7,-4.2,;18.29,-11.59,;19.69,-10.96,;18.13,-13.13,;-.97,-6.15,;-1.99,-7.29,)| Show InChI InChI=1S/C52H76N12O10S2/c1-5-74-34-17-15-33(16-18-34)26-36-45(68)60-37(25-32-11-7-6-8-12-32)47(70)63-43(30(2)3)49(72)61-38(27-41(53)65)46(69)62-39(29-75-76-52(28-42(66)58-36)21-19-31(4)20-22-52)50(73)64-24-10-14-40(64)48(71)59-35(44(54)67)13-9-23-57-51(55)56/h6-8,11-12,15-18,30-31,35-40,43H,5,9-10,13-14,19-29H2,1-4H3,(H2,53,65)(H2,54,67)(H,58,66)(H,59,71)(H,60,68)(H,61,72)(H,62,69)(H,63,70)(H4,55,56,57)/t31?,35-,36-,37+,38+,39+,40-,43-,52?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. |
J Med Chem 32: 880-4 (1989)
BindingDB Entry DOI: 10.7270/Q2ZK5FNZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50291824
(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCC(C)CC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O)cc1 |wU:23.55,27.28,35.52,wD:59.63,63.66,8.7,39.40,(5.96,4.27,;6.99,3.13,;6.51,1.66,;7.54,.52,;9.05,.84,;10.08,-.31,;9.6,-1.77,;10.63,-2.92,;10.16,-4.38,;9.13,-3.24,;7.62,-3.56,;6.59,-2.41,;7.14,-5.02,;5.64,-5.34,;5.27,-3.84,;3.8,-3.41,;2.68,-4.47,;1.2,-4.04,;3.05,-5.97,;4.52,-6.4,;5.16,-6.81,;6.19,-7.95,;7.7,-7.63,;8.73,-8.77,;10.24,-8.45,;11.27,-9.6,;10.79,-11.06,;12.77,-9.28,;12.3,-10.74,;13.33,-11.89,;12.85,-13.35,;14.83,-11.57,;13.8,-10.42,;15.31,-10.1,;16.34,-11.25,;15.78,-8.64,;14.75,-7.49,;15.23,-6.03,;16.74,-5.71,;14.2,-4.88,;14.68,-3.42,;16.18,-3.1,;17.21,-4.24,;18.72,-3.92,;19.19,-2.46,;18.16,-1.31,;16.66,-1.63,;12.69,-5.2,;11.66,-4.06,;12.14,-2.6,;17.29,-8.32,;18.32,-9.46,;17.77,-6.85,;8.25,-10.24,;9.28,-11.38,;6.75,-10.56,;5.6,-9.53,;4.27,-10.3,;4.59,-11.8,;6.12,-11.97,;6.89,-13.3,;8.43,-13.3,;6.12,-14.63,;6.89,-15.97,;6.12,-17.3,;6.89,-18.63,;6.12,-19.97,;6.89,-21.3,;8.43,-21.3,;9.2,-22.64,;9.2,-19.97,;8.43,-15.97,;9.2,-14.63,;9.2,-17.3,;10.74,-17.3,;11.51,-18.63,;13.05,-18.63,;10.74,-19.97,;8.1,-2.09,;7.07,-.95,)| Show InChI InChI=1S/C54H79N13O11S2/c1-5-78-35-17-15-34(16-18-35)26-37-47(72)63-38(25-33-11-7-6-8-12-33)49(74)66-45(31(2)3)51(76)64-39(27-42(55)68)48(73)65-40(30-79-80-54(28-44(70)61-37)21-19-32(4)20-22-54)52(77)67-24-10-14-41(67)50(75)62-36(13-9-23-59-53(57)58)46(71)60-29-43(56)69/h6-8,11-12,15-18,31-32,36-41,45H,5,9-10,13-14,19-30H2,1-4H3,(H2,55,68)(H2,56,69)(H,60,71)(H,61,70)(H,62,75)(H,63,72)(H,64,76)(H,65,73)(H,66,74)(H4,57,58,59)/t32?,36-,37-,38+,39+,40+,41-,45-,54?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. |
J Med Chem 32: 880-4 (1989)
BindingDB Entry DOI: 10.7270/Q2ZK5FNZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50020673

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C53H77N13O11S2/c1-4-77-34-19-17-33(18-20-34)26-36-46(71)62-37(25-32-13-7-5-8-14-32)48(73)65-44(31(2)3)50(75)63-38(27-41(54)67)47(72)64-39(30-78-79-53(28-43(69)60-36)21-9-6-10-22-53)51(76)66-24-12-16-40(66)49(74)61-35(15-11-23-58-52(56)57)45(70)59-29-42(55)68/h5,7-8,13-14,17-20,31,35-40,44H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,67)(H2,55,68)(H,59,70)(H,60,69)(H,61,74)(H,62,71)(H,63,75)(H,64,72)(H,65,73)(H4,56,57,58)/t35-,36-,37+,38+,39+,40-,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. |
J Med Chem 32: 880-4 (1989)
BindingDB Entry DOI: 10.7270/Q2ZK5FNZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50020667

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35-,36+,37+,38+,39-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. |
J Med Chem 32: 880-4 (1989)
BindingDB Entry DOI: 10.7270/Q2ZK5FNZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020671

(13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C46H69N11O8S2/c1-4-65-31-17-15-30(16-18-31)25-34-41(61)55-35(24-29-12-7-5-8-13-29)42(62)57-38(28(2)3)44(64)54-33(19-22-47)40(60)56-36(43(63)53-32(39(48)59)14-11-23-51-45(49)50)27-66-67-46(26-37(58)52-34)20-9-6-10-21-46/h5,7-8,12-13,15-18,28,32-36,38H,4,6,9-11,14,19-27,47H2,1-3H3,(H2,48,59)(H,52,58)(H,53,63)(H,54,64)(H,55,61)(H,56,60)(H,57,62)(H4,49,50,51)/t32-,33-,34+,35-,36-,38+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane |
J Med Chem 29: 984-8 (1986)
BindingDB Entry DOI: 10.7270/Q27M06W4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50016752

(CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C47H69N11O9S2/c1-4-67-31-17-15-30(16-18-31)23-33-41(62)55-34(22-29-12-7-5-8-13-29)43(64)58-39(28(2)3)45(66)56-35(24-37(48)59)42(63)57-36(44(65)54-32(40(49)61)14-11-21-52-46(50)51)26-68-27-69-47(25-38(60)53-33)19-9-6-10-20-47/h5,7-8,12-13,15-18,28,32-36,39H,4,6,9-11,14,19-27H2,1-3H3,(H2,48,59)(H2,49,61)(H,53,60)(H,54,65)(H,55,62)(H,56,66)(H,57,63)(H,58,64)(H4,50,51,52)/t32-,33-,34-,35-,36-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50016829
(10-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-1...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC2(CCCCC2)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O Show InChI InChI=1S/C31H39N5O7S2/c32-22(15-20-9-11-21(37)12-10-20)27(39)35-24-18-44-45-31(13-5-2-6-14-31)26(30(42)43)36-29(41)23(16-19-7-3-1-4-8-19)34-25(38)17-33-28(24)40/h1,3-4,7-12,22-24,26,37H,2,5-6,13-18,32H2,(H,33,40)(H,34,38)(H,35,39)(H,36,41)(H,42,43)/t22-,23-,24+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace delta-receptor specific radioligand [3H]DSLET |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50016829

(10-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-1...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC2(CCCCC2)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O Show InChI InChI=1S/C31H39N5O7S2/c32-22(15-20-9-11-21(37)12-10-20)27(39)35-24-18-44-45-31(13-5-2-6-14-31)26(30(42)43)36-29(41)23(16-19-7-3-1-4-8-19)34-25(38)17-33-28(24)40/h1,3-4,7-12,22-24,26,37H,2,5-6,13-18,32H2,(H,33,40)(H,34,38)(H,35,39)(H,36,41)(H,42,43)/t22-,23-,24+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace opioid receptor delta specific radioligand [3H]-DSLET |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020669
(13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C46H69N11O8S2/c1-4-65-31-17-15-30(16-18-31)25-34-41(61)55-35(24-29-12-7-5-8-13-29)42(62)57-38(28(2)3)44(64)54-33(19-22-47)40(60)56-36(43(63)53-32(39(48)59)14-11-23-51-45(49)50)27-66-67-46(26-37(58)52-34)20-9-6-10-21-46/h5,7-8,12-13,15-18,28,32-36,38H,4,6,9-11,14,19-27,47H2,1-3H3,(H2,48,59)(H,52,58)(H,53,63)(H,54,64)(H,55,61)(H,56,60)(H,57,62)(H4,49,50,51)/t32-,33+,34-,35+,36+,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane |
J Med Chem 29: 984-8 (1986)
BindingDB Entry DOI: 10.7270/Q27M06W4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace delta-receptor specific radioligand [3H]DSLET |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020665

(13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C48H72N12O9S2/c1-4-69-32-17-15-31(16-18-32)25-35-43(65)58-36(24-30-12-7-5-8-13-30)44(66)60-40(29(2)3)46(68)57-34(19-22-49)42(64)59-37(28-70-71-48(26-39(62)55-35)20-9-6-10-21-48)45(67)56-33(14-11-23-53-47(51)52)41(63)54-27-38(50)61/h5,7-8,12-13,15-18,29,33-37,40H,4,6,9-11,14,19-28,49H2,1-3H3,(H2,50,61)(H,54,63)(H,55,62)(H,56,67)(H,57,68)(H,58,65)(H,59,64)(H,60,66)(H4,51,52,53)/t33-,34+,35-,36+,37+,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane |
J Med Chem 29: 984-8 (1986)
BindingDB Entry DOI: 10.7270/Q27M06W4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016752

(CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C47H69N11O9S2/c1-4-67-31-17-15-30(16-18-31)23-33-41(62)55-34(22-29-12-7-5-8-13-29)43(64)58-39(28(2)3)45(66)56-35(24-37(48)59)42(63)57-36(44(65)54-32(40(49)61)14-11-21-52-46(50)51)26-68-27-69-47(25-38(60)53-33)19-9-6-10-20-47/h5,7-8,12-13,15-18,28,32-36,39H,4,6,9-11,14,19-27H2,1-3H3,(H2,48,59)(H2,49,61)(H,53,60)(H,54,65)(H,55,62)(H,56,66)(H,57,63)(H,58,64)(H4,50,51,52)/t32-,33-,34-,35-,36-,39+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50412340

(CHEMBL540359)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H]2CCC[N+](C)(Cc3cccc(O)c3)C2)cc1 |r| Show InChI InChI=1S/C33H40N4O6/c1-22(2)43-32(41)25-11-13-26(14-12-25)35-33(42)36-30(19-23-9-15-28(38)16-10-23)31(40)34-27-7-5-17-37(3,21-27)20-24-6-4-8-29(39)18-24/h4,6,8-16,18,22,27,30H,5,7,17,19-21H2,1-3H3,(H4-,34,35,36,38,39,40,41,42)/p+1/t27-,30-,37?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 by scintillatio... |
J Med Chem 51: 4866-9 (2008)
Article DOI: 10.1021/jm800634k
BindingDB Entry DOI: 10.7270/Q2MG7QQS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50452590

(CHEMBL3084309)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC2(CCCCC2)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O Show InChI InChI=1S/C31H39N5O7S2/c32-22(15-20-9-11-21(37)12-10-20)27(39)35-24-18-44-45-31(13-5-2-6-14-31)26(30(42)43)36-29(41)23(16-19-7-3-1-4-8-19)34-25(38)17-33-28(24)40/h1,3-4,7-12,22-24,26,37H,2,5-6,13-18,32H2,(H,33,40)(H,34,38)(H,35,39)(H,36,41)(H,42,43)/t22-,23-,24+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace delta-receptor specific radioligand [3H]DSLET |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50016760
(19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33-41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(44(62)51-32(40(49)58)15-9-12-22-47)27-65-66-46(26-38(57)50-33)20-10-6-11-21-46/h5,7-8,13-14,16-19,28,32-36,39H,4,6,9-12,15,20-27,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61)/t32-,33-,34-,35-,36-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220180
(CHEMBL243669 | N-[3-fluoro-4'-(trifluoromethyl)-4-...)Show InChI InChI=1S/C13H10F4N2O2S/c14-11-7-9(3-6-12(11)19-22(18,20)21)8-1-4-10(5-2-8)13(15,16)17/h1-7,19H,(H2,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50016830
(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H]1C(O)=O Show InChI InChI=1S/C28H35N5O7S2/c1-28(2)23(27(39)40)33-26(38)20(13-16-6-4-3-5-7-16)31-22(35)14-30-25(37)21(15-41-42-28)32-24(36)19(29)12-17-8-10-18(34)11-9-17/h3-11,19-21,23,34H,12-15,29H2,1-2H3,(H,30,37)(H,31,35)(H,32,36)(H,33,38)(H,39,40)/t19-,20-,21+,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50452524

(CHEMBL2372291)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35+,36-,37-,38?,39+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50016756

(CHEMBL265591 | c(Pmp-D-Tyr(Et)-Phe-Val-Asn-Cys)-Pr...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCCC2C(=O)NCCCCNC(N)=N)cc1 Show InChI InChI=1S/C51H75N11O9S2/c1-4-71-35-19-17-34(18-20-35)27-36-44(65)58-37(26-33-14-7-5-8-15-33)46(67)61-43(32(2)3)48(69)59-38(28-41(52)63)45(66)60-39(30-72-31-73-51(29-42(64)57-36)21-9-6-10-22-51)49(70)62-25-13-16-40(62)47(68)55-23-11-12-24-56-50(53)54/h5,7-8,14-15,17-20,32,36-40,43H,4,6,9-13,16,21-31H2,1-3H3,(H2,52,63)(H,55,68)(H,57,64)(H,58,65)(H,59,69)(H,60,66)(H,61,67)(H4,53,54,56)/t36-,37-,38-,39-,40?,43+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020654
(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35-,36-,37-,38-,39+,42+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. |
J Med Chem 29: 2425-6 (1987)
BindingDB Entry DOI: 10.7270/Q23T9G6Q |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020667

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35-,36+,37+,38+,39-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane |
J Med Chem 29: 984-8 (1986)
BindingDB Entry DOI: 10.7270/Q27M06W4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50016753
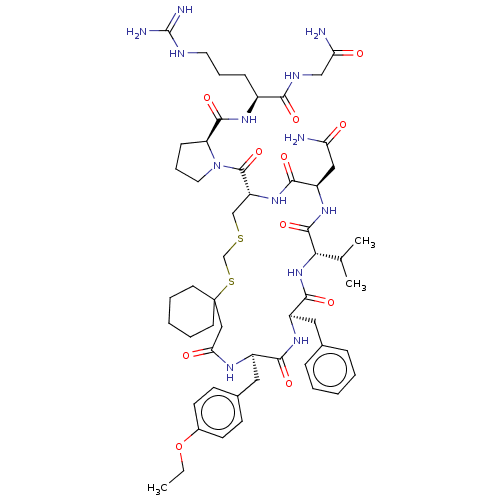
(1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#6]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C54H79N13O11S2/c1-4-78-35-19-17-34(18-20-35)26-37-47(72)63-38(25-33-13-7-5-8-14-33)49(74)66-45(32(2)3)51(76)64-39(27-42(55)68)48(73)65-40(30-79-31-80-54(28-44(70)61-37)21-9-6-10-22-54)52(77)67-24-12-16-41(67)50(75)62-36(15-11-23-59-53(57)58)46(71)60-29-43(56)69/h5,7-8,13-14,17-20,32,36-41,45H,4,6,9-12,15-16,21-31H2,1-3H3,(H2,55,68)(H2,56,69)(H,60,71)(H,61,70)(H,62,75)(H,63,72)(H,64,76)(H,65,73)(H,66,74)(H4,57,58,59)/t36-,37+,38+,39+,40+,41-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016753

(1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#6]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C54H79N13O11S2/c1-4-78-35-19-17-34(18-20-35)26-37-47(72)63-38(25-33-13-7-5-8-14-33)49(74)66-45(32(2)3)51(76)64-39(27-42(55)68)48(73)65-40(30-79-31-80-54(28-44(70)61-37)21-9-6-10-22-54)52(77)67-24-12-16-41(67)50(75)62-36(15-11-23-59-53(57)58)46(71)60-29-43(56)69/h5,7-8,13-14,17-20,32,36-41,45H,4,6,9-12,15-16,21-31H2,1-3H3,(H2,55,68)(H2,56,69)(H,60,71)(H,61,70)(H,62,75)(H,63,72)(H,64,76)(H,65,73)(H,66,74)(H4,57,58,59)/t36-,37+,38+,39+,40+,41-,45-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50016757

(1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#6]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H76N12O10S2/c1-4-74-34-19-17-33(18-20-34)26-36-45(68)60-37(25-32-13-7-5-8-14-32)47(70)63-43(31(2)3)49(72)61-38(27-41(53)65)46(69)62-39(29-75-30-76-52(28-42(66)58-36)21-9-6-10-22-52)50(73)64-24-12-16-40(64)48(71)59-35(44(54)67)15-11-23-57-51(55)56/h5,7-8,13-14,17-20,31,35-40,43H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,53,65)(H2,54,67)(H,58,66)(H,59,71)(H,60,68)(H,61,72)(H,62,69)(H,63,70)(H4,55,56,57)/t35-,36+,37+,38+,39+,40-,43-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016757

(1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#6]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H76N12O10S2/c1-4-74-34-19-17-33(18-20-34)26-36-45(68)60-37(25-32-13-7-5-8-14-32)47(70)63-43(31(2)3)49(72)61-38(27-41(53)65)46(69)62-39(29-75-30-76-52(28-42(66)58-36)21-9-6-10-22-52)50(73)64-24-12-16-40(64)48(71)59-35(44(54)67)15-11-23-57-51(55)56/h5,7-8,13-14,17-20,31,35-40,43H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,53,65)(H2,54,67)(H,58,66)(H,59,71)(H,60,68)(H,61,72)(H,62,69)(H,63,70)(H4,55,56,57)/t35-,36+,37+,38+,39+,40-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020655
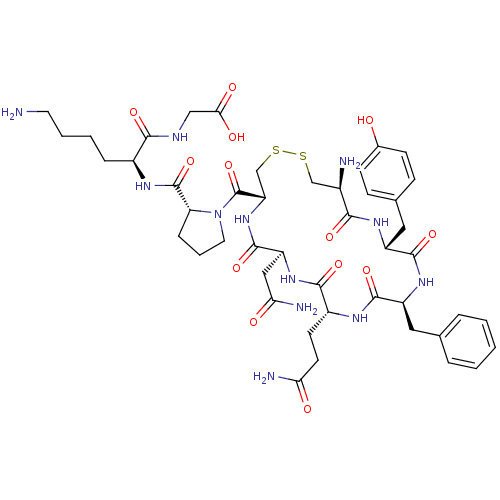
(CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...)Show SMILES NCCCC[C@H](NC(=O)[C@H]1CCCN1C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(O)=O Show InChI InChI=1S/C46H64N12O13S2/c47-17-5-4-9-29(40(65)51-22-38(62)63)53-45(70)35-10-6-18-58(35)46(71)34-24-73-72-23-28(48)39(64)54-31(20-26-11-13-27(59)14-12-26)43(68)55-32(19-25-7-2-1-3-8-25)42(67)52-30(15-16-36(49)60)41(66)56-33(21-37(50)61)44(69)57-34/h1-3,7-8,11-14,28-35,59H,4-6,9-10,15-24,47-48H2,(H2,49,60)(H2,50,61)(H,51,65)(H,52,67)(H,53,70)(H,54,64)(H,55,68)(H,56,66)(H,57,69)(H,62,63)/t28-,29+,30-,31-,32+,33+,34+,35-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. |
J Med Chem 28: 1759-60 (1986)
BindingDB Entry DOI: 10.7270/Q2CC0ZNX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020655

(CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...)Show SMILES NCCCC[C@H](NC(=O)[C@H]1CCCN1C(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(O)=O Show InChI InChI=1S/C46H64N12O13S2/c47-17-5-4-9-29(40(65)51-22-38(62)63)53-45(70)35-10-6-18-58(35)46(71)34-24-73-72-23-28(48)39(64)54-31(20-26-11-13-27(59)14-12-26)43(68)55-32(19-25-7-2-1-3-8-25)42(67)52-30(15-16-36(49)60)41(66)56-33(21-37(50)61)44(69)57-34/h1-3,7-8,11-14,28-35,59H,4-6,9-10,15-24,47-48H2,(H2,49,60)(H2,50,61)(H,51,65)(H,52,67)(H,53,70)(H,54,64)(H,55,68)(H,56,66)(H,57,69)(H,62,63)/t28-,29+,30-,31-,32+,33+,34+,35-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of hog kidney renin |
J Med Chem 28: 1759-60 (1986)
BindingDB Entry DOI: 10.7270/Q2CC0ZNX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016747
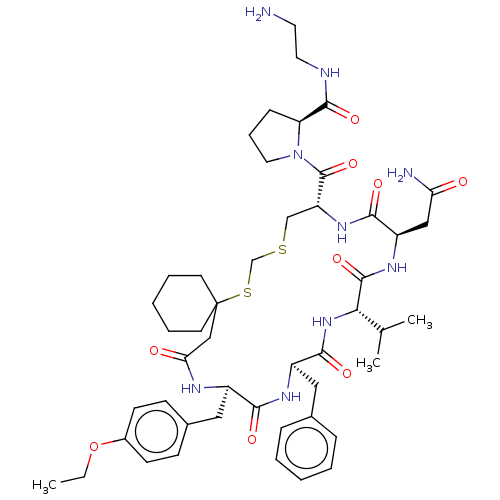
(1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)NCCN)cc1 Show InChI InChI=1S/C48H69N9O9S2/c1-4-66-33-17-15-32(16-18-33)25-34-42(60)53-35(24-31-12-7-5-8-13-31)44(62)56-41(30(2)3)46(64)54-36(26-39(50)58)43(61)55-37(47(65)57-23-11-14-38(57)45(63)51-22-21-49)28-67-29-68-48(27-40(59)52-34)19-9-6-10-20-48/h5,7-8,12-13,15-18,30,34-38,41H,4,6,9-11,14,19-29,49H2,1-3H3,(H2,50,58)(H,51,63)(H,52,59)(H,53,60)(H,54,64)(H,55,61)(H,56,62)/t34-,35-,36-,37-,38+,41+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50016747

(1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)NCCN)cc1 Show InChI InChI=1S/C48H69N9O9S2/c1-4-66-33-17-15-32(16-18-33)25-34-42(60)53-35(24-31-12-7-5-8-13-31)44(62)56-41(30(2)3)46(64)54-36(26-39(50)58)43(61)55-37(47(65)57-23-11-14-38(57)45(63)51-22-21-49)28-67-29-68-48(27-40(59)52-34)19-9-6-10-20-48/h5,7-8,12-13,15-18,30,34-38,41H,4,6,9-11,14,19-29,49H2,1-3H3,(H2,50,58)(H,51,63)(H,52,59)(H,53,60)(H,54,64)(H,55,61)(H,56,62)/t34-,35-,36-,37-,38+,41+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020675

(1-[13-Benzyl-7-carbamoylmethyl-16-(4-ethoxy-benzyl...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC(CC)(CC)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C52H77N13O11S2/c1-6-52(7-2)27-42(68)59-35(25-32-18-20-33(21-19-32)76-8-3)45(70)61-36(24-31-14-10-9-11-15-31)47(72)64-43(30(4)5)49(74)62-37(26-40(53)66)46(71)63-38(29-77-78-52)50(75)65-23-13-17-39(65)48(73)60-34(16-12-22-57-51(55)56)44(69)58-28-41(54)67/h9-11,14-15,18-21,30,34-39,43H,6-8,12-13,16-17,22-29H2,1-5H3,(H2,53,66)(H2,54,67)(H,58,69)(H,59,68)(H,60,73)(H,61,70)(H,62,74)(H,63,71)(H,64,72)(H4,55,56,57)/t34-,35-,36-,37-,38-,39+,43+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. |
J Med Chem 29: 2425-6 (1987)
BindingDB Entry DOI: 10.7270/Q23T9G6Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50016833
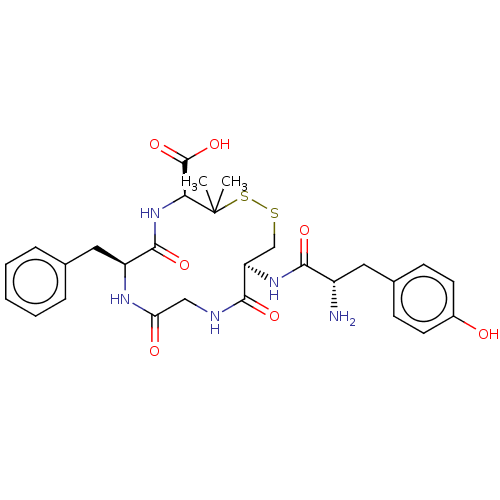
(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H35N5O7S2/c1-28(2)23(27(39)40)33-26(38)20(13-16-6-4-3-5-7-16)31-22(35)14-30-25(37)21(15-41-42-28)32-24(36)19(29)12-17-8-10-18(34)11-9-17/h3-11,19-21,23,34H,12-15,29H2,1-2H3,(H,30,37)(H,31,35)(H,32,36)(H,33,38)(H,39,40)/t19-,20-,21+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50016833

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O |r| Show InChI InChI=1S/C28H35N5O7S2/c1-28(2)23(27(39)40)33-26(38)20(13-16-6-4-3-5-7-16)31-22(35)14-30-25(37)21(15-41-42-28)32-24(36)19(29)12-17-8-10-18(34)11-9-17/h3-11,19-21,23,34H,12-15,29H2,1-2H3,(H,30,37)(H,31,35)(H,32,36)(H,33,38)(H,39,40)/t19-,20-,21+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE |
J Med Chem 32: 302-4 (1989)
BindingDB Entry DOI: 10.7270/Q2SX6DT2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016760

(19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33-41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(44(62)51-32(40(49)58)15-9-12-22-47)27-65-66-46(26-38(57)50-33)20-10-6-11-21-46/h5,7-8,13-14,16-19,28,32-36,39H,4,6,9-12,15,20-27,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61)/t32-,33-,34-,35-,36-,39+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. |
J Med Chem 28: 1759-60 (1986)
BindingDB Entry DOI: 10.7270/Q2CC0ZNX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020666

(13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CCN)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@@H](CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H69N9O8S2/c1-4-63-32-18-16-31(17-19-32)26-35-42(59)53-36(25-30-13-7-5-8-14-30)43(60)55-39(29(2)3)45(62)52-34(20-24-48)41(58)54-37(44(61)51-33(40(49)57)15-9-12-23-47)28-64-65-46(27-38(56)50-35)21-10-6-11-22-46/h5,7-8,13-14,16-19,29,33-37,39H,4,6,9-12,15,20-28,47-48H2,1-3H3,(H2,49,57)(H,50,56)(H,51,61)(H,52,62)(H,53,59)(H,54,58)(H,55,60)/t33-,34+,35-,36+,37+,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane |
J Med Chem 29: 984-8 (1986)
BindingDB Entry DOI: 10.7270/Q27M06W4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016760

(19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33-41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(44(62)51-32(40(49)58)15-9-12-22-47)27-65-66-46(26-38(57)50-33)20-10-6-11-21-46/h5,7-8,13-14,16-19,28,32-36,39H,4,6,9-12,15,20-27,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61)/t32-,33-,34-,35-,36-,39+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016756

(CHEMBL265591 | c(Pmp-D-Tyr(Et)-Phe-Val-Asn-Cys)-Pr...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCCC2C(=O)NCCCCNC(N)=N)cc1 Show InChI InChI=1S/C51H75N11O9S2/c1-4-71-35-19-17-34(18-20-35)27-36-44(65)58-37(26-33-14-7-5-8-15-33)46(67)61-43(32(2)3)48(69)59-38(28-41(52)63)45(66)60-39(30-72-31-73-51(29-42(64)57-36)21-9-6-10-22-51)49(70)62-25-13-16-40(62)47(68)55-23-11-12-24-56-50(53)54/h5,7-8,14-15,17-20,32,36-40,43H,4,6,9-13,16,21-31H2,1-3H3,(H2,52,63)(H,55,68)(H,57,64)(H,58,65)(H,59,69)(H,60,66)(H,61,67)(H4,53,54,56)/t36-,37-,38-,39-,40?,43+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220182
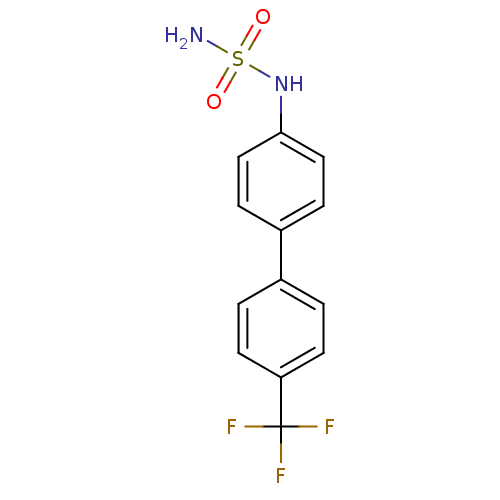
(CHEMBL390629 | N-[4'-(trifluoromethyl)-4-biphenyly...)Show InChI InChI=1S/C13H11F3N2O2S/c14-13(15,16)11-5-1-9(2-6-11)10-3-7-12(8-4-10)18-21(17,19)20/h1-8,18H,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020673

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C53H77N13O11S2/c1-4-77-34-19-17-33(18-20-34)26-36-46(71)62-37(25-32-13-7-5-8-14-32)48(73)65-44(31(2)3)50(75)63-38(27-41(54)67)47(72)64-39(30-78-79-53(28-43(69)60-36)21-9-6-10-22-53)51(76)66-24-12-16-40(66)49(74)61-35(15-11-23-58-52(56)57)45(70)59-29-42(55)68/h5,7-8,13-14,17-20,31,35-40,44H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,67)(H2,55,68)(H,59,70)(H,60,69)(H,61,74)(H,62,71)(H,63,75)(H,64,72)(H,65,73)(H4,56,57,58)/t35-,36-,37+,38+,39+,40-,44-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane |
J Med Chem 29: 984-8 (1986)
BindingDB Entry DOI: 10.7270/Q27M06W4 |
More data for this
Ligand-Target Pair | |
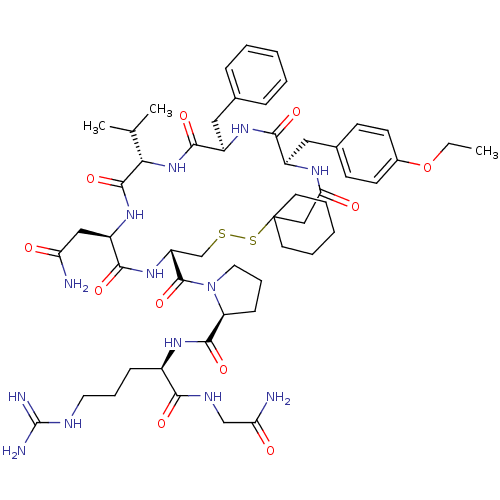
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020653
(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C53H77N13O11S2/c1-4-77-34-19-17-33(18-20-34)26-36-46(71)62-37(25-32-13-7-5-8-14-32)48(73)65-44(31(2)3)50(75)63-38(27-41(54)67)47(72)64-39(30-78-79-53(28-43(69)60-36)21-9-6-10-22-53)51(76)66-24-12-16-40(66)49(74)61-35(15-11-23-58-52(56)57)45(70)59-29-42(55)68/h5,7-8,13-14,17-20,31,35-40,44H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,67)(H2,55,68)(H,59,70)(H,60,69)(H,61,74)(H,62,71)(H,63,75)(H,64,72)(H,65,73)(H4,56,57,58)/t35-,36-,37-,38-,39-,40+,44+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. |
J Med Chem 28: 1759-60 (1986)
BindingDB Entry DOI: 10.7270/Q2CC0ZNX |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50020653

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(N)=O)cc1 Show InChI InChI=1S/C53H77N13O11S2/c1-4-77-34-19-17-33(18-20-34)26-36-46(71)62-37(25-32-13-7-5-8-14-32)48(73)65-44(31(2)3)50(75)63-38(27-41(54)67)47(72)64-39(30-78-79-53(28-43(69)60-36)21-9-6-10-22-53)51(76)66-24-12-16-40(66)49(74)61-35(15-11-23-58-52(56)57)45(70)59-29-42(55)68/h5,7-8,13-14,17-20,31,35-40,44H,4,6,9-12,15-16,21-30H2,1-3H3,(H2,54,67)(H2,55,68)(H,59,70)(H,60,69)(H,61,74)(H,62,71)(H,63,75)(H,64,72)(H,65,73)(H4,56,57,58)/t35-,36-,37-,38-,39-,40+,44+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. |
J Med Chem 29: 2425-6 (1987)
BindingDB Entry DOI: 10.7270/Q23T9G6Q |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
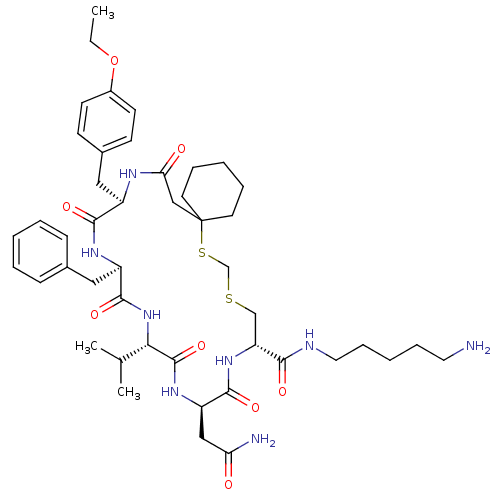
(Homo sapiens (Human)) | BDBM50016755
(20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)NCCCCCN)cc1 Show InChI InChI=1S/C46H68N8O8S2/c1-4-62-33-18-16-32(17-19-33)25-34-42(58)51-35(24-31-14-8-5-9-15-31)44(60)54-40(30(2)3)45(61)52-36(26-38(48)55)43(59)53-37(41(57)49-23-13-7-12-22-47)28-63-29-64-46(27-39(56)50-34)20-10-6-11-21-46/h5,8-9,14-19,30,34-37,40H,4,6-7,10-13,20-29,47H2,1-3H3,(H2,48,55)(H,49,57)(H,50,56)(H,51,58)(H,52,61)(H,53,59)(H,54,60)/t34-,35-,36-,37-,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016755

(20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)NCCCCCN)cc1 Show InChI InChI=1S/C46H68N8O8S2/c1-4-62-33-18-16-32(17-19-33)25-34-42(58)51-35(24-31-14-8-5-9-15-31)44(60)54-40(30(2)3)45(61)52-36(26-38(48)55)43(59)53-37(41(57)49-23-13-7-12-22-47)28-63-29-64-46(27-39(56)50-34)20-10-6-11-21-46/h5,8-9,14-19,30,34-37,40H,4,6-7,10-13,20-29,47H2,1-3H3,(H2,48,55)(H,49,57)(H,50,56)(H,51,58)(H,52,61)(H,53,59)(H,54,60)/t34-,35-,36-,37-,40+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50016763

(1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N2CCC[C@H]2C(=O)NCCCCN)cc1 Show InChI InChI=1S/C50H73N9O9S2/c1-4-68-35-19-17-34(18-20-35)27-36-44(62)55-37(26-33-14-7-5-8-15-33)46(64)58-43(32(2)3)48(66)56-38(28-41(52)60)45(63)57-39(30-69-31-70-50(29-42(61)54-36)21-9-6-10-22-50)49(67)59-25-13-16-40(59)47(65)53-24-12-11-23-51/h5,7-8,14-15,17-20,32,36-40,43H,4,6,9-13,16,21-31,51H2,1-3H3,(H2,52,60)(H,53,65)(H,54,61)(H,55,62)(H,56,66)(H,57,63)(H,58,64)/t36-,37-,38-,39-,40+,43+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































