

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50002471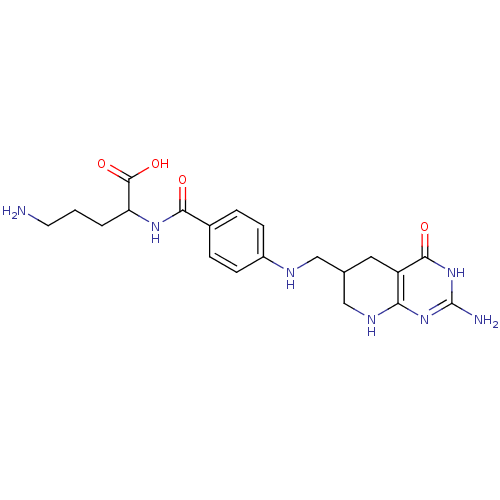 (5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50003896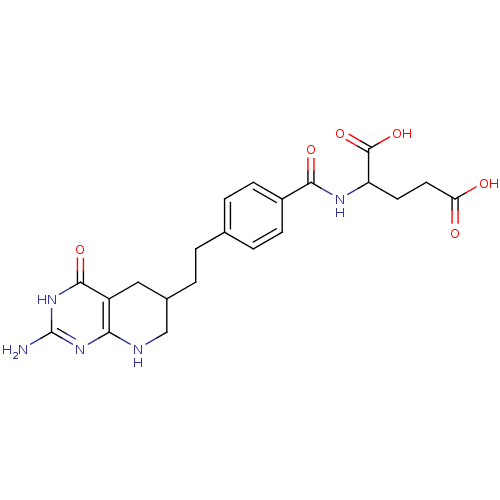 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase | J Med Chem 35: 4450-4 (1992) BindingDB Entry DOI: 10.7270/Q2RR1ZV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50005868 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50024475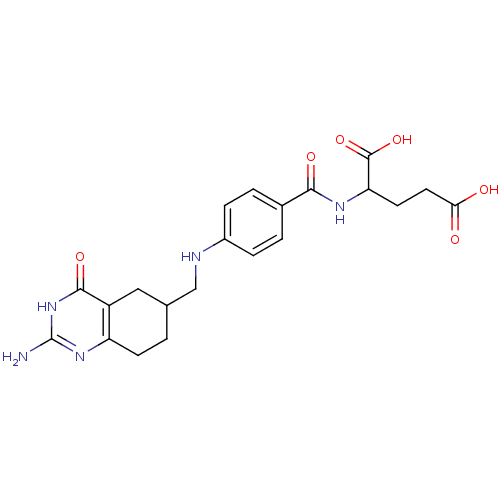 (2-{4-[(2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse GAR transformylase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50006906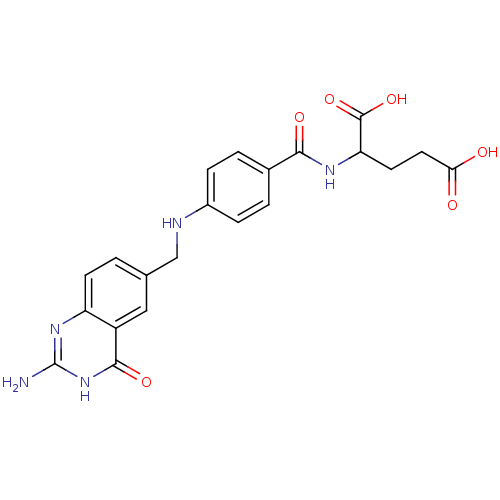 (2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity of the compound towards Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 2626-30 (1992) BindingDB Entry DOI: 10.7270/Q2DV1HVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016343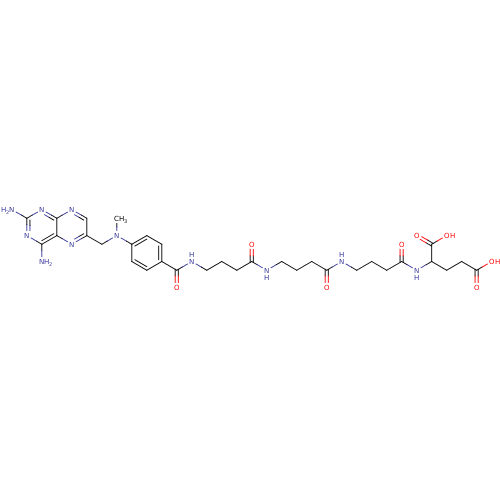 (2-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50011885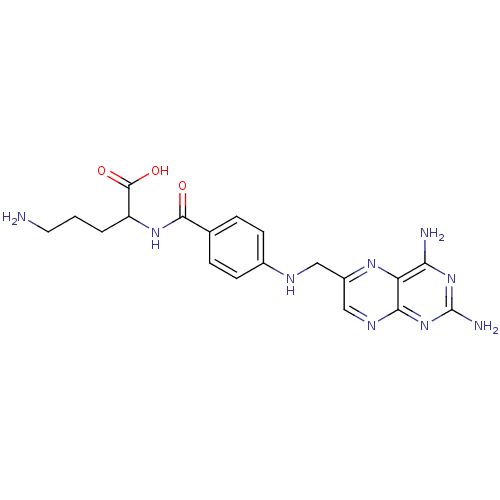 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver | J Med Chem 29: 655-60 (1986) BindingDB Entry DOI: 10.7270/Q2RR1ZTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016340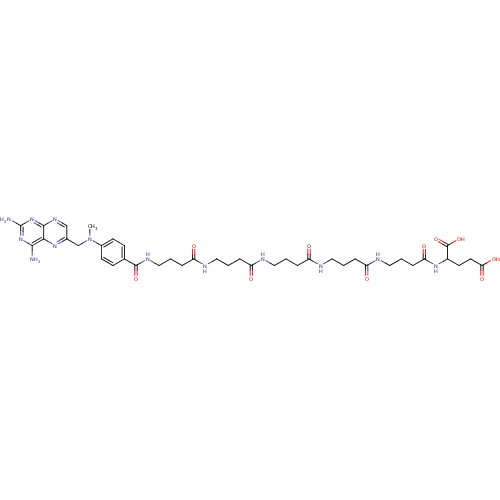 (2-[4-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016339 (2-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50005865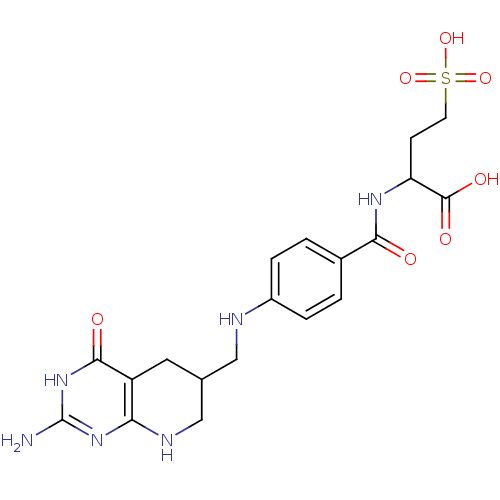 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50080450 ((S)-5-Amino-2-{5-[(2-amino-4-oxo-3,4,5,6,7,8-hexah...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity of the compound as Competitive substrate in the presence of 50 gmM aminopterin towards recombinant Human Folyl-polyglutamate synthas... | J Med Chem 42: 3510-9 (1999) Article DOI: 10.1021/jm9807205 BindingDB Entry DOI: 10.7270/Q2X929GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016342 (2-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50020107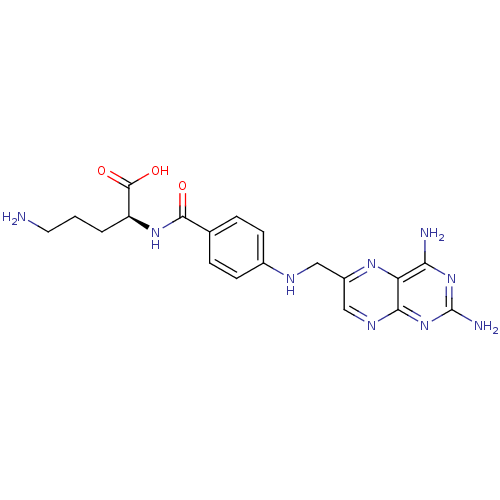 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from mouse | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50020107 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from human | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016341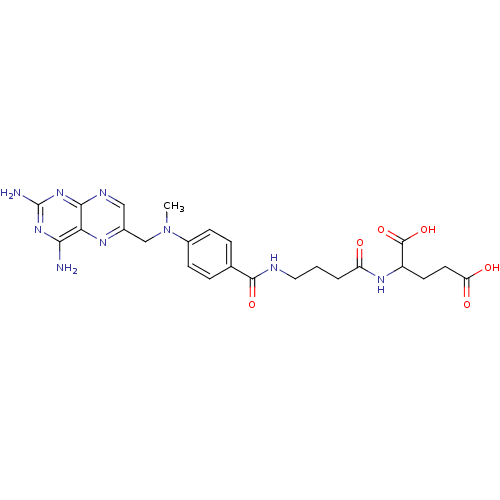 (2-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18796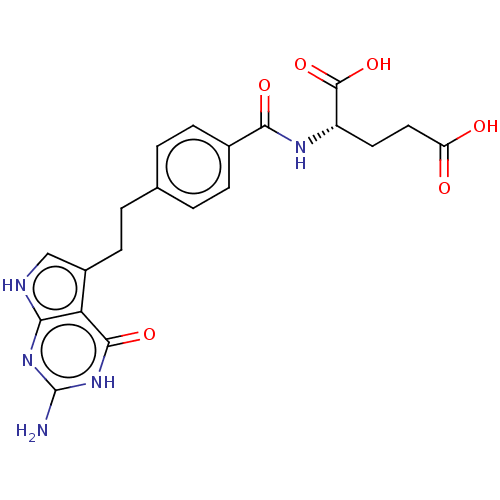 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase | J Med Chem 35: 4450-4 (1992) BindingDB Entry DOI: 10.7270/Q2RR1ZV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50020101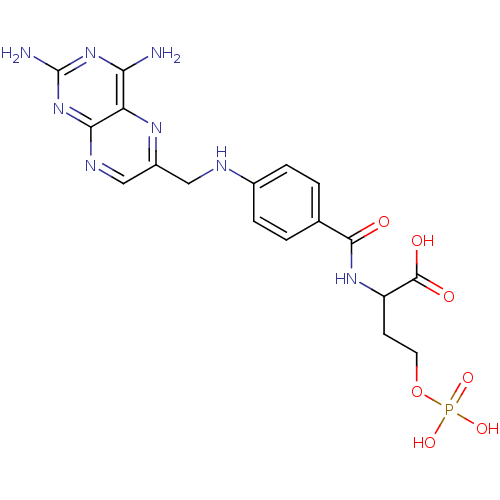 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-ben...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from human | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50006907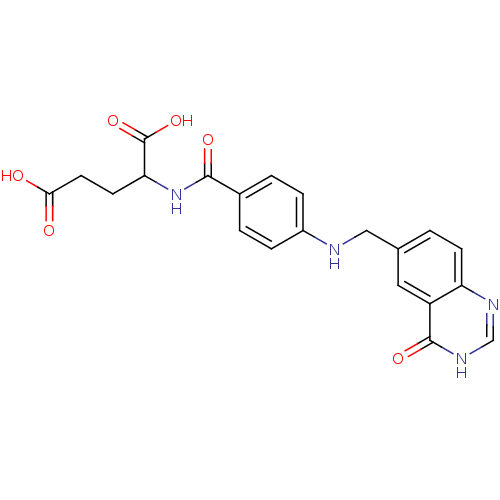 (2-{4-[(4-Oxo-3,4-dihydro-quinazolin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity of the compound towards Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 2626-30 (1992) BindingDB Entry DOI: 10.7270/Q2DV1HVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50006905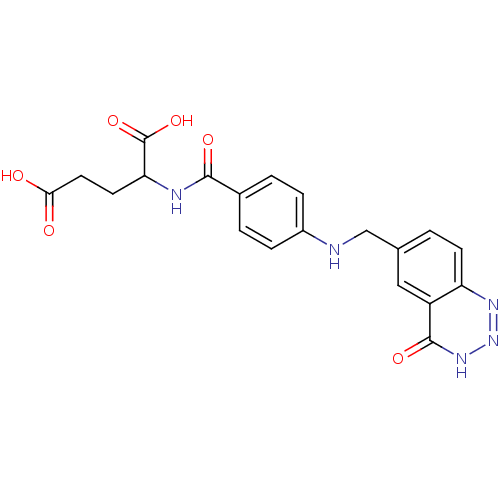 (2-{4-[(4-Oxo-3,4-dihydro-benzo[d][1,2,3]triazin-6-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity of the compound towards Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 2626-30 (1992) BindingDB Entry DOI: 10.7270/Q2DV1HVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50005868 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50002471 (5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50020102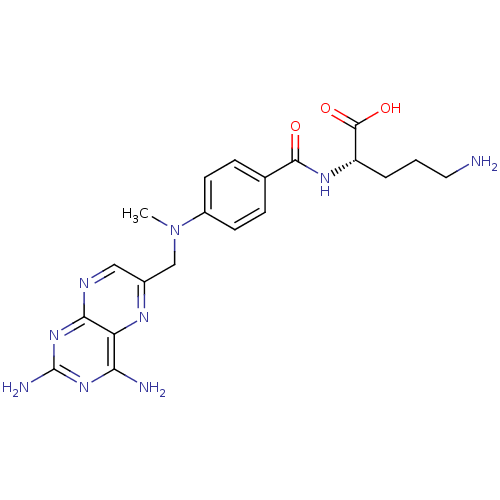 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from rat | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50020102 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from human | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50005859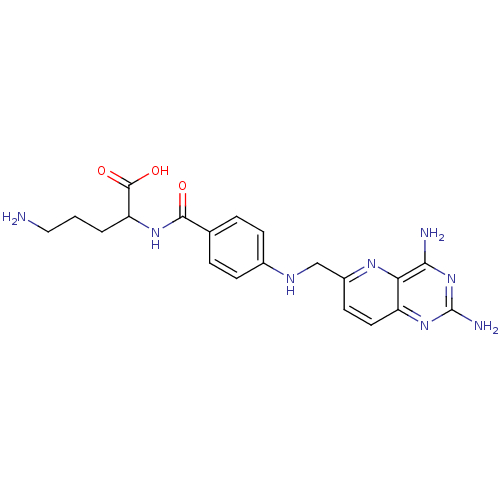 (5-Amino-2-{4-[(2,4-diamino-pyrido[3,2-d]pyrimidin-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50020108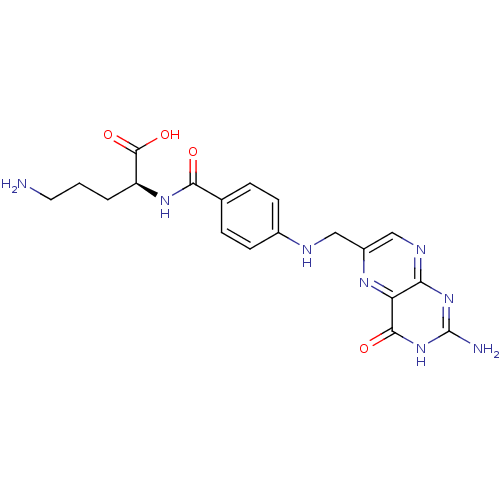 (5-Amino-2-{4-[(2-amino-4-hydroxy-pteridin-6-ylmeth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from hog | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50020101 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-ben...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from mouse | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50005867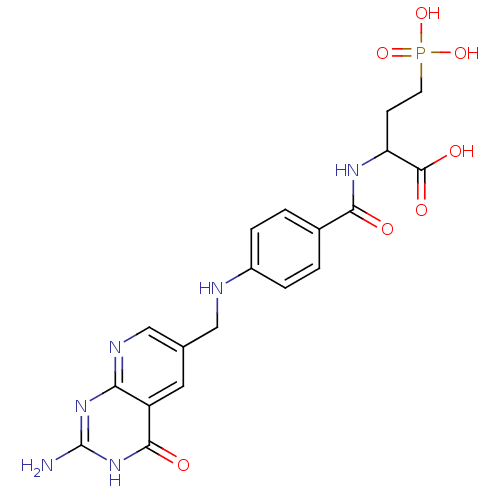 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-pyrido[2,3-d]pyri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50020109 (2-{4-[(2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-pteridi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from hog | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver | J Med Chem 29: 655-60 (1986) BindingDB Entry DOI: 10.7270/Q2RR1ZTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50020102 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from mouse liver | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50011886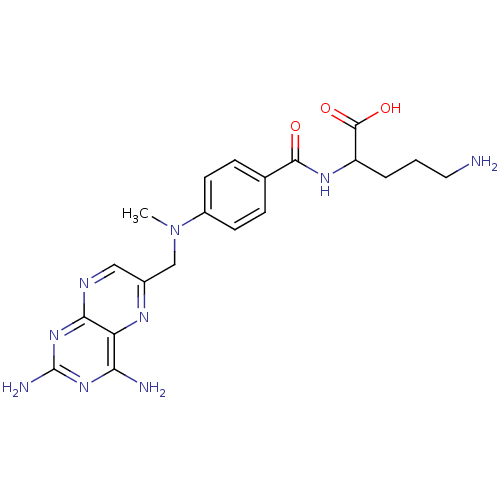 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver | J Med Chem 29: 655-60 (1986) BindingDB Entry DOI: 10.7270/Q2RR1ZTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50005865 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006905 (2-{4-[(4-Oxo-3,4-dihydro-benzo[d][1,2,3]triazin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound was tested for the inhibition of recombinant mouse thymidylate synthase, competitive with 5,10-methylenetetrahydrofolate as variable substra... | J Med Chem 35: 2626-30 (1992) BindingDB Entry DOI: 10.7270/Q2DV1HVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50028119 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-ben...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mouse liver folyl polyglutamate synthetase. | J Med Chem 27: 600-4 (1984) BindingDB Entry DOI: 10.7270/Q2PR7V0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50023710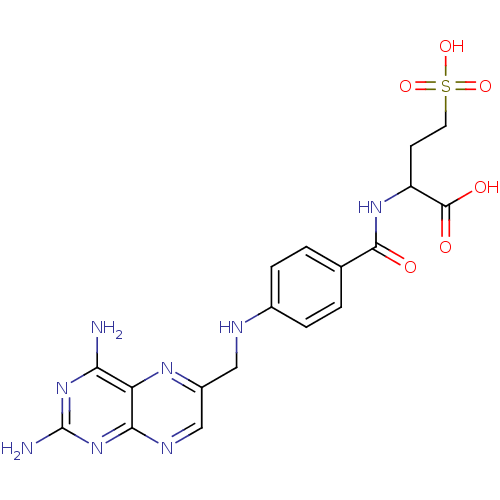 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-ben...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Folyl-polyglutamate synthase from mouse liver | J Med Chem 31: 1326-31 (1988) BindingDB Entry DOI: 10.7270/Q21N8049 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50023710 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-ben...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mouse liver folyl polyglutamate synthetase. | J Med Chem 27: 600-4 (1984) BindingDB Entry DOI: 10.7270/Q2PR7V0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50080449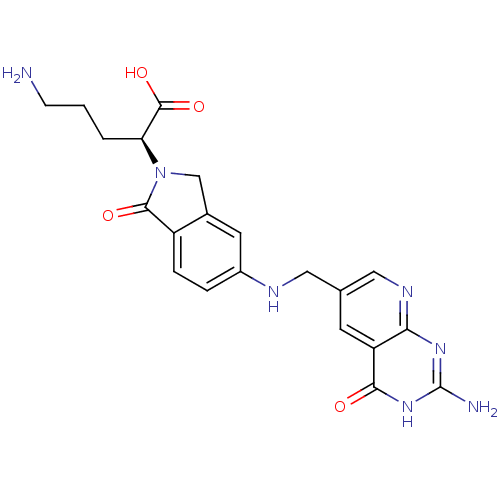 ((S)-5-Amino-2-{5-[(2-amino-4-oxo-3,4-dihydro-pyrid...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity of the compound as Competitive substrate in the presence of 50 gmM aminopterin towards recombinant Human Folyl-polyglutamate synthas... | J Med Chem 42: 3510-9 (1999) Article DOI: 10.1021/jm9807205 BindingDB Entry DOI: 10.7270/Q2X929GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50005860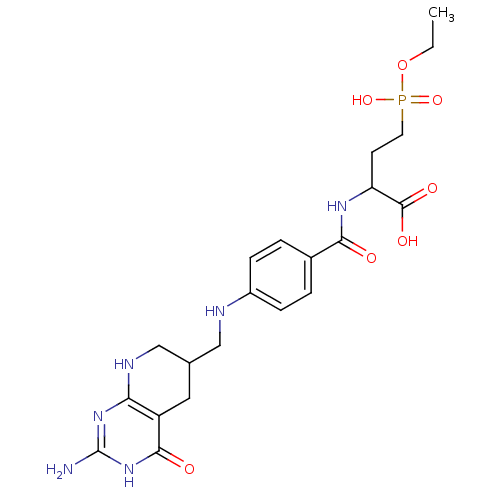 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50028118 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mouse liver folyl polyglutamate synthetase. | J Med Chem 27: 600-4 (1984) BindingDB Entry DOI: 10.7270/Q2PR7V0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver | J Med Chem 29: 655-60 (1986) BindingDB Entry DOI: 10.7270/Q2RR1ZTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50023709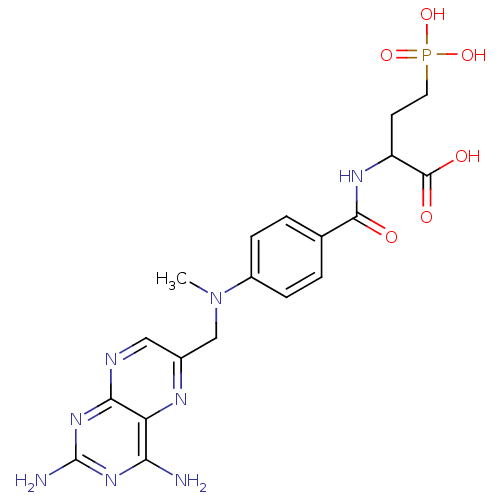 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Folyl-polyglutamate synthase from mouse liver | J Med Chem 31: 1326-31 (1988) BindingDB Entry DOI: 10.7270/Q21N8049 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50023711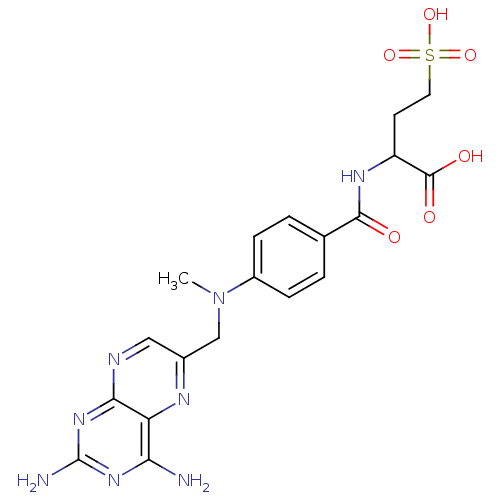 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Folyl-polyglutamate synthase from mouse liver | J Med Chem 31: 1326-31 (1988) BindingDB Entry DOI: 10.7270/Q21N8049 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50023711 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to mouse liver folyl polyglutamate synthetase. | J Med Chem 27: 600-4 (1984) BindingDB Entry DOI: 10.7270/Q2PR7V0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50020099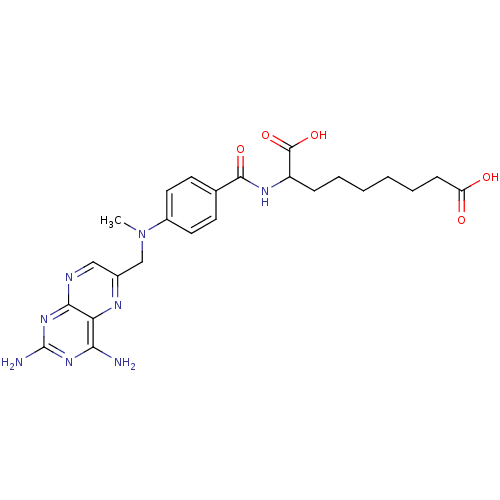 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase from mouse liver | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50005863 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-pyrido[2,3-d]pyri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50005866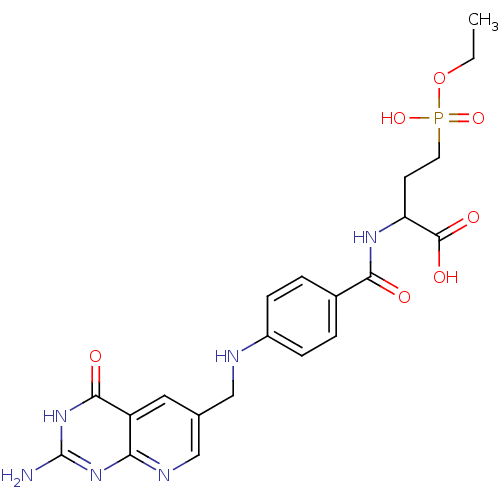 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-pyrido[2,3-d]pyri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25013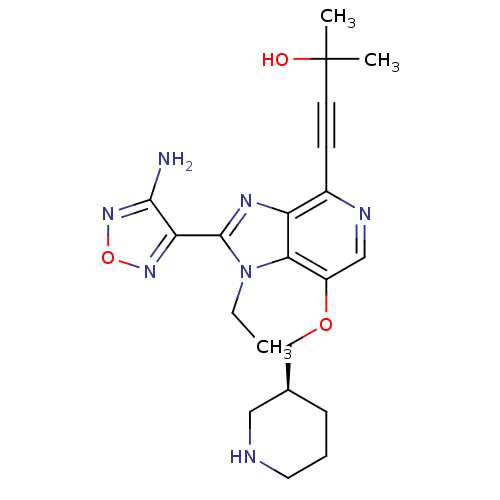 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of AKT1 (unknown origin) after 1 hr by Z'-LYTE kinase assay in presence of 75 uM ATP | ACS Med Chem Lett 5: 462-7 (2014) Article DOI: 10.1021/ml500088x BindingDB Entry DOI: 10.7270/Q2VT1TNG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Bos taurus (Cattle)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against beef liver dihydrofolate reductase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase(DHFR) isolated from L1210 murine leukemia cells | J Med Chem 31: 763-8 (1988) BindingDB Entry DOI: 10.7270/Q24J0FPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of human dihydrofolate reductase (DHFR) | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 138 total ) | Next | Last >> |